Traditionally, placental vascular anastomoses are associated with monochorionic twin gestations (Hubinont et al., Reference Hubinont, Lewi, Bernard, Marbaix, Debieve and Jauniaux2015; Miller, Reference Miller2021; Quintero, Reference Quintero2003). Pathologies associated with placental blood sharing, such as twin to twin transfusion syndrome (TTTS) and twin anemia polycythemia sequence (TAPS), are also thought to be associated with monochorionic twin gestations almost exclusively (Baschat & Miller, Reference Baschat and Miller2022; Miller, Reference Miller2021; Moaddab et al., Reference Moaddab, Nassr, Espinoza, Ruano, Bateni, Shamshirsaz, Mandy, Welty, Erfani, Popek, Belfort and Shamshirsaz2016; Quintero, Reference Quintero2003). These complications are thought to impact 5−15% of monochorionic pregnancies (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010).
Dichorionic twin gestations are generally thought to not develop such pathologies due to the presence of distinct placentas and vasculature (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010). This was illustrated in a prospective study published by this journal in 2016 (Zhao et al., Reference Zhao, Lipa, Wielgos, Cohen, Middeldorp, Oepkes and Lopriore2016). The authors identified placentas as either dichorionic or monochorionic on prenatal ultrasound, which was confirmed by postnatal histopathological exam, and then injected placentas with vascular dyes to assess for the presence of vascular anastamoses. They noted vascular anastomoses in 133/134 (99%) of monochorionic placentas and 0/124 (0%) of dichorionic placentas.
However, despite these findings, there have been a number of reports of placental anastomoses between fetuses in dichorionic diamniotic twin gestations. These connections have largely been recognized when the clinical appearance TTTS or TAPS has developed in dichorionic diamniotic pregnancies (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Chmait et al., Reference Chmait, Floyd and Benirschke2011; Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021; King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019). We recently had a case of dichorionic twins with placental vascular anastamoses at our institution that prompted review of the literature and raised awareness of the potential complications surrounding this rare physiology.
Clinical Vignette
We noted a case of dichorionic diamniotic twins conceived via in vitro fertilization from a frozen day 6 single embryo. Chorionicity was determined by a first trimester ultrasound at 11 weeks and 6 days with a twin peak sign (Figure 1). Ultrasounds later in pregnancy revealed concordant sex, fetal growth restriction, and bilateral talipes in both fetuses. Additionally, at 24 weeks, Twin A was also noted to have a sacral mass measuring 1.0 cm x 0.7 cm without Doppler flow suspicious for a skin tag, and Twin B was noted to have echogenic bowel. The patient elected for amniocentesis for both twins due to fetal anomalies, and given the discordance of the anomalies, to evaluate for the possibility of frozen embryo transfer and simultaneous natural conception. Results showed normal karyotype (46, XX) and normal microarray. Exome sequencing revealed a maternally inherited MYPBC1 c.1631T>C:p.I544T variant of uncertain significance in both twins. Pathogenic variants in MYPBC1 are associated with distal arthrogryposis, but this variant has never been reported in an individual with arthrogryposis, and the patient herself has no features of arthrogryposis. On further examination, this variant was determined to be benign by our Genetics team. Exome sequencing also noted that the twins had matching genomes. As a result, it was determined that this pregnancy was monozygotic.
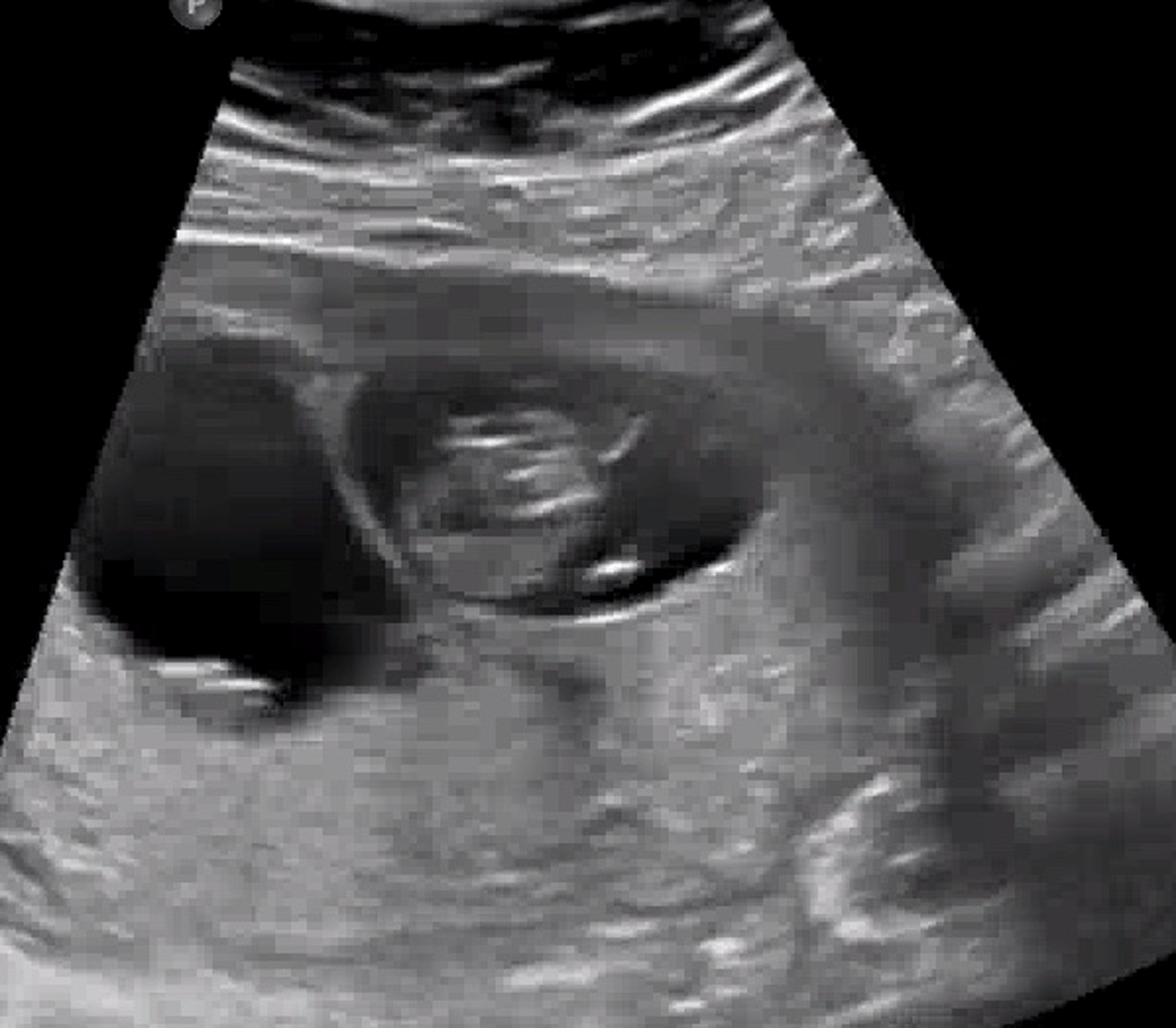
Figure 1. Eleven-week ultrasound with lambda sign indicating dichorionicity.
The patient was followed for sequential growth ultrasounds and twice weekly biophysical profile and umbilical artery Doppler testing due to the presence of fetal anomalies and the diagnosis of fetal growth restriction of both twins at 24 weeks and 1 day. At diagnosis, Twin A had an estimated fetal weight (EFW) of 531 g (3rd percentile) and normal umbilical artery Doppler (UAD), whereas Twin B had an EFW of 495 g (1st percentile), and intermittent reversal of UAD. Fetal weight discordance was 7% at that time.
At 32 weeks and 3 days, an ultrasound showed twins had weights of 1399 g (normal UAD) 1391 g (elevated UAD), for twin A and B respectively, which were both less than the 1st percentile. Twin B had new polyhydramnios with a maximum vertical pocket (MVP) of 14.8 cm. The MVP for Twin A was 5.5 cm. The patient presented twice more for ultrasounds, which showed stable fluid volumes and UAD. At 33 weeks and 6 days gestation, the patient presented for antenatal ultrasound. At this time, Twin A was demised. Twin B had persistent polyhydramnios, and a non-stress test showed decelerations. She was transferred to Labor & Delivery, and on arrival, Twin B was noted to have demised as well.
After delivery, autopsies were performed on both neonates. Twin A appeared pale and had a body weight of 1390 g, which was small for gestational age. Bilateral talipes and a 2 cm sacral hamartoma were noted. Twin B appeared congested with dark red skin and had a body weight of 1270 g, which was also small for gestational age. Bilateral talipes and thoracolumbar scoliosis were noted. Placental histopathology evaluation by a pediatric pathologist revealed two amnions and two chorions, confirming dichorionic diamniotic placentas. The placentas were fused along one edge. Placenta A consisted of 30% of the total combined placental areas and Placenta B consisted of 70%. There was also a deep artero-venous vascular connection seen between the two twin circulations.
Of note, in our case there are multiple possible contributors to the fetal demise of both gestations. This could include the unequal placental area division as noted above, and/or the vascular connection also noted above. However, fetal demise could have also been due to fetal growth restriction (possibily in the setting of poor placental functioning), noted fetal anomalies, and/or an unidentified genetic condition.
The Incidence of Placental Anastamoses in Dichorionic Gestations
The incidence of placental anastomotic connections in dichorionic diamniotic gestations is overall rare. However, their presence has been reported multiple times in the literature dating back to the 1980s, with increasing reports over the past 10 years. We noted the case above at our institution, and on further review, noted 10 additional cases of placental vascular anastomoses in dichorionic twin gestations in the literature (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Chmait et al., Reference Chmait, Floyd and Benirschke2011; Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021; King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019).
The increased awareness of abnormal intertwin placental vasculature most likely coincides with the increased incidence of twin gestations in general. This has been due to increased maternal age at conception and higher usage of assisted reproductive technologies over the past few decades (American College of Obstetricians & Gynecologists [ACOG], 2021; Hubinont et al., Reference Hubinont, Lewi, Bernard, Marbaix, Debieve and Jauniaux2015). Additionally, the incidence is also likely correlated with increased identification. This can be attributed to improvements in ultrasound technology allowing for easier identification of pathologies. Our ability to diagnose these pathologies was also bolstered by the characterization of TTTS in 1999 by Quintero (Reference Quintero, Walter, Allen, Bornick, Johnson and Kruger1999) and the introduction of TAPS in 2006 by Robyr et al. (Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006).
Determination of Chorionicity in Affected Pregnancies
All reported cases of dichorionic gestations with placental vascular anastomoses used ultrasound to established chorionicity (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Chmait et al., Reference Chmait, Floyd and Benirschke2011; Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021; King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019). There was clear evidence of a ‘twin peak’ or ‘lambda’ sign in all gestations. Placentas were all subsequently evaluated after delivery and found to also be dichorionic in almost all cases where appropriate histologic studies could be performed (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021; King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019). There was one case of a hybrid dichorionic, monochorionic placenta as noted on histologic examination with a dividing chorion covering only part of the amniotic membrane (Chmait et al., Reference Chmait, Floyd and Benirschke2011). One case could not determine chorionicity via histopathology due to a fixation error (Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016).
Evaluation of Placental Vascular Anastamoses
After delivery of these pregnancies, some researchers additionally performed histopathological evaluation of the placental for vascular anastomoses. In eight cases, the placentas were injected with dye, and connections were identified (Chmait et al., Reference Chmait, Floyd and Benirschke2011; King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019). One case had undergone laser ablation of all connections, so no anastomoses were seen on postnatal dye studies (Chmait et al., Reference Chmait, Floyd and Benirschke2011). One case did not report on the presence of placental anastomoses (Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021).
Overview of TTTS and TAPS in Dichorionic Pregnancies as Reported in the Literature
There are numerous case reports of either TTTS or TAPS occurring in dichorionic diamniotic twin gestations in the literature. TTTS has been more frequently reported (six cases; Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Chmait et al., Reference Chmait, Khan, Benirschke, Miller, Korst and Goodwin2010; Lage, Reference Lage, Vanmarter and Mikhail1989; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010) than TAPS (three cases; Kanagaretnam et al., Reference Kanagaretnam, Nayyar and Zen2021; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016; Zilliox et al., Reference Zilliox, Koch, Favre and Sananes2019). There was one case of intertwin transfusion noted during evaluation for selective termination, and subsequently postnatally identified placental anastomoses without evidence of TTTS or TAPS (King et al., Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995). Three of the TTTS cases were identified as Quintero Stage III by ultrasound (Chmait et al., Reference Chmait, Khan, Benirschke, Miller, Korst and Goodwin2010; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016). Another case was stage I (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010), a further case was stage II (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019), and the third case of TTTS was only suspected after postnatal evaluation (Lage, Reference Lage, Vanmarter and Mikhail1989). Two of the TAPS cases were identified by discrepant peak systolic velocities of middle cerebral artery (MCA) Dopplers. Zilliox et al. (Reference Zilliox, Koch, Favre and Sananes2019) obtained MCA Dopplers due to noted fetal hydrops, and Kanagaretnam et al. (Reference Kanagaretnam, Nayyar and Zen2021) did not explain why these were obtained in their case. In another case of TAPS, the diagnosis was only identified postnatally (Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021).
Role of Laser Photocoagulation
Fetoscopy with subsequent laser photocoagulation was offered to and performed in three cases with TTTS (Chmait et al., Reference Chmait, Floyd and Benirschke2011; Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010). All cases recognized the uniqueness of using laser photocoagulation for treatment of TTTS in dichorionic twin gestations. In these reports, multiple anastomotic connections were noted across the placentas, which were then photocoagulated. In two of these cases, authors then reported resolution of TTTS (Lanna et al., Reference Lanna, Faiola, Casati and Rustico2019; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010), and the third case does not comment on this (Chmait et al., Reference Chmait, Floyd and Benirschke2011). One case specifically mentioned the consideration of laser once Stage III TTTS was identified, but they did not pursue this therapy option due to its unknown benefit in dichorionic gestations (Murata et al., Reference Murata, Takano, Kagawa, Fujiwara, Sumie and Nakata2016).
Theories Regarding Physiologic Formation of Placental Anastomoses
There are a few theories speculating why monochorionic gestations form vascular connections between fetuses. These theories may provide insight into the formation of placental anastomoses in dichorionic gestations. Quintero et al. (Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010) stated that all monochorionic pregnancies have such connections. However, only certain gestations have sequela including TTTS and TAPS. They postulated that aberrations in placental vasculature formation leads to the lack of anastomotic connections from recipient to donor, or to a discrepancy in quantity/size of connections from donor to recipient (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010). Furthermore, the extravillous trophoblast, which may promote ‘chorionic leave’ in monochorionic pregnancies, is a robust producer of vascular endothelial growth factor, placental growth factor, and angiopoietin-2. These factors promote placental and vascular growth and should eventually become inhibitors of angiogenesis due to surrounding mediators and factors. However, in cases where inhibition does not occur, or trophoblasts leave despite continued expression of such factors, vascular connections may flourish. This could explain the persistence of anastomotic connections in dichorionic pregnancies. (Charnock-Jones et al., Reference Charnock-Jones, Kaufmann and Mayhew2004; Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010; Zhou et al., Reference Zhou, Bellingard, Feng, McMaster and Fisher2003). Additionally, monochorionic twins are almost always monozygotic (Trombetta et al., Reference Trombetta, Fabbro, Demori, Driul, Damante and Xodo2022). As a result, such connections may be further explained by monozygosity itself, and the persistence of a placental bridge, as described below (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010).
The Role of Monozygosity
Zygosity testing was performed in four cases. All four of these cases revealed monozygotic twin gestations with dichorionicity. Our case also revealed monozygozity. In three cases that disclosed the sex of the neonates, but did not perform zygosity testing, the sexes were concordant (Cavazza et al., Reference Cavazza, Lai, Sousa and Pina2019; Lage, Reference Lage, Vanmarter and Mikhail1989; Tollenaar et al., Reference Tollenaar, Prins, Beuger, Slaghekke, Oepkes and Lopriore2021). Assessing for zygosity in these cases — when same sex dichorionic diamniotic twins are noted — may be useful in to identifying dichorionic pregnancies at risk for development of TTTS or TAPS. This is likely due to the embryologic origins of monozygotic dichorionic twin gestations. Such twins occur when a cleavage-stage embryo divides within 3 days of fertilization. When a blastocyst-stage embryo divides on subsequent days, monochorionic twins develop (Konno et al., Reference Konno, Murakoshi, Miura and Masuzaki2020; Sundaram et al., Reference Sundaram, Ribeiro and Noel2018). Quintero et al. (Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010) suggested the possibility of a persistent placental bridge of vascularity that remains when a zygote divides within 3 days of fertilization, which could lead to the formation of placental anastomoses (Quintero et al., Reference Quintero, Kontopoulos, Barness, Steffensen, Hilbelink, Chmait, Benirschke and Bornick2010). This theory suggests the importance of monozygozity, as opposed to chorionicity, as as more significant determinant of risk for placental vascular anastamoses.
Monozygotic dichorionic twin gestations can be due to spontaneous conception or also occur in the setting of assisted reproductive technologies. This is thought to occur due to alterations in the zona pellucida during intracytoplasmic sperm injection (ICSI) or changes in the inner cell mass during preimplantation genetic testing or assisted hatching (Konno et al., Reference Konno, Murakoshi, Miura and Masuzaki2020). In vitro fertilization itself is known to increase the incidence of monozygotic pregnancies (Sundaram et al., Reference Sundaram, Ribeiro and Noel2018). Other risk factors for monozygotic twins include oocyte age under 35 years old, manipulation via ICSI or hatching, and blastocyst transfer in fresh cycles as opposed to cleavage-stage transfer (Konno et al., Reference Konno, Murakoshi, Miura and Masuzaki2020; Sundaram et al., Reference Sundaram, Ribeiro and Noel2018).
As a result, proposed risk factors for placental vascular anastomoses in dichorionic twin gestations include use of assisted reproductive technologies, use of oocyte age less than 35 years old, and monozygosity. Consideration can be given to assessing for zygosity in cases of concordant sex dichorionic twin gestations, especially when assisted reproductive technologies were used. Non-invasive prenatal testing (NIPT) has been successfully used to assess for zygosity, and makes assessing for zygosity more accessible (Bai et al., Reference Bai, Zhao, Lin, Huang, He, Wang and Ou2020; Norwitz et al., Reference Norwitz, McNeill, Kalyan, Rivers, Ahmed, Meng, Vu, Egbert, Shapira, Kobara, Parmar, Goel, Prins, Aruh, Persico, Robins, Kirshon, Demko, Ryan and Hedriana2019). When single nucleotide polymorphisms were used for assessment, zygosity was correctly predicted in 100% of cases (Norwitz et al., Reference Norwitz, McNeill, Kalyan, Rivers, Ahmed, Meng, Vu, Egbert, Shapira, Kobara, Parmar, Goel, Prins, Aruh, Persico, Robins, Kirshon, Demko, Ryan and Hedriana2019).
Other Considerations
Additionally, the possibility of anastomotic connections in dichorionic pregnancies has implications for blood sharing between fetuses without the development of TTTS or TAPS. Foschini et al (Reference Foschini, Gabrielli, Dorji, Kos, Lazzarotto, Lanari and Landini2003) described how a case of congenital CMV was noted via amniocentesis in one fetus, and which then ultimately spread to the other and was noted at birth, in a dichorionic dizygotic twin gestation. King et al. (Reference King, Soothill, Montemagno, Young, Sams and Rodeck1995) also described a case of blood sharing between fetuses without the development of TTTS or TAPS that was noted prior to administration of potassium chloride for selective termination. This has implications for cases in which selective feticide is being considered.
Lastly, Zhao et al. (Reference Zhao, Lipa, Wielgos, Cohen, Middeldorp, Oepkes and Lopriore2016) illustrated that the vast majority of monochorionic gestations have vascular anastomoses (99% in their study), but not all pregnancies develop complications related to this. Similarly, we would like to make clear the possibility of the presence of vascular anastomoses in dichorionic gestations that do not develop any sequela of shared vasculature, and therefore go unnoticed.
Future Directions
Data is still sparse for which dichorionic cases are at risk for the development of placental vascular anastomoses. Additionally, it is unclear how many of these placentas with vascular anastomoses then develop associated pathologies such as TTTS and TAPS. Regardless, this is an important pathology to be aware of, and future research should focus on identifying risk factors for abnormal vasculature in dichorionic twin gestations so we can risk stratify patients.
Funding
There are no financial or funding disclosures for this study.
Competing of interests
None
Ethical disclosures
None. We obtained written consent from the patient discussed in the case at our institution.




