Research involving human subjects is an essential component of furthering the knowledge of the etiology, characteristics, and treatment of human medical conditions and disorders. The realization of research relies on the ethical concerns for, and participation of, the study subjects. Informed consent –– or an explicit waiver of the need for it from an ethical review board –– is a cornerstone for recruitment of study participants for medical research (Rothstein & Shoben, Reference Rothstein and Shoben2013). When the choice to consent is dependent on characteristics pertaining to the individual or context, the selection of participants for the study is consequently non-random, which introduces the possibility of bias in estimations of associations within the sample (Groenwold et al., Reference Groenwold, van der Graaf and van Delden2013; Hernan et al., Reference Hernan, Hernandez-Diaz and Robins2004)
It has previously been demonstrated that individuals who consent to medical research participation may be different in several ways from those who decline participation, but consistent patterns are lacking (Kho et al., Reference Kho, Duffett, Willison, Cook and Brouwers2009). For example, several studies have shown that males were more likely to consent (Damery et al., Reference Damery, Ryan, McManus, Warmington, Draper and Wilson2011; Knies et al., Reference Knies, Burton and Sala2012; Matsui et al., Reference Matsui, Kita and Ueshima2005; Schwartz et al., Reference Schwartz, Brecher, Whyte and Klein2005; Woolf et al., Reference Woolf, Rothemich, Johnson and Marsland2000), whereas others have not demonstrated any sex difference in consent rates (Al-Shahi et al., Reference Al-Shahi, Vousden and Warlow2005; Baker et al., Reference Baker, Shiels, Stevenson, Fraser and Stone2000; Beebe et al., Reference Beebe, Ziegenfuss, Jenkins, Haas and Davern2011; Buckley et al., Reference Buckley, Murphy, Byrne and Glynn2007; Huang et al., Reference Huang, Shih, Chang and Chou2007; Klassen et al., Reference Klassen, Lee, Barer and Raina2005; McKinney et al., Reference McKinney, Jones, Parslow, Davey, Darowski and Chaudhry2005) and some have seen a higher likelihood of consent among females (Dunn et al., Reference Dunn, Jordan, Lacey, Shapley and Jinks2004; Kho et al., Reference Kho, Duffett, Willison, Cook and Brouwers2009). The age of the potential study participants may play a part in the choice to consent. Previous studies have shown that relatively older individuals may be more (Beebe et al., Reference Beebe, Ziegenfuss, Jenkins, Haas and Davern2011; Damery et al., Reference Damery, Ryan, McManus, Warmington, Draper and Wilson2011; Woolf et al., Reference Woolf, Rothemich, Johnson and Marsland2000) or less (Dunn et al., Reference Dunn, Jordan, Lacey, Shapley and Jinks2004; Huang et al., Reference Huang, Shih, Chang and Chou2007; Knies et al., Reference Knies, Burton and Sala2012; Matsui et al., Reference Matsui, Kita and Ueshima2005; Schwartz et al., Reference Schwartz, Brecher, Whyte and Klein2005) likely to give consent compared to younger individuals in the same study; however, variation in the age ranges of the study populations complicates comparisons between the studies. Interestingly, an individual's health status could have an effect on consent choice. Some studies have described that being afflicted with some medical conditions or disabilities was associated with higher likelihood of consent (Buckley et al., Reference Buckley, Murphy, Byrne and Glynn2007; Dunn et al., Reference Dunn, Jordan, Lacey, Shapley and Jinks2004; Klassen et al., Reference Klassen, Lee, Barer and Raina2005; McKinney et al., Reference McKinney, Jones, Parslow, Davey, Darowski and Chaudhry2005). However, it is possible that this is to some degree specific to the conditions in question, as other studies report no association between their health outcome under study and consent (Baker et al., Reference Baker, Shiels, Stevenson, Fraser and Stone2000; Damery et al., Reference Damery, Ryan, McManus, Warmington, Draper and Wilson2011) or that those suffering from certain diseases could indeed be less likely to provide consent (Huang et al., Reference Huang, Shih, Chang and Chou2007; Jacobsen et al., Reference Jacobsen, Xia, Campion, Darby, Plevak, Seltman and Melton1999; Schwartz et al., Reference Schwartz, Brecher, Whyte and Klein2005). Due to their focused scopes, these studies do not allow for deductions regarding whether the specific disease or its severity is a more important contributor to consent choice. An additional factor of importance for consent to research participation is the educational level of the potential study subject. Most reports including such information have concluded that individuals with a higher level of education are more inclined to agree to research participation (Huang et al., Reference Huang, Shih, Chang and Chou2007; Knies et al., Reference Knies, Burton and Sala2012; Schwartz et al., Reference Schwartz, Brecher, Whyte and Klein2005).
Requests for consent to extract information from an individual's medical health records do not require in-person contact with the study participant. In this sense it entails low risk and little required effort for the individual compared to that associated with intervention studies or elements of observational studies requiring active participation (Kho et al., Reference Kho, Duffett, Willison, Cook and Brouwers2009). In addition, medical health records contain more objective measures compared to self-reported data (Knies et al., Reference Knies, Burton and Sala2012). Nevertheless, individuals may have concerns regarding the purposes of such research (Hill et al., Reference Hill, Turner, Martin and Donovan2013). Very little is known about factors predicting consent to collection of medical health records from adolescents.
Following consent to extraction of information from medical health records, the subsequent retrieval and collection of these records may pose a further challenge to the representative selection of data available for a study, adding an additional dimension of potential bias. When conducting epidemiologic research, a systematic misrepresentation of the general population within a study population may cause problems with the external validity of the study results.
In this study, we investigated the extent to which an adolescent's choice to consent to collection of their child and school medical health records is associated with sex, health status, and parental factors, in order to identify risk for potential introduction of consent bias. Additionally, we evaluated the result of the subsequent data collection in terms of potential regional differences.
Materials and Methods
This study was carried out within a cohort of twins from The CATSS. The CATSS framework encompasses several studies described below, also illustrated in Figure 1.
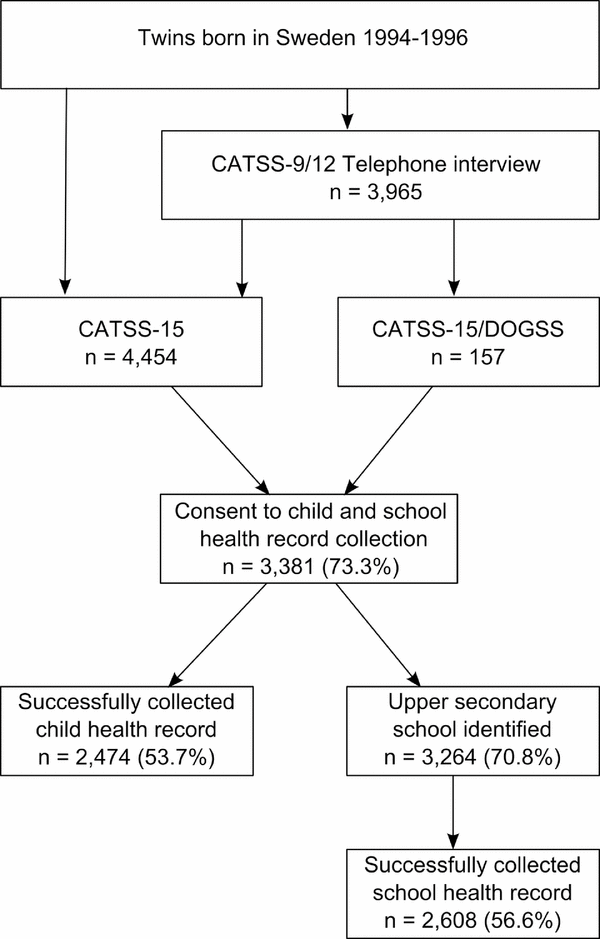
FIGURE 1 Flow chart describing the CATSS studies and the child and school health record consent-collection process.
CATSS-9/12
CATSS-9/12 is an ongoing data collection aimed at twins born and living in Sweden on their 9th birthday. Starting in July 2004, parents of twins born from 1992 onwards were contacted for a telephone interview including a wide variety of questions on childhood characteristics and health such as height, weight, history of neonatal care, lactose intolerance, celiac disease, asthma, allergic diseases, and use of prescribed drugs. The interview also includes the Autism–Tics, AD/HD and other Comorbidities Inventory (A-TAC), a parental interview designed specifically to screen for neurodevelopmental problems (Anckarsater et al., Reference Anckarsater, Lundstrom, Kollberg, Kerekes, Palm, Carlstrom and Lichtenstein2011; Larson et al., Reference Larson, Anckarsater, Gillberg, Stahlberg, Carlstrom, Kadesjo and Gillberg2010, Reference Hill, Turner, Martin and Donovan2013). During the first three years of the study, parents of 12-year-old twins (born July 1992 to June 1995) and 9-year-old twins (born from July 1995 onwards) were contacted in parallel, and after that only 9-year-old twins were included. To date, information on >24,000 twins has been collected, and the average response rate has been 80% (Anckarsater et al., Reference Anckarsater, Lundstrom, Kollberg, Kerekes, Palm, Carlstrom and Lichtenstein2011). Zygosity (monozygotic or dizygotic same- or opposite-sexed twins) was determined either using an interview-based algorithm or genetic testing (Magnusson et al., Reference Magnusson, Almqvist, Rahman, Ganna, Viktorin, Walum and Lichtenstein2013).
CATSS-15 and CATSS-15/DOGSS
At 15 years of age, twins eligible for CATSS-9/12 were invited to one of two follow-up-studies. Starting with twins born in 1993, CATSS-15/DOGSS (Developmental Outcomes in a Genetic twin Study in Sweden) was a targeted study based on screen-positivity in A-TAC for either autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (AD/HD), tic disorders (TD), learning disorders (LD), developmental coordination disorder (DCD), obsessive compulsive disorder (OCD), oppositional defiant disorder (ODD), conduct disorder (CD), or eating disorder (ED) in at least one twin of a same-sex pair. In addition, CATSS-15/DOGSS included randomly selected healthy controls from CATSS-9/12. It was primarily a clinical study, but an additional component included a paper questionnaire –– here referred to as the ‘age 15 questionnaire’. CATSS-15/DOGSS included 452 individual twins born 1993–1995, but only twins born between 1994 and 1995 who responded to the questionnaire were included in the current study (n = 157). To be a CATSS-15 participant, previous participation in CATSS-9/12 was not a prerequisite. Rather, starting with twins born in 1994, all 15-year-old twins who were not already participants in CATSS-15/DOGSS were invited to respond to a questionnaire. For the purposes of the current study, CATSS-15 twins born 1994–1996 (n = 4,454) were included. The age 15 questionnaires in CATSS-15 and in CATSS-15/DOGSS were largely identical, including questions on current height and weight, asthma and allergic diseases. Both questionnaires were distributed to twins as well as their parents (either parent in CATSS-15 or both in CATSS-15/DOGSS); however, for the purposes of the current study, a response from the twins was sufficient.
Consent to Health Record Collection
Twins who responded to the age 15 questionnaire in either CATSS-15 or CATSS-15/DOGSS received the question ‘May the Swedish Twin Registry request information from your child- and school health records?’ In Swedish law, 15 is the age when an individual is expected to provide their own consent to participation in medical research, given that they understand what this entails on their behalf. All study participants received standardized information including details about the intended use of the data, data anonymization, and confidentiality. The study was approved by the Regional Ethical Review board in Stockholm, Sweden.
Child and School Health Records
The Swedish Child and School Healthcare systems follow children from birth up to graduation from either 9th or 12th grade. The medical health records established within these systems include regular height and weight measurements of the children. Practices for the handling of these medical health records differ somewhat across Sweden. Commonly, however, the child health record is sent to the county archive either when a child moves outside the county, upon the child's transfer to school healthcare, or when the child leaves 9th grade and continues on to upper secondary school (Grade 10 to 12). The first school health record is established when the child enters the school system, but a new record may be created when there is a transfer between schools, such as between 9th and 10th grade.
The Record Collection Process
Child health records were requested from county council archives (Swedish: Landstingsarkiv) in the county of residence for twins born 1993–1995. Records of twins born in 1996 were requested from the county of residence and the county of birth. Lists of twins who had consented to record collection were sent to the Swedish National Board of Student Aid (Centrala Studiestödsnämnden, CSN), a government organization supplying financial aid for studies, including at upper secondary school level. As a result, the identity of the school for any given individual can be retrieved through the CSN, which was possible in n = 3,264 cases, 96.5% of all consenting twins. School health records were requested in a letter addressed to the school health department or nurse at the upper secondary school of the twin, as identified by the CSN. In some instances, child health records were retrieved along with the school health records, and sometimes missing school health records could be retrieved from the county council archive. For the purposes of this study, a collected record of either type, independent of source, was considered successful.
Quantitative Variables
Parents reported height and weight of the twins at the CATSS-9/12 telephone interview. Twins reported their current height and weight in the age 15 questionnaire. Height and weight was used to calculate body mass index (BMI, kg/m2) at each time point. BMI was categorized into low/normal or high based on age- and sex-specific cut-off values proposed by Cole et al. (Reference Cole, Bellizzi, Flegal and Dietz2000). For example, at age 9, the cut-off value between low/normal and high was 19.07 kg/m2 for females and 19.10 kg/m2 for males.
Register Linkages
In addition to the information available through CATSS-9/12 and CATSS-15 or CATSS-15/DOGSS, the study participants’ data were linked to population-based registers using the personal identification numbers (Ludvigsson et al., Reference Ludvigsson, Otterblad-Olausson, Pettersson and Ekbom2009). Specifically, the multi-generation register was used to identify the parents, the Medical Birth Register was used to find information on maternal BMI at the first antenatal visit, and finally parental education levels were found through the Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA by Swedish acronym). All data were de-identified prior to analyses.
Statistical Analysis
Two primary outcomes were studied: (1) consent to collection of medical health records (individual level analyses) and (2) successful collection of medical health records of the consenting twins (group level analyses). Concerning the outcome of consent, distributions of the covariates were presented for the full study population as well as separately for consenters and non-consenters. Logistic regression was used to estimate odds ratios (OR) with 95% confidence intervals (CI) for the associations between consent and explanatory variables. The sandwich estimator for standard errors was used to account for clustering within twin pairs. The outcome of a successfully collected medical (child and school) health record was analyzed using logistic regression for grouped data. Counties were categorized by quartiles of the distribution of the proportion of at least one parent with college education across counties: low (counties in which <42.5% of twins had at least one college educated parent), low middle (42.5–<47.7%), high middle (47.7 to 52.9%) and high (52.9% and above). This was referred to as the educational level quartile. Robust standard errors were estimated to account for clustering of observations within counties. All analyses were performed using Stata 12 (StataCorp, Tx.)
Results
The study population included n = 4,611 twins born 1994–1996 who had participated in either CATSS-15 or CATSS-15/DOGSS. Of these, 3,965 (86.0%) were also previous participants of CATSS-9/12. In total, 3,381 (73.3%) twins gave consent to collection of their child and school health record. A total of 4,486 twins (97.3%) belonged to pairs where both individuals responded to the age 15 questionnaire (full pairs). Most commonly, both twins within full pairs responding gave the same response to the question for consent as their co-twin, but 443 pairs (in total 886 individual twins) were consent-discordant, meaning one twin consented to child and school health record collection but not the other.
Table 1 shows individual characteristics of the study participants presented for the full study population, as well as separately for consenters and non-consenters. Males were significantly less likely to consent to record collection compared to females (OR 0.74, 95% CI 0.64–0.85). Twins whose co-twin also responded to the age 15 questionnaire were not significantly more likely to consent. However, among these full pairs, twins whose participating co-twin consented were significantly more likely to consent themselves (OR 10.9, 95% CI 8.76–13.5). Monozygotic twins were slightly more likely to give consent than dizygotic same-sexed twins (OR 1.50, 95% CI 1.20–1.88).While a high parent- or self-reported BMI at either age 9/12 or at age 15 was not associated with the likelihood of consent, twins who did not provide enough information in the age 15 questionnaire in order for their BMI to be calculated were less likely to consent (OR 0.05, 95% CI 0.04–0.07).
TABLE 1 Distribution of Individual Characteristics of CATSS-15 and CATSS-15/DOGSS Participants Born 1994–1996* and Their Association With Consent to Child and School Health Record Collection
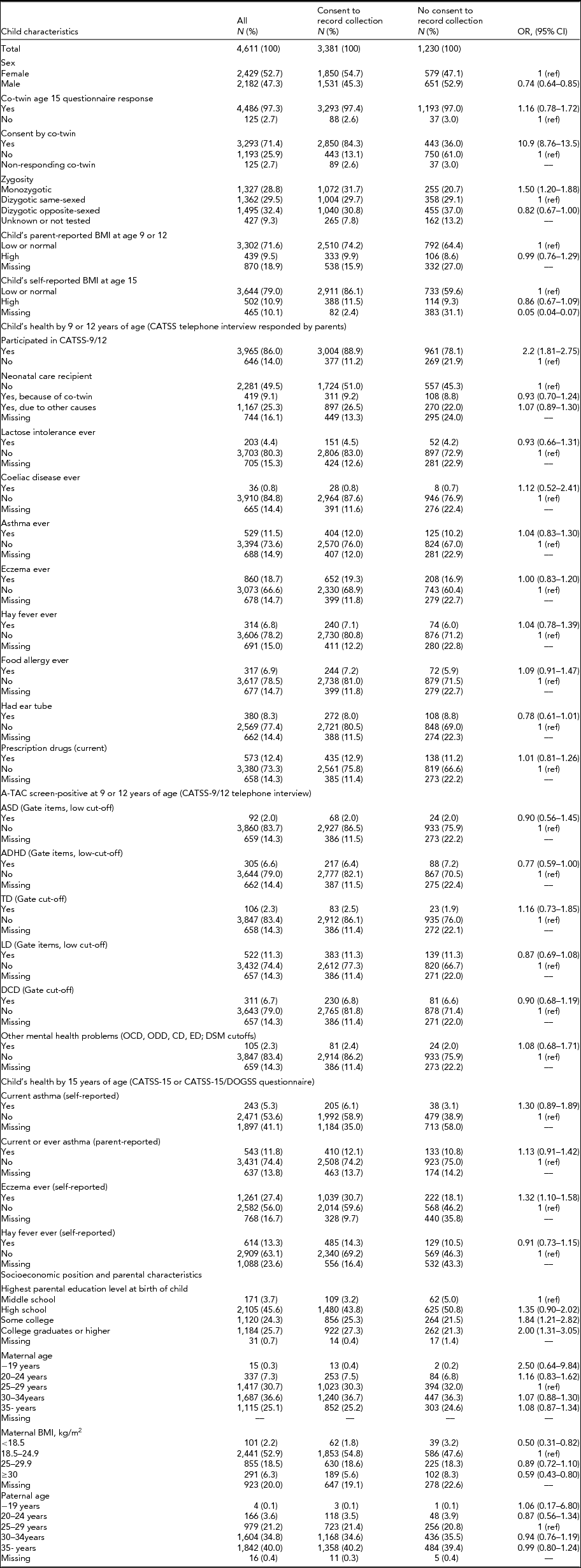
*Child specific and those associated with socioeconomic position, including parental education, age and BMI. BMI = body mass index; CATSS = child and adolescent twin study in Sweden; DOGSS = developmental outcomes of neurodevelopmental problems in a genetic twin study in Sweden; ASD = autism spectrum disorder; ADHD = attention-deficit/hyperactivity disorder; LD = learning disorders; TD = tic disorders; DCD = developmental coordination disorder; ODD = oppositional defiant disorder; OCD = obsessive compulsive disorder; CD = conduct disorder; ED: eating disorder.
Previous participation in CATSS-9/12 was positively associated with consent to record collection at age 15 (OR 2.2, 95% CI 1.81–2.75). There were no significant associations between outcomes related to the child's somatic or neurodevelopmental health in CATSS-9/12 and consent; however, there was a tendency for individuals who were A-TAC screen-positive for ADHD to be less likely to provide consent to record collection (OR 0.76, 95% CI 0.59–1.00). At age 15, neither self-reported current asthma nor parent-reported asthma ever were associated with consent to record collection, but twins reporting ever having had eczema were more likely to give consent (OR 1.32, 95% CI 1.10–1.58).
Some parental characteristics were associated with consent. Compared to twins whose parents’ highest educational level at birth of child was middle school, twins whose parents had experienced some college or were college graduates were more likely to consent (OR for college graduate parents 2.00, 95% CI 1.31–3.05). Twins whose mother had a BMI of <18.5 or ≥30 at the first antenatal care visit were less likely to consent to record collection (OR for maternal BMI <18.5 was 0.50, 95% CI 0.31–0.82, and for maternal BMI ≥30 OR was 0.59, 95% CI 0.43–0.80).
Table 2 shows the overall results of collection of child and school health records respectively, presented by twins’ county of residence. In total, 2,474 child health records (73.2%) and 2,608 (77.2%) school health records were successfully collected. The range of successfully collected child health records was 11.4–97.8% (median 79.1%), whereas the range of successfully collected school health records was 65.5–94.9% (median 79.6%). The highest proportion of successfully collected child health records was found in Östergötland (97.8% of requested records successfully retrieved), whereas the county from which the highest proportion of successfully collected school health records was Gotland with 94.9%.
TABLE 2 Results of Child and School Health Record Collection Presented Overall as well as Divided by the County of Birth of the Children Whose Records Were Requested

Presented also (n) are requests of each type of record per county and the proportion of individuals whose highest parental education level was at least some college education. The educational level quartile is assigned following the distribution of the proportion of twins with at least one college educated parent.
Table 3 shows collection results grouped on the educational level quartile of the twins’ county of residence. Using the lowest quartile as the reference group child health records were more often successfully collected from counties belonging to all other categories, but the effects did not reach statistical significance (e.g., OR for low middle compared to low was 2.30, 95% CI 0.70–7.55). A similar pattern was seen for the school health records.
TABLE 3 Results for Child and School Health Record Collection Divided by Educational Level Quartiles of Twins’ County of Residence
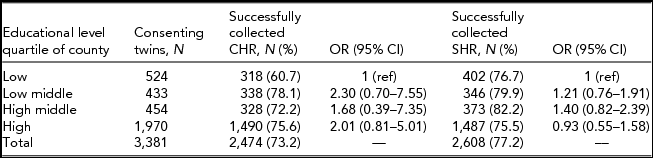
CHR = child health record, SHR = school health record.
Discussion
We show that the most important predictors for adolescents’ consent to participate in a research study are related to their families’ past or present choices to participate in research. Individuals whose twin sibling consented to record collection or whose parents had participated in an earlier study within the same framework were significantly more likely to consent. Parental college education was associated with higher odds of consent. Covariates associated with lower odds of consent were male sex, very low or very high maternal pre-pregnancy BMI and a twin's choice of not providing self-reported height or weight. Moreover, for the association between various dimensions of past and current health and adolescent twins’ choices to consent to the extended study, the only studied health outcome associated with consent was self-reported eczema ever at age 15. In contrast, however, parent-reported eczema ever at age 9 or 12 was not significantly associated with consent.
The subsequent attempt to collect child and school health records featured varying degrees of success between regions. These regional differences in turn slightly skewed the study population towards a selection with higher educational level, even though this effect did not reach statistical significance. This raises some concern regarding the representativeness of the final sample. It has been argued, however, that lack of representativeness is mainly of concern when the biological mechanism under study is potentially so different between two groups that inferences from one of them cannot reasonably be assumed to apply also in the other (Rothman et al., Reference Rothman, Gallacher and Hatch2013). Whether this is true in any given situation should be evaluated on a case-by-case basis. Given that educational achievement is a consequence of a sequence of events and circumstances, however, it seems unlikely in most scenarios that biological mechanisms should be unique between different levels. That said, in some cases, parents’ education may be associated with both diagnosis of disease and medication use in its treatment, which may make a skewed selection problematic if not for biological then possibly for social reasons (Gong et al., Reference Gong, Lundholm, Rejno, Mood, Langstrom and Almqvist2014).
Previous studies including age, sex and zygosity as a potential predictor for consent to use of medical health records for research have most often shown that males are more likely to consent than females (Damery et al., Reference Damery, Ryan, McManus, Warmington, Draper and Wilson2011; Knies et al., Reference Knies, Burton and Sala2012; Matsui et al., Reference Matsui, Kita and Ueshima2005; Woolf et al., Reference Woolf, Rothemich, Johnson and Marsland2000), although some previous studies demonstrate higher likelihood for consent in females compared to males. A British study including participants aged 18 and above found that among people below the age of 50, females were more likely to consent, but that the pattern was reversed among older individuals (Dunn et al., Reference Dunn, Jordan, Lacey, Shapley and Jinks2004). Previous studies have shown a tendency for younger females and older males to be most positive towards research participation (Hill et al., Reference Hill, Turner, Martin and Donovan2013). Thus, a reasonable explanation for our finding that females were more likely to consent to medical health record collection may lie in the fact that our study population consists only of adolescents, a group that has previously been underrepresented in similar investigations. Our findings also imply that monozygous twins are slightly more likely to give consent, which may reflect that their twinship makes them more positive towards research and confirms a recent study on the genetic effect of missingness (Schwartz & Beaver, Reference Schwartz and Beaver2014).
Past investigations of predictors for consent to record collection have been carried out primarily in the United Kingdom (Baker et al., Reference Baker, Shiels, Stevenson, Fraser and Stone2000; Damery et al., Reference Damery, Ryan, McManus, Warmington, Draper and Wilson2011; Dunn et al., Reference Dunn, Jordan, Lacey, Shapley and Jinks2004; Knies et al., Reference Knies, Burton and Sala2012; Tate et al., Reference Tate, Calderwood, Dezateux and Joshi2006) or the United States (Beebe et al., Reference Beebe, Ziegenfuss, Jenkins, Haas and Davern2011; Jacobsen et al., Reference Jacobsen, Xia, Campion, Darby, Plevak, Seltman and Melton1999; Schwartz et al., Reference Schwartz, Brecher, Whyte and Klein2005; Woolf et al., Reference Woolf, Rothemich, Johnson and Marsland2000). Our study presents a Northern European perspective, including similarities as well as differences from previous studies and providing added information for future comparisons of international differences in research participation patterns.
This study has several strengths and some limitations. It is one of the first studies on factors associated with consent to research involving collection of medical health records among adolescents. Inclusion of twin siblings and the longitudinal cohort design in CATSS allows us to study the influence of families’ past or present choices, which is a unique feature of our study. At the same time, we cannot exclude that this type of study design may have some influence on our results. The CATSS studies contain extensive information concerning health outcomes and other covariates (Larson et al., Reference Larson, Lundstrom, Nilsson, Selinus, Rastam, Lichtenstein and Kerekes2013) for consenters as well as non-consenters, making several comparisons between these groups possible. At the same time, some of the CATSS questions are not validated and for those the specificity for actual clinical diagnosis may be low. There was an association between declining to report sufficient information to calculate BMI at age 15 and not consenting to child and school health record collection. As the longitudinal recording of height and weight is central to the child and school health records, and it was explicitly stated in connection to the question for consent that this was the primary research interest, it is possible that the study participants who considered this information especially sensitive declined consent and did not report height or weight for the same reasons. The current data do not allow us to study whether a more general wording of the question would have yielded similar results.
In conclusion, adolescents’ choice to consent is not independent of certain covariates. Non-random selection in itself does not automatically result in selection or participation bias, however, as it requires the intended exposure as well as outcome of the future study to be associated with consent (Hernan et al., Reference Hernan, Hernandez-Diaz and Robins2004). Lack of representativeness may be of varying importance depending on the research question of interest (Rothman et al., Reference Rothman, Gallacher and Hatch2013). Awareness of these inherent issues in observational studies is essential to the design and interpretation of results.
Acknowledgments
Financial support was provided from the Swedish Research Council (C.A., grant number 2011-3060) and through the Swedish Initiative for research on Microdata in the Social And Medical sciences (SIMSAM) framework (C.A., grant number 340-2013-5867), the Strategic Research Program in Epidemiology at Karolinska Institutet (C.A.), the Swedish Heart-Lung Foundation (C.A.) the Swedish Asthma and Allergy Association's Research Foundation (C.A., grant numbers 2010048-K, 2011051-K, 2012056-K, 2013049-K), Stiftelsen Frimurare Barnhuset Stockholm (C.A.), grants provided by the Stockholm County Council (C.A., ALF project no 520344 and 530422, and C.H.G., no 20100336.) and Sahlgrenska Universitetssjukhuset (H.A., S.L., A.L.F., project no ALFGBG138111), the Swedish Council for Working Life and Social Research (S.L., H.A., grant number 2006-0547), Systembolaget (S.L.), the National Board of Forensic Medicine (S.L., H.A., grant number X10-90078), Bank of Sweden Tercentenary Foundation (S.L., H.A., grant number P2006-0390:1-E), and Hedlunds stiftelse (C.H.G.). The Ministry for Higher Education financially supports the Swedish Twin Registry.






