The transition from adolescence into adulthood is a particularly formative period for a number of behaviors. In the case of substance use, both initial experimentation and continued use are thought to be due to a combination of genetic and environmental influences. Similar to other phenotypes, it is likely that the magnitudes of these influences vary across time and context. While several twin studies have examined the extent to which genes and environment influence substance use at various ages, differences across samples and measures make the results less interpretable than findings from prospective developmental studies.
An essential aspect of understanding influences on the frequency of substance use behavior is to first look at what motivates trying substances for the first time. Ever having tried a particular substance will herein be referred to as ‘use’ if tried, and ‘no use’ if never tried. Estimates of the proportion of genetic and environmental influences on use/no use appear to vary by age of sample. For example, in a sample of male and female twins in adulthood (mid-thirties) the heritability for liability to use tobacco was 0.73 (Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004). In a younger sample (age 17–18 years; Han et al., Reference Han, McGue and Iacono1999), which may not be fully past the ‘age of risk’ (Lopez-Leon & Raley, Reference Lopez-Leon, Raley and Rosner2012), the heritability of tobacco use was estimated at 0.11 (females) and 0.59 (males), with shared environment estimates of 0.71 and 0.18 for females and males, respectively. Parameter estimates for alcohol use were similar in that sample (Han et al., Reference Han, McGue and Iacono1999). When splitting a twin sample into three age groups (i.e., twins aged 13–15, 16–17, and 18–20 years), heritability estimates for ‘ever’ using marijuana declined with age while shared environmental influences increased (Distel et al., Reference Distel, Vink, Bartels, Van Beijsterveldt, Neale and Boomsma2011). A similar increase in the magnitude of shared environmental influences was found when comparing 12- to 14-year-old twins to 15- to 16-year-old pairs for initiation of alcohol use, especially among females (Koopmans et al., Reference Koopmans, Lorenz and Boomsma1997). Evidence for age-moderated influences suggests that these parameter estimates should be interpreted within the context of specific life stages, in which differential environmental or genetic influences may be of importance. The authors of a recent meta-analysis of twin studies of marijuana use acknowledged the possible moderating effect of age on estimates of genetic and environmental influences across time, although the findings are limited by the relatively small number of genetically informative longitudinal samples currently available (Verweij et al., Reference Verweij, Zietsch, Lynskey, Medland, Neale, Martin and Vink2010).
Similar developmental issues exist in the literature on the frequency of substance use, where most reported results are also cross-sectional. In a sample of twin pairs ranging from 8 to 16 years, Maes and colleagues found moderate to high heritabilites for past month substance use (0.60, 0.56, and 0.27) and a small-to-moderate proportional influence of shared environment (0.18, 0.17, and 0.35) for tobacco, alcohol, and marijuana, respectively (Maes et al., Reference Maes, Woodard, Murrelle, Meyer, Silberg, Hewitt and Eaves1999). In a combined twin, sibling, and adoptive sample of adolescents (mean age 15.85, SD 2.08 years), moderate-to-high heritabilities for regular tobacco and marijuana use were reported, with no genetic influences on regular alcohol use (Rhee et al., Reference Rhee, Hewitt, Young, Corley, Crowley and Stallings2003).
While cross-sectional studies have been informative, more powerful longitudinal designs measure substance use at several ages or developmental stages, and eliminate the problems associated with cross-sample comparisons. In one quasi-longitudinal cross-sectional study, a life history calendar approach was used to bolster retrospective recall of average monthly use for nicotine, alcohol, and marijuana at various life stages (Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008). Shared environmental influences on frequency of alcohol and marijuana use were important through adolescence, and genetic influences increased in relative importance into adulthood. For frequency of cigarette use, shared environment influences were only evident for very early use and then declined steadily from age 15 years as genetic influences became increasingly important (Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008). A one-year longitudinal study of the FinnTwin16 cohort found substantial shared environmental influences on alcohol use at age 16 (0.79) and 17 (0.76), with smaller estimates for frequency of alcohol use across the same time span, (0.35 and 0.22 at ages 16 and 17 years, respectively; Viken et al., Reference Viken, Kaprio, Koskenvuo and Rose1999). Following the FinnTwin12 and FinnTwin16 cohorts up to age 25 years, the relative importance of shared environment for females increased while the heritability for the frequency of alcohol use decreased. Estimates for males remained stable from ages 17 to 25 years (Pagan et al., Reference Pagan, Rose, Viken and Pulkkinen2006). Finally, a longitudinal study tracking smoking, alcohol, and illicit drug use across adolescence showed some increase in heritability across ages (Baker et al., Reference Baker, Maes, Larsson, Lichtenstien and Kendler2011).
Like twin studies, adoption designs also capitalize on the varying degrees of genetic similarity of sibling pairs to estimate the extent of genetic and environmental influences on a given trait. Biological sibling pairs reared in the same home who share on average 50% of their alleles identical by descent may be similar on a given phenotype because of shared environment or shared genes. In the absence of selective placement, any similarity between adopted sibling pairs, who are not genetically related, must be attributed to shared environment. Thus, adoption studies can provide a direct estimate of the influence of shared environment on a phenotype — an estimate that can be used as a powerful anchor for comparison with findings from twin studies. Similarly, parent–offspring designs are useful for estimating the magnitude of shared environmental influence by comparing similarity of children to their biological and adoptive parents. Parent–offspring and sibling-based adoption designs differ in several ways; most notably are the specific sources and magnitude of shared environment. While neither parent–offspring nor adoptive-sibling designs rely on the equal environments assumption of twin studies, there are also notable differences in the source and magnitude of shared environmental effects between twins and siblings. For example, because twins are the same age, they tend to spend more time together than non-twin siblings. Sibling-based adoptive designs can be influenced by factors such as test age differences between adoptive and biological sibling groups. While these design differences could lead to slightly different estimates, the comparison is still warranted.
While there have been a few cross-sectional adoption studies that have investigated substance use at specific points during adolescence (Buchanan et al., Reference Buchanan, McGue, Keyes and Iacono2009; McGue et al., Reference McGue, Sharma and Benson1996), and one recent parent–offspring longitudinal study (McGue et al., Reference McGue, Malone, Keyes and Iacono2014), no sibling-based adoption study has investigated the stability or change of these influences from adolescence into adulthood.
The current study had several aims. We sought to corroborate previously described estimates of biometrical parameters based on twin research using an adoptive sample, which provides a direct estimate of shared environmental influences common to siblings. Further, as the first comprehensive longitudinal sibling-based adoption study of substance use spanning adolescence to early adulthood (i.e., age 18 years), we examined whether the estimates of heritability and environmental influences change as adolescents transition through significant biological or socio-environmental life stages. Finally, we tested a series of biometrical models to determine the extent to which the change in estimates over time is due to stable or novel genetic and environmental influences. We were particularly interested in the transition from adolescence to early adulthood (i.e., age 18 years) as changing cultural attitudes, increased independence, and changes in legal rights (e.g., ability to legally purchase cigarettes) may underlie important environmental changes during this time.
Materials and Methods
Sample
Participants were from the Colorado Adoption Project (CAP), a longitudinal study following adoptive children, matched controls, and their families (Plomin & DeFries, Reference Plomin and DeFries1983; Reference Plomin and DeFries1985) approximately yearly from infancy into adulthood. Adoptive probands were ascertained through two Denver adoption agencies, while control probands were recruited from hospitals and matched to adoptive families based on sex of proband, number of children in the family, age and occupation of father, and father's years of education. Enrollment in the CAP occurred between 1976 and 1983, and resulted in a final sample of 245 adoptive families and 245 matched control families (Rhea et al., Reference Rhea, Bricker, Corley, DeFries and Wadsworth2013). The most proximal younger sibling of the proband (if available) was also recruited into the study as they reached the age of the proband at first assessment, so that sibling pair similarity could be compared across adoptive and control families. While proband assessments at any age (e.g., age 14 years) generally clustered within a given year, there was variation in the birth years of the siblings tested at a given age. Siblings were also assessed approximately annually, so that it was possible to compare measures taken when both the proband and the sibling were at a given age (e.g., 14 years). In contrast, cross-sectional studies compare sibling similarity within a given test year (e.g., proband at age 14 years, sibling at age 11 years) when influences on substance use may vary in both source and magnitude. (For further details of the CAP recruitment and assessment protocols, refer to Rhea et al., Reference Rhea, Bricker, Corley, DeFries and Wadsworth2013.) Table 1 shows the number of control and adoptive sibling pairs tested at each age.
TABLE 1 Descriptive Statistics at Each Age

The CAP includes early and frequent interviews for substance use, biannually from ages 12 to 18 years. Due to low prevalence of any substance use in early adolescence, we began analysis with the age 14 years assessment. We used data from probands and siblings who were tested at the same age (i.e., age at time of assessment of the sibling was within one year of the proband's test age; see Table 1). When individuals had multiple assessments within a year (starting at age 15 years), we selected those assessments that would minimize the test age gap within sibling pairs. Although we used identical procedures for adoptive and control families, there was a trend (in three out of five waves) for the mean difference between the test age of a proband and his/her sibling to be greater in adoptive families compared to control families. These mean differences were small and generally not significant, with the exception of the age 18 assessment (control age difference, M = 0.39, SD = 0.25; adoptive age difference, M = 0.52, SD = 0.25; t (96) = 2.53, p = .013). Though significant, this difference corresponds to a mean test age difference of approximately 50 days at the age 18 years assessment.
Measures
Substance use was assessed two different ways: use versus no use ever (use/no use), and the quantity/frequency of use in the past month. Wording of the measures varied slightly depending on the assessment wave in which the data was collected.
Use/No Use
Use/no use was coded into dichotomous responses (0 = No, never; 1 = Yes) for cigarettes, alcohol, and marijuana, based on the following questions: ‘Have you ever smoked cigarettes?’, ‘Have you ever had a drink of beer, wine, or liquor?’, and ‘Have you ever tried marijuana?’ respectively. At some assessment waves, quantity/frequency measures (described below) were collapsed into use/no use categories for alcohol and marijuana when not asked directly (e.g., quantity/frequency assessments include none and never to identify non-users). Prevalence of use of cigarettes, alcohol, and marijuana at each age are shown in Table 2.
TABLE 2 Percent of Participants who Report Having Tried Substances at Least Once at Each Age

Quantity/Frequency of Substance Use
Quantity/frequency of cigarette use in the past month was measured by the following question: ‘How frequently have you smoked cigarettes during the past 30 days?’ This item was coded on a seven-point scale (1 = none, 2 = less than 1 cigarette a day, 3 = 1–5 cigarettes a day, 4 = ½ pack a day, 5 = 1 pack a day, 6 = 1½ packs a day, 7 = 2 packs a day).
Quantity/frequency of alcohol and marijuana use was also assessed ‘in the past month’ when direct assessments of that time frame were available; however, some assessments only asked about quantity/frequency of use ‘in the past 6 months’ or ‘in the past year’. In such cases, we recoded responses into estimates of past month use in order to maintain consistency in the measure across time points. We assumed use was relatively stable across months and divided reported use by 6 or 12 to estimate use within a single month. Ultimately, ‘past month’ quantity/frequency responses (either directly assessed or estimated) were measured on a 7-point scale (1 = 0 times, 2 = 1–2 times, 3 = 3–5 times, 4 = 6–9 times, 5 = 10–19 times, 6 = 20–39 times, 7 = 40 or more times). Notably, Table 3 shows a general trend of increasing means and standard deviations for quantity/frequency across ages. This is consistent with the increasing prevalence of use across age in Table 2.
TABLE 3 Mean and Standard Deviation Quantity/Frequency of Use by at Each Age (Raw Scores)

quantity/frequency of use was measured on a 7-point scale (1 = 0 times, 2 = 1–2 times, 3 = 3–5 times, 4 = 6–9 times, 5 = 10–19 times, 6 = 20–39 times, 7 = 40 or more times) in the past month. Table entries include the Ns, and mean ± standard deviation.
Data Transformation
For the analysis of dichotomous use/no use data, potential prevalence differences in substance use conditional on age, sex, and adoptive status were accommodated by estimating thresholds separately for adoptive versus control sibling pairs, and at each age. As seen in Table 2, there are strong age trends in the prevalence of use with greater use at older ages. There is also a trend (though less strong) for a higher prevalence of use among adoptive probands compared to non-adoptive probands. No significant sex differences in prevalence were observed across this age range.
Quantity/frequency data were transformed to minimize skewness. Within each subgroup (e.g., control probands, adoptive probands, control siblings, and adoptive siblings), we regressed quantity/frequency scores on sex and obtained residuals. A constant of 5 was added to each standardized residual to remove negative values, and the residuals were then log transformed to minimize the positive skew. Finally, log-transformed scores were standardized to facilitate interpretation of model parameter estimates. For descriptive purposes, raw scores are reported in Table 3. However, all biometrical analyses were conducted on standardized, transformed scores.
Analyses
Descriptive statistics and Pearson's product moment correlations quantifying sibling resemblance for quantity/frequency of substance use in the past month were calculated using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 19.0). Genetic analyses were conducted using the software package Mx (Neale et al., Reference Neale, Boker, Xie and Maes2006). Tetrachoric (sibling pair) correlations for substance use/no use were computed allowing for separate thresholds for probands and siblings, adoption status, and different thresholds for each assessment age.
Biometrical models accounted for the genetic covariance structure implicit in the adoption design. Briefly, the covariance between control/biological siblings at a given time point can be parsed into additive genetic influences (a2), and common environmental influence (c2). Within adoptive sibling pairs, phenotypic similarity can only be due to common environmental influence in the absence of selective placement. Non-shared environmental influences (e2) only contribute to the overall variance in a trait in a population; the total variation in the population is assumed to be the sum of a2, c2, and e2. Due to sparse data issues, it was not possible to fit multivariate models to the longitudinal ‘use’ data. Although we fit models to raw data, many of the 10×10 tetrachoric matrices (proband five waves × sibling five waves for each substance) were not positive definite. For both adoptive and sibling pairs, some cells of the matrices were empty or yielded correlations of ±1.0. For this reason only univariate models for use/no use were conducted for the three substances at each of the five time points.
For multivariate models, a series of nested models were compared for goodness of fit using standard chi-square difference tests (e.g., Neale & Cardon, Reference Neale and Cardon1992). A basic Cholesky decomposition was used as a base model (see Figure 1; Neale & Cardon, Reference Neale and Cardon1992). Since these models are a full decomposition of the variance-covariance matrix across all measurement occasions, they will necessarily provide a good fit to the data structure (i.e., the Cholesky decomposition is just-identified). Subsequent models were considered to have good fit if the additional parameter constraints did not result in a significant decrement in fit compared to the model fit of the corresponding Cholesky decomposition.
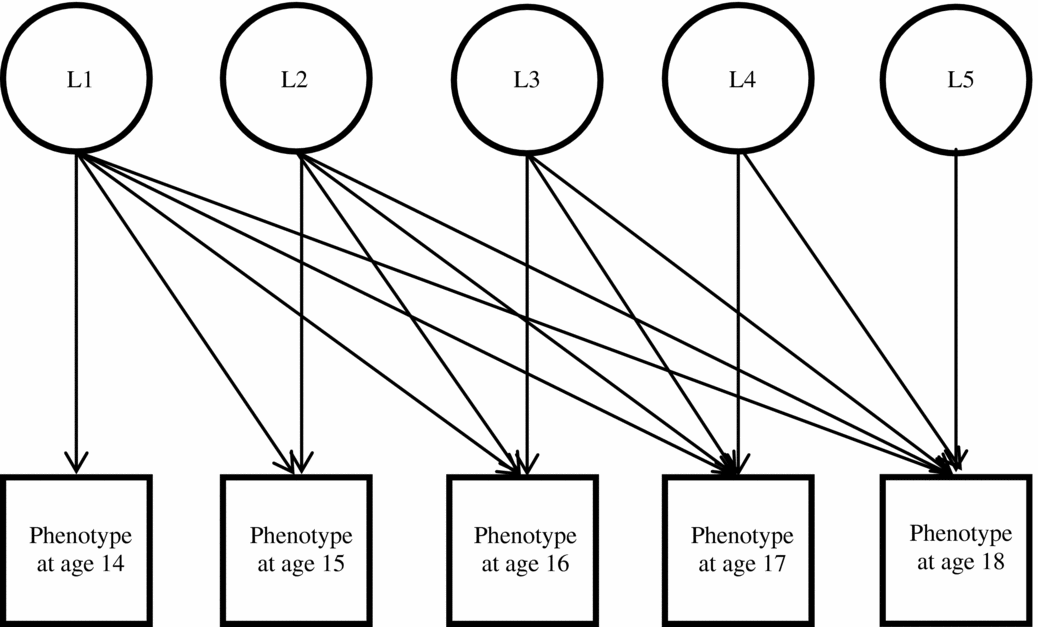
FIGURE 1 Basic cholesky decomposition model.
The independent pathway model estimates additive genetic (A), shared environmental (C), and non-shared environmental (E) factors that are common across all time points, as well as age-specific influences (or residuals) that only explain variation at specific measurement occasions (see Figure 2). These models allow the common genetic and environmental factors to influence the measured traits to different extents. Age-specific influences also may reflect important developmental changes across adolescence, such as novel influences coming ‘on-line’ at older ages.

FIGURE 2. Independent pathway model.
Several constraints were added to the general independent pathway model to empirically test developmental trends. Specifically, we tested whether (1) all age-specific influences were significant, and (2) whether the common influences affected each age to the same degree or whether the magnitude of these influences increase/decrease across adolescence.
Results
Sibling Correlations for ‘Use/No Use’
Table 4 shows estimated tetrachoric sibling pair correlations for control pairs (who share both genetic and environmental influences) and adoptive pairs (who share only environmental influences) at assessment ages 14 through 18 years. Across these ages, there was a consistent trend where control sibling pairs were more highly correlated for substance use than adoptive sibling pairs (see Table 4).
TABLE 4 Sibling Correlationsa and Univariate Parameter Estimates for Use/No Use at Each Age

atetrachoric correlations.
Univariate Estimates for ‘Use/No Use’
Although confidence intervals are quite broad due to the dichotomous nature of the data and the limited samples sizes at each age, the point estimates suggest substantial genetic influences (a2) on the liability to use alcohol and marijuana, but only modest effects on cigarette use/no use. Shared environmental (c2) estimates suggest small-to-moderate influence of the shared environment across substances and across ages. However, for alcohol use, there is some evidence for increasing shared environmental influences from age 14 to 18 years.
Sibling Correlations for ‘Quantity/Frequency’
Again, with a few exceptions (e.g., the youngest ages), control sibling pairs were generally more highly correlated for quantity/frequency of substance use than adoptive sibling pairs. Cross time point correlations were generally more strongly correlated with proximal time points compared to more distal ones. Table 5 shows the full proband-sibling correlation matrix across the five time points. Adoptive proband-sibling correlations are shown above the diagonal and control proband-sibling correlations below.
TABLE 5 Correlations for Quantity/Frequency of Use in Past Month at Each Age

Bold type indicates adoptive family correlations, normal typeface indicates control family correlations. Within-proband correlations are in top left quadrant, sibling-proband correlations are in bottom left quadrant (control) and top right quadrant (adoptive), and within-sibling correlations are in bottom right quadrant.
Multivariate Biometrical Results
We reported raw scores for substance use quantity/frequency in Table 3 to illuminate several trends (e.g., increasing means and variances across ages). However, substance use variables were log-transformed and standardized prior to multivariate biometrical analysis so that path loadings across ages could be interpreted on the same scale. Unfortunately, sparse data issues, though not as severe as with our use/no use data, precluded fitting simplex models to the longitudinal data. It was necessary to utilize Cholesky decomposition and independent pathway models, which are more robust to sparse data issues.
Model fitting comparisons are presented in Table 6. Compared to the base Cholesky decomposition (Model 1), the more parsimonious independent pathway model (Model 2) did not result in a significant decrement of fit for quantity/frequency of use of cigarettes, alcohol, or marijuana-assessed at five measurement occasions. Thus, we used the independent pathway as the base model for subsequent model comparisons to explore possible developmental trends.
TABLE 6 Model Comparisons of Biometrical Models for Five Ages With Standardized Variables

asample size adjusted BIC.
As a test of the significance of age-specific sources of variance, we compared a series of models where either the additive genetic (A), shared environmental (C), or non-shared environmental (E) specifics were dropped from the base independent pathway models (Models 3–5). Specifics were dropped independently (e.g., Model 3 dropped additive genetic specifics while shared environmental and non-shared environmental specifics remained in the model). Across all substances, there was a significant decrement in fit only when dropping the age-specific non-shared environmental variance components (Model 5). There were no significant age-specific additive genetic or shared environmental influences. Although we had limited power, it can be seen from Table 7 that the point estimates for specific A and C, with few exceptions, are small and quite often zero.
TABLE 7 Standardized Variance Estimates, Standardized Path Coefficients, 95% Confidence Intervals for Independent Pathway Results (Model 2)

a2, c2, and e2 reflect the total additive genetic, shared environmental, and non-shared environmental variance (e.g., common and specific combined). Standardized variance estimates may not add up to 1.00 due to rounding.
To test the stability of common influences, we also tested a series of models where the common additive genetic, shared environmental, or non-shared environmental pathways were constrained to be equal (Models 6–8). Across all substances, the additive genetic and shared environmental influences could be constrained to be equal; indicating substantial stability across adolescence. However, some caution in interpretation is warranted, given power issues. Non-shared environmental pathways across ages were the most variable and could not be constrained to be equal across age for all three substances.
Standardized parameter estimates and confidence intervals for the base (full ACE) independent pathway models for each substance are shown in Table 7. The total proportion of variance explained by additive genetic (a2), shared environmental (c2), and non-shared environmental (e2) factors (i.e., common plus specific influences combined) are also reported.
Discussion
The current study utilized a longitudinal adoption design to examine the magnitude and developmental patterns of genetic and environmental influences on substance use from ages 14 to 18 years. Importantly, results from adoption studies can be used to anchor estimates of environmental influences that are indirectly assessed from twin studies, but directly estimated in sibling adoption designs.
Due to limited sample sizes, multivariate analysis of the use/no use data was not feasible. Age-specific, univariate analyses of substance use/no use at each age yielded parameter estimates with large confidence intervals. However, the point estimates suggested interesting trends. In contrast to many twin studies, which tend to show more evidence of environmental influences during the adolescent years (Rose et al., Reference Rose, Dick, Viken, Pulkkine and Kaprio2001), the pattern of sibling pair tetrachoric correlations from age 14 to 18 years indicates moderate heritabilites for liability to use cigarettes, alcohol, and marijuana in adolescence. Heritability decreased in magnitude for cigarette and alcohol use across adolescence, but increased for marijuana use. Shared environmental influences were relatively modest for cigarette use/no use across adolescence. For alcohol use, there is a trend for increasing shared environmental influences, with the greatest influence at age 18 years, where access to substances may be more readily available. In comparison, a recent longitudinal adoptive parent–offspring study found significant shared environmental (parent–offspring) influences on drinking behavior at this age, while genetic influences were important in early adulthood (McGue et al., Reference McGue, Malone, Keyes and Iacono2014). A twin study by Kendler et al. (Reference Kendler, Schmitt, Aggen and Prescott2008) also found that shared environmental influences on liability to use alcohol remain well into the young adult years. In contrast, Koopmans et al. (Reference Koopmans, Lorenz and Boomsma1997) found substantial early (age 12–14 years) shared environmental influences for males only, while female alcohol use had strong early genetic influences. In contrast to Kendler et al. (Reference Kendler, Schmitt, Aggen and Prescott2008), our study found that shared environmental influences on liability to use marijuana were modest across the range from age 14 to 18 years. Similarly, Baker et al. (Reference Baker, Maes, Larsson, Lichtenstien and Kendler2011) described a common factor model with substantial genetic effects on marijuana and illicit drug use/no use at age 13–14 years, with few additional innovative genetic affects emerging at ages 16–17 and 19–20 years.
Our adoptive and control sibling correlations for quantity/frequency of substance use generally suggest genetic influences, with only modest effects of the shared environment, particularly at early ages when prevalence of use was lower. Additive genetic factors have also been shown to contribute substantially to substance use across development. A meta-analysis by Bergen et al. (Reference Bergen, Gardner and Kendler2007) found an increase in the heritability for multiple phenotypes from adolescence into adulthood but no significant increases for two substance use measures (i.e., nicotine initiation and alcohol consumption).
Although substance use is correlated across measurement occasions, it is possible that particular environmental shifts (e.g., starting high school) or biological changes (e.g., beginning puberty) may influence behavior at specific periods of adolescence. Thus, we fitted multivariate biometric models to test whether use patterns across five ages had common influences or age-specific influences.
Overall, all age-specific genetic and shared environmental influences could be dropped from cigarette, alcohol, and marijuana quantity/frequency of use models (e.g., Models 3–5 in Table 6). Age-specific, non-shared environmental influences may reflect measurement error rather than unique environmental influences that could influence substance use at multiple waves.
While most variance was due to common influences, it is possible that common factors could have varying degrees of influence over adolescence. We tested this by constraining loadings from common factors to be equal across ages (Models 6–9). There were some non-significant trends for common additive genetic influences, in that the proportion of variance explained for cigarette and alcohol quantity/frequency of use appeared to increase as participants aged (p = .06, .09, respectively). For marijuana, these influences were the largest at ages 15 and 16, though path loadings could be constrained across ages without significant decrement in fit compared to the base independent pathway model (p = .07). Common shared environmental pathways were stable across ages for all substances (p = .24–.66). Common non-shared environmental pathways were highly variable and could not be constrained for any substance. Given that few age-specific influences were detected, the total proportion of variance explained by additive genetics and shared environment follow similar trends.
There are several limitations to consider when interpreting these results. A potential confound of the CAP sample was that there are more same-sex sibling pairs in the control families, while the adoptive families included more opposite-sex pairs. If same-sex sibling pairs are more similar than opposite-sex pairs on substance use behaviors, the increased similarity of the control families (due to greater numbers of same-sex siblings) could bias our estimates of variance due to genetic effects upward. To test this, we ran a series of regression analyses to test the effect of adoption versus control status, same sex versus opposite sex status, and their interaction on sibling pair difference scores for quantity/frequency of use. Across five time points for each substance, same sex pairs were not significantly more similar than opposite sex pairs, nor were these effects different across adoptive and control families. For use/no use, we used logistic regression to test the same effects on pair concordance and discordance. Across five time points for each substance, the test of the same sex/opposite sex effect was significant only once. However, the effect was in the opposite direction than expected. Opposite sex pairs were more similar for age 17 years alcohol use than same sex pairs, and this was more true for adoptive pairs than control pairs. Thus, there is no evidence in our data to suggest that the greater similarity of control siblings compared to adoptive siblings can be explained by the difference in same-sex versus opposite-sex pairs.
Second, we did not have identical assessment questions throughout the length of the study. Our transformation from 6-month use or past-year use variables into past-month variables required some assumptions; namely, that average use over the past month was consistent with the given time span. For example, if a participant reported using marijuana once a month on average over the past 6 months (or year), they would have been coded as using once during the past month, although they may have used more or less during different peak times over the year.
Finally, the numbers of adoptive and non-adoptive sibling pairs available at each age were relatively small in this study. This was primarily due to the requirement that both proband and sibling be tested within the same test age year-which was necessary for yearly assessment of the sibling pairs. This led to some sparse data issues that limited our approaches to data analysis (e.g., multivariate analysis of the use/no use was not possible; and multivariate analysis of the quantity/frequency data required use of methods that were robust to sparse data issues).
Despite these limitations, our study provides a unique contribution to the literature on genetic and environmental influences on substance use behavior. As the first sibling-based longitudinal adoption study of substance use, our estimates provide a test of the role of environment on use of cigarettes, alcohol, and marijuana from adolescence into early adulthood. These estimates corroborate the point estimates of cross-sectional twin studies and other prospective designs. Importantly, the general trend of increasing genetic influences in late adolescence/early adulthood for quantity/frequency of alcohol use mirrors results reported from a recent parent–offspring longitudinal adoptive design (McGue et al., Reference McGue, Malone, Keyes and Iacono2014). In conclusion, results of the present study indicate that individual differences in substance use from 14 to 18 years of age are largely due to common influences. Moreover, although the sample of adopted and control sibling pairs was relatively small, our findings suggest that frequency/quantity of substance use during adolescence are due substantially to genetic influences, and that new genetic influences may emerge for cigarette and alcohol use in late adolescence.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards if the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgments
We greatly appreciate the participation of the CAP families and the work of the Institute for Behavioral Genetics interview staff. This research was supported by grants HD010333, HD036773, & AG046938. Author Huibregtse was supported by T32 DA017637-11.











