Twin Types
In the context of twin research, zygosity refers to the genetic similarity between members of a twin pair. Monozygotic (MZ) twins arise from a single zygote, which splits into two after a number of cell divisions (Hall, Reference Hall2003); these twins are often described as ‘identica’. Dizygotic (DZ) twins arise from two separate zygotes and are often described as ‘fraternal’ or ‘non-identical’ (Figure 1). DZ twins are no more or less genetically similar than any two siblings from the same parents; on average, they share 50% of their genetic variation. Approximately half of DZ twin pairs are the same sex and half are opposite sexes. Like singletons, each member of a DZ pair develops its own chorion (thick outer sac continuous with the placenta, Figure 1). Members of ‘dichorionic’ twin pairs develop in two placentas, while ‘monochorionic’ twins share the same placenta (Hall, Reference Hall2003; McNamara et al., Reference McNamara, Kane, Craig, Short and Umstad2016). All DZ twins are dichorionic, but approximately two-fifths of MZ twins are dichorionic and three-fifths are monochorionic. About 1% of MZ twins are both monochorionic and monoamniotic (sharing a single amniotic sac) and are known popularly as ‘MoMo’ twins. Certain rare types of twins and twin-related phenomena, as well as the origin of twins, are discussed in detail elsewhere (Hall, Reference Hall2003; McNamara et al., Reference McNamara, Kane, Craig, Short and Umstad2016).
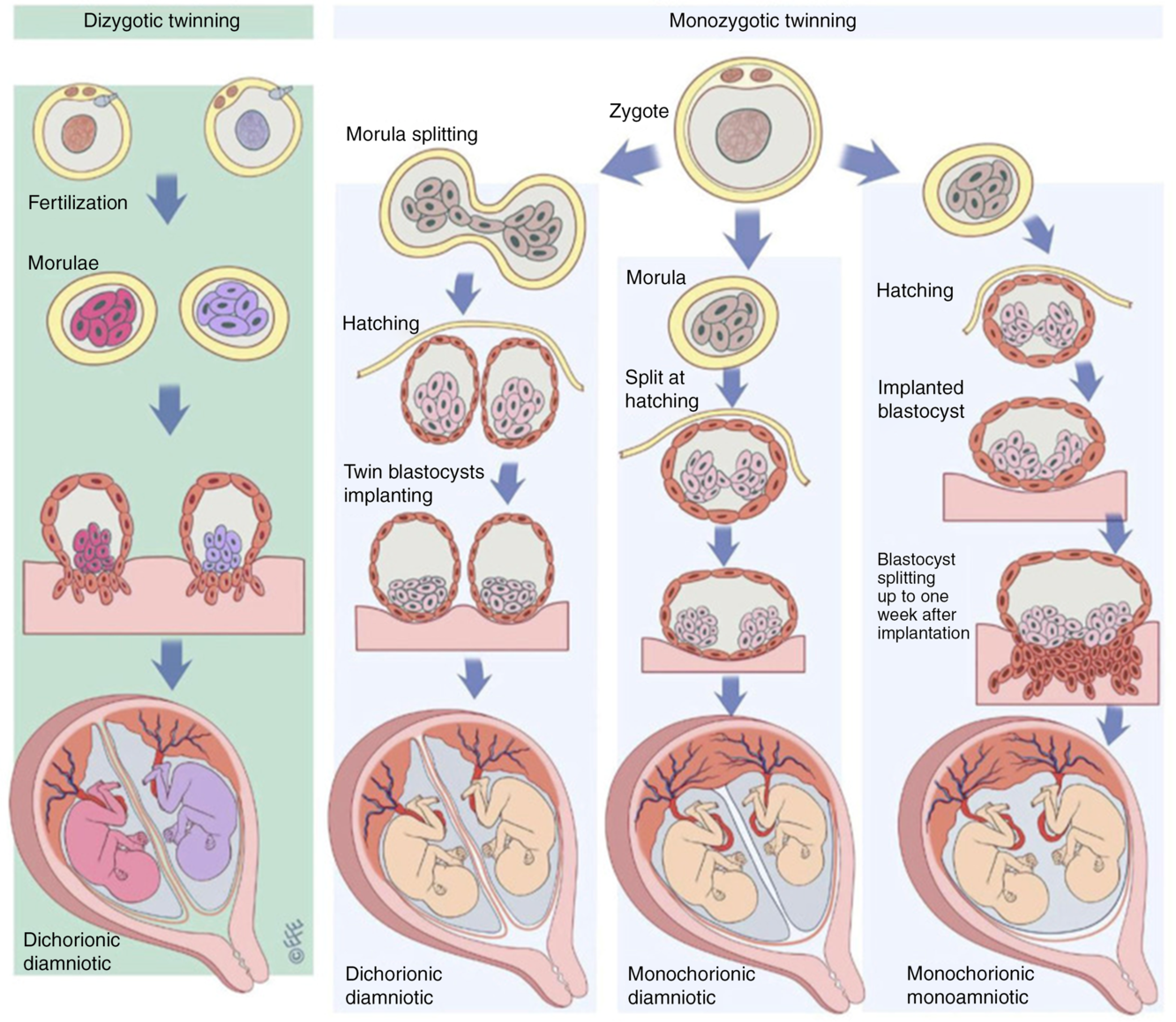
Fig. 1. The formation of dizygotic and monozygotic twins. Dizygotic twins are the product of two fertilization events resulting in dichorionic diamniotic twins with each twin developing to become a genetically distinct individual. Monozygotic twins result from post-zygotic splitting of the product of a single fertilization event. Dichorionic diamniotic twins result from splitting up to the morula stage, monochorionic diamniotic twins from splitting at the blastocyst hatching stage and monochorionic monoamniotic from splitting at the implantation stage. Reproduced with permission from McNamara et al. (Reference McNamara, Kane, Craig, Short and Umstad2016).
The Value and Variety of Twin Study Designs
With rare exceptions, findings from twin studies are applicable to singletons. For example, twins and singletons share a similar incidence of chronic diseases (Martin et al., Reference Martin, Boomsma and Machin1997) and neurodevelopmental disorders (Lorenz, Reference Lorenz2012). These similarities underscore the value of twin research for the population as a whole. However, monochorionic twins have a higher rate of morbidity and mortality than singletons, whereas twins of all types tend to have shorter gestation and lower birth weight than singletons. Although these factors have little effect on chronic disease incidence, studies of neurodevelopmental disorders are advised to adjust for them (Lorenz, Reference Lorenz2012). To date, cerebral palsy is the only condition with a higher incidence in twins after adjusting for gestational age and birth weight (Bonellie et al., Reference Bonellie, Currie and Chalmers2005), prompting caution when findings on this condition in twins are extrapolated to singletons.
Classical Twin Models
Various analytic models have capitalized on characteristics unique to twins (reviewed in Boomsma et al. (Reference Boomsma, Busjahn and Peltonen2002)). Early twin studies compared phenotypic similarity within MZ twin pairs to that within DZ pairs to establish the proportion of variance in a given trait that is due to genetic variation within a population (also known as heritability) and the proportion due to environmental variation. Such heritability studies are often called ‘classical twin models’. In 1993, using a classical twin model with both MZ and DZ pairs, Berkovic et al. (Reference Berkovic, Howell, Hay and Hopper1993) confirmed an earlier finding that epilepsy is highly heritable and challenged the causal role of perinatal factors in this illness. They also found that twins and singletons have a similar risk of epileptic seizures, a result with important implications for medical practice. A few years later, a study using a similar model calculated a high heritability for autism spectrum disorders (Bailey et al., Reference Bailey, Le Couteur, Gottesman, Bolton, Simonoff, Yuzda and Rutter1995) which had previously been ascribed to lack of maternal warmth.
Classical twin models can also estimate, for a given phenotype, the proportion of variance due to genetics (the sequence of DNA itself), shared environment (all exposures that twins share) and nonshared environment (exposures and stochastic effects that differ within pairs). Such models were in their infancy 33 years ago. They use statistical techniques in which phenotypic variance is partitioned into variance due to additive genetic effects (abbreviated A), common or shared environment (C) and nonshared or unique environment (E) (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002). These three types of variance are usually expressed as ratios that sum to one. ‘Additive’ implies that the number of genetic variants correlates with the severity of the phenotype in a linear manner. Additive factors, along with other genetic effects such as dominance, are used in estimating heritability. Heritability is a proportion rather than an absolute number, while genotype is largely fixed at conception. Therefore, the estimate of heritability is inversely proportional to the total variation in phenotype that can be increased by geographic and age-related factors. For example, height is less heritable in developing countries with higher levels of environmental adversity than in developed countries with less adversity (Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003), while the heritability of body mass index (BMI) increases from birth to childhood and then declines in adulthood (Elks et al., Reference Elks, den Hoed, Zhao, Sharp, Wareham, Loos and Ong2012). These and other issues have led many researchers to advise caution when interpreting heritability estimates (Hopper, Reference Hopper1993; Turkheimer, Reference Turkheimer2011). Details of other types of statistical methods applied to the analysis of variance components in twin studies are reported elsewhere (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002; Hopper, Reference Hopper1993).
Twin Differences Models
‘Twin differences’ models, which focus on comparisons within MZ pairs, were developed in the early 1940s but have not been used as extensively as classical twin models. Within-pair analysis of MZ twins controls for paternity, maternal factors during pregnancy, gestational age, location and season of birth, postnatal familial factors, age, ethnicity, sex and genetics (Hall, Reference Hall2003; McNamara et al., Reference McNamara, Kane, Craig, Short and Umstad2016). Within-pair analysis of DZ twin pairs controls for all these factors with the exception of genetics, for which only 50% of variation is controlled, and sex that is controlled only in same-sex pairs.
Although within-DZ pair designs have been used less often than MZ designs, one approach is worth highlighting. Within-pair analysis of opposite-sex DZ pairs can address sex differences in the risk, severity, outcomes and response to intervention of a specific disease or disorder. For example, a study of opposite-sex DZ pairs concordant for autism spectrum disorder (Robinson et al., Reference Robinson, Lichtenstein, Anckarsater, Happe and Ronald2013) found that boys had more severe symptoms than their twin sisters. These results corroborated a previous hypothesis that female sex could protect against autism. Other notable applications of this approach include findings that males are more vulnerable to delinquency than females (Newsome et al., Reference Newsome, Vaske, Gehring and Boisvert2016) and that males and females differ on some causes of depression (Kendler & Gardner, Reference Kendler and Gardner2014).
The co-twin control model is a type of twin differences model that focuses exclusively on MZ pairs who are discordant either for a disease or for an exposure, such as an environmental factor. This model has recently been used to investigate environmental contributions to the associations between BMI and cardiovascular disease (Song et al., Reference Song, Lee and Sung2015), between height and asthma (Protudjer et al., Reference Protudjer, Lundholm and Almqvist2015) and between pain risk factors and low back pain (Oliveira et al., Reference Oliveira, Ferreira, Refshauge, Maher, Griffin, Hopper and Ferreira2015). One notable study with this design demonstrated that smoking was associated with a reduction of 5–10% in bone density, thereby conferring a high risk of fracture (Hopper & Seeman, Reference Hopper and Seeman1994).
Clinical Trials
An especially efficient version of the co-twin control model has been used in clinical trials, usually those aimed at establishing the effectiveness of a medical intervention. This approach can use either MZ pairs exclusively or MZ and same-sex DZ pairs in combination. One member of each twin pair is randomized to the treatment group and the other to the control group, and then the association between exposure and outcome is analyzed from the perspective of within-pair differences. This model can achieve up to seven times more statistical power to detect an intervention effect than can traditional randomized controlled trials (Carr et al., Reference Carr, Martin and Whitfield1981)
Unfortunately, twins remain underrepresented in clinical trials (Yelland et al., Reference Yelland, Sullivan and Makrides2015). Even when they are included, analytical methods are often inadequately described or do not account for twin clustering, which is a function of the common environment and genetic background shared by each twin pair (Hibbs et al., Reference Hibbs, Black, Palermo, Cnaan, Luan, Truog and Ballard2010). One notable exception was a meta-analysis of 34 randomized controlled trials with an aggregated sample of 14,106 singletons and 2578 twins (both MZ and DZ; Saccone et al., Reference Saccone, Saccone and Berghella2015) This analysis found insufficient evidence to support the routine use of Omega-3 supplements during pregnancy to prevent preterm birth and other complications, such as preeclampsia. Another example is an ongoing trial to test an intervention to improve sleep quality in patients with low back pain (Pinheiro et al., Reference Pinheiro, Morosoli, Ferreira, Madrid-Valero, Refshauge, Ferreira and Ordonana2018). A further example is a clinical trial that found that dietary fat intake is the main influencer of fat taste sensitivity, which is a marker of satiety and a contributory factor for obesity (Costanzo et al., Reference Costanzo, Nowson, Orellana, Bolhuis, Duesing and Keast2018).
Gene–Environment Interactions
Gene–environment correlation occurs when exposure to a given environment is associated with a specific genotype. Gene–environment interaction occurs when people with different genotypes respond to the same environment in different ways. Studies investigating such phenomena are essential to understanding the interplay between genes and environment, and they have great potential to inform the personal tailoring of medical care. A 2015 study of MZ and DZ twins found that genetic effects on musical accomplishment were most pronounced among musicians who practiced frequently (Hambrick & Tucker-Drob, Reference Hambrick and Tucker-Drob2015). Another study found that a genetic disposition to depression in MZ and DZ co-twins significantly interacted with environmental triggers, such as stressful life events, to precipitate depressive episodes (Kendler et al., Reference Kendler, Kessler, Walters, MacLean, Neale, Heath and Eaves1995). Another, using an unspecified number of MZ and DZ twin pairs, found that gene–environment interactions can influence levels of gene expression (Buil et al., Reference Buil, Brown, Lappalainen, Vinuela, Davies, Zheng and Dermitzakis2015).
Cause Versus Association
Some twin models are well suited to studies that seek to differentiate between cause and association in human health (reviewed in Hrubec & Robinette, Reference Hrubec and Robinette1984; and van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012). Their sensitivity stems from the unique capacity of twin designs, especially the MZ co-twin design, to control for variables that often confound population studies. For example, a study with 1196 MZ and 1352 DZ twin pairs showed that even though regular exercise is associated with reductions in symptoms of anxiety and depression in the general population, the association is not due to the effects of exercise (De Moor et al., Reference De Moor, Boomsma, Stubbe, Willemsen and de Geus2008). This conclusion was based on findings that MZ twin pairs discordant for physical activity reported similar levels of anxiety and depression. A study with a similar design in 157 MZ twin pairs found that later bedtimes and shorter sleep duration predicted subsequent development of depression, anxiety and self-injury (Matamura et al., Reference Matamura, Tochigi, Usami, Yonehara, Fukushima, Nishida and Sasaki2014). A longitudinal study of 2464 elderly Danish twins noted associations among smoking, BMI and longevity, but concluded that the links between smoking and mortality and between BMI and mortality were more likely to result from environmental effects than from genetics (Herskind et al., Reference Herskind, McGue, Iachine, Holm, Sorensen, Harvald and Vaupel1996). More recently, a study of 503 MZ twin pairs found a causal relationship between measures of stress and tension and subsequent depression and anxiety (Davey et al., Reference Davey, Lopez-Sola, Bui, Hopper, Pantelis, Fontenelle and Harrison2016). Therefore, interventions that successfully minimize stress and tension should also have positive effects on depression and anxiety.
Emerging Uses of Twins in Medical Research
Epigenetics and Twins
Epigenetics is the study of changes in an organism over time caused by the addition and removal of small molecules in DNA and DNA-packaging proteins, without any changes in DNA sequence. Well-studied examples of epigenetic processes include the covalent addition of a methyl group (CH3) to the cytosine nucleotide of a cytosine–guanine sequence (CpG site) and the covalent addition of an acetyl group (C2H3O) to DNA-packaging histone proteins. Epigenetic changes are intrinsic to mammalian development and are often susceptible to influence by environmental conditions that can be internal (e.g., fetal nutrient availability) or external (e.g., socioeconomic status; Chiarella et al., Reference Chiarella, Tremblay, Szyf, Provencal and Booij2015). Studies involving genome-wide analysis of DNA methylation have begun to identify causal mechanisms and diagnostic biomarkers for complex human diseases (Mikeska & Craig, Reference Mikeska and Craig2014).
Twin studies in particular can yield valuable insights in epigenetics and its application to the epigenome — that is, the sum total of epigenetic marks in any given tissue. This research has been reviewed elsewhere (Bell & Saffery, Reference Bell and Saffery2012; Chiarella et al., Reference Chiarella, Tremblay, Szyf, Provencal and Booij2015; Martin et al., Reference Martin, Boomsma and Machin1997; van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012), so we limit our focus to epigenome-wide studies involving twins, especially studies of the etiology of human disease and the variance components of DNA methylation itself. We discuss the role of epigenetics in the developmental origins of disease in the next section.
Epigenome-wide association studies analyze the associations between DNA methylation and a wide variety of environments, phenotypes and diseases (Bell & Saffery, Reference Bell and Saffery2012; Martin et al., Reference Martin, Boomsma and Machin1997; van Dongen et al., Reference van Dongen, Slagboom, Draisma, Martin and Boomsma2012). Most have used microarrays (DNA sequences laid out on glass slides) that enable quantification of DNA methylation in regions of the genome that regulate gene activity. Given the possibility of environment- and phenotype-discordant co-twin designs, epigenome-wide association studies with MZ twins offer greater power than studies with singletons, because MZ twins present essentially no variation in the DNA sequence itself. Such studies are recommended as a first step in the epigenetic analysis of human disease phenotypes (Rakyan et al., Reference Rakyan, Down, Balding and Beck2011), and notable recent examples include epigenome-wide association studies of twins discordant for cerebral palsy (Mohandas et al., Reference Mohandas, Bass-Stringer, Maksimovic, Crompton, Loke, Walstab and Craig2018), multiple sclerosis (Souren et al., Reference Souren, Gerdes, Lutsik, Gasparoni, Beltran, Salhab and Walter2019) and BMI (Li et al., Reference Li, Wong, Bui, Nguyen, Joo, Stone and Hopper2019).
However, epigenome-wide association studies with exposure–discordant pairs are uncommon, and to our knowledge, the only study of this kind performed to date involved 20 MZ twin pairs discordant for smoking (Allione et al., Reference Allione, Marcon, Fiorito, Guarrera, Siniscalchi, Zijno and Matullo2015). Even with this limited sample, the study identified eight loci previously associated with smoking in singletons.
The DNA methylation level at each CpG site in the genome in any given tissue can be treated as a separate phenotype and analyzed accordingly by statistical modeling, with the caveat that levels of DNA methylation within a region of hundreds of base pairs are often highly correlated. Studies using this approach have revealed a wide range in the influence of genetic, shared and nonshared environments on variation in DNA methylation. Using microarrays, researchers have found that the average heritability of DNA methylation across the genome is relatively low (McRae et al., Reference McRae, Powell, Henders, Bowdler, Hemani, Shah and Montgomery2014; van Dongen et al., Reference van Dongen, Nivard, Willemsen, Hottenga, Helmer, Dolan and Boomsma2016). A recent study of DNA methylation at more than 410,000 CpG sites in peripheral blood leukocytes of 769 MZ and 424 DZ twin pairs found a heritability of .19, a nonshared environmental component of .81 and negligible evidence for an effect of shared environment (van Dongen et al., Reference van Dongen, Nivard, Willemsen, Hottenga, Helmer, Dolan and Boomsma2016). Another study using the same microarray technology and tissue with 614 participants, including 69 MZ and 11 DZ twin pairs, found that highly heritable CpG sites were influenced by genetic variants within hundreds of base pairs (McRae et al., Reference McRae, Powell, Henders, Bowdler, Hemani, Shah and Montgomery2014). A much smaller study using more than 19,000 CpG sites in multiple tissues from 22 MZ and 12 DZ newborn twin pairs also found that the nonshared environment was by far the largest contributor to DNA methylation (Gordon et al., Reference Gordon, Joo, Powell, Ollikainen, Novakovic, Li and Saffery2012). The key role played by the nonshared environment suggests that some combination of stochastic factors, including random biological variation or ‘noise’ (Little et al., Reference Little, Tikhonov and Gregor2013) and twin-specific factors, is the most common influence on the epigenome. The likelihood that most of this variation happens in utero is reviewed in the next section.
The Developmental Origins of Health and Disease
Accumulated evidence from human and animal studies has shown beyond reasonable doubt that most of the environmental components of chronic illness, including cardiometabolic and psychiatric diseases and some cancers, originate early in life (Gluckman et al., Reference Gluckman, Hanson and Buklijas2010). It is also understood that humans are affected by the environment throughout the lifespan, although this influence diminishes over time. The field of developmental origins of health and disease, as it is widely known, arguably originated with the discovery of the link between low birth weight (a proxy for intrauterine growth) and elevated risk of cardiometabolic disease (Barker & Osmond, Reference Barker and Osmond1988) This field has since expanded to include such concepts as developmental mismatch, in which the prenatal maternal environment to which a fetus is exposed differs markedly from the postnatal environment (Gluckman et al., Reference Gluckman, Hanson and Buklijas2010).
Twins have played a prominent role in this research. At a fundamental level, twin studies enable the partitioning of variance in phenotypic outcomes (such as low birth weight) into genetic, shared and nonshared environments. Only twin research can differentiate between the latter two. Except in rare circumstances, all twin pairs share the same mother and father. In addition, the shared environment to which twins are exposed in utero includes aspects of maternal diet and lifestyle, since soluble factors in the mother’s body — including nutrients, oxygen, inflammatory factors, some infectious agents and hormones — are passed to both twins via the placenta and umbilical cords. All twins also share the same season of birth, and most share the same early postnatal family environment. Studies of environmental risk factors involving siblings or unrelated singletons are unable to adequately control for these shared factors.
Nonetheless, despite the important contribution of shared factors to chronic disease risk, nonshared factors are likely to be more influential, as they are in epigenetics. A recent meta-analysis of data on 28 chronic diseases from more than 150,000 European twins found that the sum of shared genetics and shared environment accounted on average for only 18.5% of variance in disease phenotype (Rappaport, Reference Rappaport2016). A recent in-depth study of more than 200 immune parameters in 201 healthy twins found that 58% of these parameters were almost completely determined by nongenetic factors and that nonshared environment dominated (Brodin et al., Reference Brodin, Jojic, Gao, Bhattacharya, Angel, Furman and Davis2015). One seminal study (Plomin & Daniels, Reference Plomin and Daniels1987) from the 1980s highlighted the high rate of discordance for behavioral phenotypes within MZ pairs, concluding that many factors must be capable of producing health differences between identical twins. Research over the ensuing decades has addressed the intrauterine environment as a critical source of influences on the development of individual members of a twin pair (Martin et al., Reference Martin, Boomsma and Machin1997; Stromswold, Reference Stromswold2006).
In almost all twins, the placenta and umbilical cords can be classified as contributors to the nonshared environment because of their physical and biological variability within pairs. Such variation can arise from differences in the uterine implantation site; in the length, width, torsion, knotting and number of vessels of the umbilical cord; and in the location where the umbilical cord is inserted. All these factors have the potential to affect the growth rate and the rate of transplacental transport of oxygen, nutrients and teratogens in individual twins. In addition, any twin pair can be discordant for prenatal (Dickinson et al., Reference Dickinson, Keil and Charles2006; Pimentel et al., Reference Pimentel, Szymanski, Samuel and Meier2012) or perinatal (Jamieson et al., Reference Jamieson, Read, Kourtis, Durant, Lampe and Dominguez2007) infection, whether viral (Dickinson et al., Reference Dickinson, Keil and Charles2006) or bacterial (Pimentel et al., Reference Pimentel, Szymanski, Samuel and Meier2012) or concordant for infection but discordant for severity of symptoms (Schiesser et al., Reference Schiesser, Sergi, Enders, Maul and Schnitzler2009). Inflammation of the umbilical cord (funisitis) and the placenta (chorioamnionitis) can be confined to the placenta of a single twin (Dickinson et al., Reference Dickinson, Keil and Charles2006). If infectious agents enter the embryo via the placenta and cord, this process might be a mechanism for discordant placental inflammation, which would lead to discordant prenatal infection. Another potential source of discordance could be the ability of each placenta to induce a separate maternal immune response (Yusuf & Kliman, Reference Yusuf and Kliman2008). Once an infection appears in the amniotic sac of one twin, layers of amnion — and in dichorionic twins, of chorion, in addition to the co-twin’s placenta — could protect the co-twin from its spread.
Hrubec and Robinette (Reference Hrubec and Robinette1984) reported that MZ co-twin studies had already revealed associations linking low birth weight with cerebral palsy as well as schizophrenia. Since then, studies using this design have found associations linking higher birth weight with cognition in very early childhood (Halling et al., Reference Halling, Malone, Breathnach, Stewart, McAuliffe and Morrison2015) and with higher BMI in adolescence (Yokoyama et al., Reference Yokoyama, Jelenkovic, Sund, Sung, Hopper, Ooki and Silventoinen2016) and adulthood (Pietilainen et al., Reference Pietilainen, Kaprio, Rasanen, Rissanen and Rose2002), as well as associations linking lower birth weight with higher blood pressure in childhood (Dwyer et al., Reference Dwyer, Blizzard, Morley and Ponsonby1999), with early onset of puberty (Schulte et al., Reference Schulte, Wolfle, Schreiner, Stoffel-Wagner, Peter, Bartmann and Gohlke2016) and with risk of type 2 diabetes in adulthood (Poulsen et al., Reference Poulsen, Vaag, Kyvik, Moller Jensen and Beck-Nielsen1997). Dwyer et al. (Reference Dwyer, Blizzard, Morley and Ponsonby1999) concluded that the critical step in the causal pathway from low birth weight to hypertension must involve mechanisms in the placenta, cord and fetus, with maternal exposures operating only as antecedents or primers. Collectively, these data add to the evidence that prenatal factors have long-lasting effects on health. In addition, birth weight is correlated with placenta weight, with a higher correlation within pairs than between pairs (Gielen et al., Reference Gielen, Lindsey, Derom, Loos, Derom, Nijhuis and Vlietinck2007). This again suggests that nonshared factors have more bearing on later health outcomes than do shared factors. In a within-pair model, higher birth weight has also been associated with a more central umbilical cord insertion (Gielen et al., Reference Gielen, Lindsey, Derom, Loos, Derom, Nijhuis and Vlietinck2006), which in turn has been linked with more favorable long-term health outcomes (Antoniou et al., Reference Antoniou, Derom, Thiery, Fowler, Southwood and Zeegers2011).
Epigenomic studies have also been used to study the early-life origins of disease in twins. One set of studies found that at birth, the epigenetic mark of DNA methylation exhibits a range of within-pair variation throughout the genome (Gordon et al., Reference Gordon, Joo, Powell, Ollikainen, Novakovic, Li and Saffery2012). Results also showed that a small number of MZ twin pairs are more epigenetically discordant even than some unrelated individuals, emphasizing the formative nature of time spent in the womb. In addition, these studies have found that within-pair differences in DNA methylation and expression of genes involved in lipid metabolism, immune function and cardiometabolic function are associated with birth weight discordance. This finding is consistent with the previously established role of DNA methylation in mediating the link between low birth weight and chronic disease risk (Gluckman, Reference Gluckman2012).
Microbiota
Recent research using DNA sequencing has revealed the existence of a large number of previously unrecognized species of microbiota that cannot be grown in culture. This work has identified roles for microbiota in regulating metabolism, immunity and brain function (Spector, Reference Spector2015). Central to this developing field is the question of whether genetic or environmental variation plays a larger role in the diversity of microbiota in each somatic niche. It is therefore no surprise that twin studies have already made prominent contributions to this field.
A study of the gut microbiome in 416 MZ and DZ adult twin pairs found that host genetics plays a role in the frequency of numerous bacterial species, even though bacteria vary widely in heritability (Goodrich et al., Reference Goodrich, Waters, Poole, Sutter, Koren, Blekhman and Ley2014) However, a similar study of the dental plaque microbiome from 242 MZ and DZ twin pairs in childhood found that although most variation in the microbiome was determined by environmental factors, highly heritable bacterial species were also present (Gomez et al., Reference Gomez, Espinosa, Harkins, Leong, Saffery, Bockmann and Nelson2017). Another recent study investigated MZ twins in Malawi who were discordant for kwashiorkor, a malnutrition disorder (Smith et al., Reference Smith, Yatsunenko, Manary, Trehan, Mkakosya, Cheng and Gordon2013). When germ-free mice were given a combination of the Malawian diet and fecal transplant from either affected or healthy twins, kwashiorkor developed only in mice that received the transplant from affected twins. This finding implicated microbial imbalance as a major causal factor for this disorder. Using a co-twin control model, Cernada et al. (Reference Cernada, Bauerl, Serna, Collado, Martinez and Vento2016) studied bacterial DNA and gene expression in exfoliated host epithelial cells in the gut from DZ twins discordant for neonatal sepsis. They found that gut microbiomes differed within pairs at birth, and that microbiota in affected twins induced the expression of genes involved in inflammatory and oxidative stress pathways in gut epithelial cells. A similar co-twin model was used to investigate neonatal fecal microbiomes in a pair of same-sex dichorionic twins of unknown zygosity who were discordant for necrotizing enterocolitis, a serious disease that occurs when intestinal tissues become damaged and begin to die (Hourigan et al., Reference Hourigan, Ta, Wong, Clemency, Provenzano, Baveja and Niederhuber2016). Results showed that the twins had distinctly different microbiomes, and that changes in microbiome complexity preceded clinical symptoms. Large, longitudinal studies of twins with a range of pathologies will doubtless reveal much more about the bacterial, viral and fungal origins of disease.
Stem Cell Models of Disease
Patient-derived induced pluripotent stem cells (those capable of giving rise to several different cell types) are used with increasing frequency to model disease in vitro. In this method, patient cells, typically from skin or blood, are de-differentiated in vitro to a state in which they can be re-differentiated into disease-relevant cell types while still retaining genetic and possibly epigenetic signatures of disease. Stages of early development can then be recapitulated in the desired cell type, and genotype–phenotype associations can be quantified. To our knowledge, only three studies have adopted this approach with twins.
In a study of neurons derived from stem cells contributed by MZ twins discordant for Parkinson’s disease, the affected twin’s neurons showed lower levels of dopamine, increased expression of the monoamine oxidase gene whose product degrades dopamine and impaired neural network activity (Woodard et al., Reference Woodard, Campos, Kuo, Nirenberg, Nestor, Zimmer and Noggle2014). These findings guided the development of an in vitro treatment that could eventually lead to in vivo treatment for Parkinson’s. Another study of MZ twins discordant for trisomy 21 found epigenetic changes in the promoter regions of genes on other chromosomes involved in the growth of embryonic organs, yielding important insights into the mechanism by which this condition develops (Sailani et al., Reference Sailani, Santoni, Letourneau, Borel, Makrythanasis, Hibaoui and Antonarakis2015). Finally, neurons derived from stem cells were used to study MZ twins discordant for Rett syndrome, which affects intellectual and physical development, primarily in females. Findings identified associated this syndrome with defects in the behavior of astrocytes (support cells in the brain) accompanied by changes in the expression of several genes (Andoh-Noda et al., Reference Andoh-Noda, Akamatsu, Miyake, Matsumoto, Yamaguchi, Sanosaka and Okano2015). A surprising result from this work is that epigenetic and genetic differences within MZ pairs were maintained in lineages derived from stem cells. These and similar results underscore the critical importance of using twin samples in stem cell models of human disease.
Twin Registries and International Networks
Over the past 36 years, twin registries have become increasingly sophisticated. Today, they play an important role in facilitating twin studies for medical research, especially by recruiting twin participants through population-based and volunteer-based methods. In addition, they increasingly collect and store biological samples along with other twin data, applying complex systems for database management, data handling and confidentiality (Hur & Craig, Reference Hur and Craig2013).
The International Network of Twin Registries was recently formed to foster scientific collaboration and promote twin research globally by expanding the resources of participating twin registries, most notably in the form of a catalogue of twin data and biospecimens available for new twin studies (Buchwald et al., Reference Buchwald, Kaprio, Hopper, Sung, Goldberg, Fortier and Harris2014). The Network was created in 2011 as a working group of the International Society of Twin Studies, which was established only eight years before Hrubec and Robinette’s review. Network members share expertise in managing twin registries, conducting twin studies and analyzing data.
Working in conjunction with these organizations, the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) has assembled the largest international dataset ever created for twin analyses. CODATwins unites representatives from more than 40 twin cohorts in 22 countries who share data on more than 400,000 twins, including more than 800,000 measures of height and weight (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). Collaborators aim to study the variance components and early-life antecedents of height and BMI, as well as their variation by age, ethnicity and country. To date, researchers with CODATwins have found that DZ twins in childhood are taller and have higher BMI than MZ twins (Jelenkovic et al., Reference Jelenkovic, Yokoyama, Sund, Honda, Bogl, Aaltonen and Silventoinen2015), that genetic factors play a major role in the variation of BMI among adolescent populations of different ethnicities exposed to different obesogenic factors (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016) and that first-born twins are slightly taller and have higher BMI than their second-born co-twins (Yokoyama et al., Reference Yokoyama, Jelenkovic, Sund, Sung, Hopper, Ooki and Silventoinen2016). Finally, the ‘Mothers of Twins Clubs’ cited by Hrubec and Robinette (Reference Hrubec and Robinette1984) as a key resource to identify twins for research engagement have since evolved into multiple birth associations established across the world, with global representation through the International Council of Multiple Birth Organisations.
Conclusion
In 1984, Hrubec and Robinette described the application of twin models in medical research. Our review discusses how far this endeavor has progressed over the past three and a half decades by singling out the studies that in our view illustrate the best new work. Twin studies now benefit from an expanded set of statistical models and a concerted global effort to coordinate twin research projects. They have altered the way we think about the etiology of such disorders as epilepsy, autism and schizophrenia. They have also demonstrated that heritability can differ according to age, socioeconomic status and total phenotypic variance. Research involving twins is poised to yield great benefit to medicine, especially by applying findings from twins to non-twin populations in order to illuminate the variety of factors that make us human.
Acknowledgments
The authors thank Raymond M. Harris, PhD, for his invaluable reviews of the manuscript. JMC is supported by grants from the Australian National Health and Medical Research Council (grant numbers 1011070 and 1083779), the Financial Markets Foundation For Children (grant number 032–2007) and the Murdoch Children’s Research Institute, which is funded by the Victorian Government’s Operational Infrastructure Support Program. LCF and JMC are supported by a Centre of Research Excellence Grant (1079102) from the National Health and Medical Research Council of Australia. LCF is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education, Brazil.



