The treatment of TTTS has evolved through the years and has included expectant-medical management (Jones et. al., Reference Jones, Sbarra, Dilillo, Cetrulo and D'Alton1993), sectio parva (Urig et al., Reference Urig, Simpson, Elliott and Clewell1988), serial amniocentesis (Dennis & Winkler, Reference Dennis and Winkler1997; Saunders et al., Reference Saunders, Snijders and Nicolaides1992; Wax et al., Reference Wax, Blakemore, Blohm and Callan1991), and the current treatment using laser photocoagulation of the placental vascular anastomoses (De Lia et al., Reference De Lia, Cruikshank and Keye1990; De Lia et al., Reference De Lia, Kuhlmann, Harstad and Cruikshank1995; Hecher et al., Reference Hecher, Diehl, Zikulnig, Vetter and Hackeloer2000; Quintero et al., Reference Quintero, Morales, Mendoza, Allen, Kalter, Giannina and Angel1998; Ville et al., Reference Ville, Hecher, Ogg, Warren and Nicolaides1992; Ville et al., Reference Ville, Hyett, Hecher and Nicolaides1995). The rationale for the use of laser photocoagulation in TTTS stems from the fact that: (1) TTTS occurs via placental vascular anastomoses, which are responsible for the net imbalance sharing of blood volume between two (or more) fetuses; (b) as a corollary, TTTS does not occur in the absence of placental vascular anastomoses (e.g., dichorionic twins); (c) TTTS should disappear if the anastomoses are ablated.
Therefore, the goal of the laser surgery is to correctly identify and ablate all of the vascular anastomoses. This raises two issues: (1) can all of the placental vascular anastomoses be identified? (2) can all of the placental vascular anastomoses be ablated?
Step 1: Identification of the Placental Vascular Anastomoses
Classic placental pathology studies have shown the presence of three different types of placental vascular anastomoses: arterio-venous (A-V; so-called ‘deep anastomoses’) and arterio-arterial (A-A) or veno-venous (V-V; so-called ‘superficial’) anastomoses (Benirschke & Kaufmann, Reference Benirschke and Kaufmann1995). All of these anastomoses can be seen on the surface of the placenta, even if the actual anastomotic exchange occurs deep within the substance of the placenta. While some authors have suggested that in fact there are anastomoses deep within the placental parenchyma that are not visible from the surface (Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006), these surgical pathology arguments have been raised more to explain the failure of laser surgery from certain centers, rather than plausible surgical pathology explanations. In actuality, all of the different types of vascular anastomoses should be visible endoscopically from the fetal surface, with exceedingly few exceptions, provided obviously that they are within the reach of the endoscope.
How can the anastomoses be identified? In the early days of laser surgery, this was an issue of contention. The original reports suggested that the anastomoses could be identified based on their appearance, that is, based on specific patterns, angles, with drawings intended to aid in this process (identification based on pattern recognition; De Lia et al., Reference De Lia, Kuhlmann, Cruikshank and O'Bee1993). Unfortunately, this was not conducive, given the myriad of patterns that the anastomoses can actually have. Furthermore, a pair of vessels could look exactly like an anastomosis, yet they could both trace back to the same fetus and thus not be an anastomosis. The lack of a practical way of identifying placental vascular anastomoses was one of the most important hindrances in the beginning of the era of laser surgery for TTTS. This led to the development of the so-called ‘non-selective technique’.
The Non-Selective Technique
The non-selective technique was based on lasering all of the vessels that would cross the dividing membrane. By definition, this technique did not attempt to differentiate anastomotic from non-anastomotic vessels, but rather to catch as many anastomoses as possible, based on three assumptions: (1) the dividing membrane lies parallel to the vascular equator; (2) all of the vessels crossing the dividing membrane are vascular anastomoses; (3) the vascular anastomoses (vascular equator) are all within the sac of the recipient twin (where the endoscope is inserted).
In actuality, although many cases could fall within these three assumptions, the assumptions do not guarantee that only anastomotic vessels will be targeted. First, the location of the dividing membrane may or may not be parallel to the so-called vascular equator, the area of the placenta where the anastomoses are located. Indeed, the location of the dividing membrane relative to the fetal placental surface may be: (a) at an angle to the vascular equator (such that some of the anastomoses may be in the sac of the donor twin and some in the sac of the recipient twin); (b) completely within the sac of the donor twin, such that all of the anastomoses are inside the sac of the donor twin.
Second, and as a corollary, not all of the vessels crossing the dividing membrane are anastomotic vessels. Therefore, by lasering all of the vessels that would cross the dividing membrane, the risk of ablating normal vessels (i.e., non-anastomotic) could be high. Third, in the rare cases where all of the anastomoses are within the sac of the donor twin, lasering of all the vessels crossing the dividing membrane from within the sac of the recipient twin would aim only at recipient vessels (with or without anastomoses), with certain demise of this fetus. Clinical proof of the potential for harm to either twin from the use of a non-selective technique was shown in the report by Ville et al. (Reference Ville, Hyett, Hecher and Nicolaides1995), where the use of the non-selective technique was associated with a dual fetal survival of only 35%, and a rate of single intrauterine fetal demise of 35% as well (Ville et al., Reference Ville, Hyett, Hecher and Nicolaides1995). Subsequent clinical studies showed that the use of a non-selective technique, which unnecessarily targeted vessels that were not involved in blood exchange between the fetuses, was associated with an increased risk for demise of one or both twins (Ville et al., Reference Ville, Hyett, Hecher and Nicolaides1995). Clearly, using the dividing membrane as a surrogate for the identification of the placental vascular anastomoses was suboptimal, albeit an improvement over the previously non-descriptive reports. Thus, a better way of identifying the actual anastomoses was needed.
The Selective Technique
In 1998, Quintero et al. described the selective laser photocoagulation of communicating vessels technique (coined as ‘SLPCV’; Quintero et al., Reference Quintero, Morales, Mendoza, Allen, Kalter, Giannina and Angel1998). This was the first description of a precise anatomical way of identifying the placental vascular anastomoses endoscopically and differentiating them from non-anastomotic vessels. The technique required a systematic assessment of the vascular equator from one end of the placenta to the other. Basically, this meant following each and every vessel on the surface of the placenta to its terminal end. If the terminal end of an artery of one twin was accompanied by a returning vein to the same fetus, this was labeled as a non-anastomotic pair. On the other hand, if the terminal end of an artery was accompanied by a returning vein to the other twin, this was labeled as an A-V anastomosis. A-A anastomoses were apparent, since the artery of one twin would continue as an artery to the other twin. Similarly, V-V anastomoses could be identified by following a vein from one twin to the other. The identification of these anastomoses did not rely on the location of the dividing membrane on the surface of the placenta. This avoided missing anastomoses located in the sac of the donor, whether this meant a few or even all of the anastomoses. Visualization of the anastomoses through the dividing membrane within the sac of the donor was also shown to be possible. This was in contrast to previous reports, where presumably the anastomoses within the sac of the donor were not visible due to the presence of two layers of amnion (De Lia et al., Reference De Lia, Kuhlmann, Cruikshank and O'Bee1993). In fact, the two layers of amnion do not preclude visualization of the anastomoses within the sac of the donor twin, particularly when there is severe oligohydramnios or anhydramnios in the sac of that fetus. The selective technique provided a reproducible way of identifying all of the vascular anastomoses, independent of their location, a first step in the proper performance of the laser surgical technique. R. Quintero, L. Quintero L., (Reference Quintero, Quintero, Pivatelli, Bornick, Allen and Johnson2000) also reported how to document the vascular anastomoses. This included mentioning the size (hair, small, medium, large, extra-large) of the anastomoses, as well as their direction (from donor to recipient or from recipient to donor). The direction of flow in A-A or V-V anastomoses cannot be determined in most cases, unless significant discoloration is present in these vessels from differences in fetal SpO2 (Quintero et al., Reference Quintero, Chmait, Carver, Bornick, Allen and Kontopoulos2008). In cases with significant discoloration differences, the collision front between the two fetal circulations within an A-A or a V-V anastomosis led to the concept of the ‘hemodynamic equator’ (HE; Chang et al., Reference Chang, Chmait, Bornick, Allen and Quintero2006). The HE allowed, for the first time, a better understanding of the actual behavior of A-A or V-V anastomoses, which until then were thought to be bidirectional in all cases. Indeed, if the HE moves back and forth between draining vessels of either twin, the behavior of the A-A or V-V anastomosis is truly bidirectional. On the other hand, if the HE only reaches a draining vessel of one twin, the function of the superficial vessel is essentially no different than an AV anastomosis (and, as such, labeled as fAVDR if from donor to recipient, or fAVRD if from recipient to donor). Lastly, if the HE does not reach any draining vessel, the net exchange of blood between the fetuses through that vessel is zero.
The selective technique also assumed that once the vascular equator was entirely mapped, the anastomoses could all be photocoagulated (a two-step process).
The acronym used for the selective technique, that is, SLPCV, defined the systematic approach that needs to take place to identify and photocoagulate all of the vascular anastomoses. Other acronyms used to describe the performance of the laser surgery may or may not be similar to the Quintero SLPCV technique. For example, while performing SLPCV, the dividing membrane is always respected. Purposeful injury to the dividing membrane, or so-called ‘septostomy’, is not part of the SLPCV technique (Harkness & Crombleholme, Reference Harkness and Crombleholme2005). Though not implicit, the performance of a laparotomy to access the amniotic cavity is also not part of the SLPCV technique. While general anesthesia was originally used in our cases (Rossi et al., Reference Rossi, Kaufman, Bornick and Quintero2008), surgery can be best performed under local anesthesia (Ville et al., Reference Ville, Hecher, Gagnon, Sebire, Hyett and Nicolaides1998). Therefore, the acronym SLPCV should apply only to those surgeries in which access to the amniotic cavity is performed under local anesthesia, percutaneously, and following a systematic and thorough identification and obliteration only of placental vascular anastomoses (Chmait et al., Reference Chmait, Kontopoulos, Korst, Llanes, Petisco and Quintero2011; Quintero, Reference Quintero and Quintero2002; Quintero et al., Reference Quintero, Morales, Mendoza, Allen, Kalter, Giannina and Angel1998).
Connecting the Dots: The ‘Solomon’ Technique
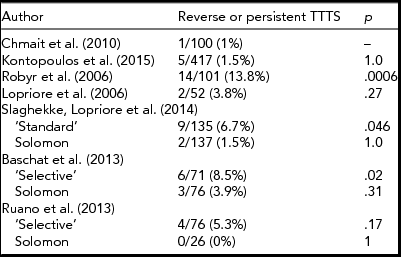
As other centers began to adopt the selective technique, one particular issue of concern became apparent. Indeed, the reported incidence of residual patent placental vascular anastomoses after laser surgery on surgical pathology analysis of the placentas varied significantly between centers (Table 1). Similarly, the incidence of failed surgery, defined as PRTTTS, which is the clinical manifestation of residual patent placental vascular anastomoses after laser surgery, also varied significantly (Table 2). In a study by Robyr et al. (Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006), the rate of failed surgery was 22%. Anemia after demise of one of the fetuses was also noted as a complication, which is indirect evidence of residual patent placental vascular anastomoses as well. Lopriore et al. (Reference Lopriore, Middeldorp, Oepkes, Klumper, Walther and Vandenbussche2006) reported an incidence of 33% of residual patent placental vascular anastomoses in 52 TTTS patients treated with laser at their institution. In comparison, the rate of residual patent placental vascular anastomoses after SLPCV by our group, using similar technique and technology, has consistently been less than 5%, with no anemia after demise of the co-twin, and an incidence of reverse or persistent TTTS of only 1–1.5% (USFetus Consortium; Quintero, Reference Quintero and Quintero2007).
TABLE 1 Reported Incidence of Residual Patent Placental Vascular Anastomoses After Laser Surgery on Surgical Pathology Analysis of the Placentas. Comparisons Made Relative to the Lowest Reported Rate (P1) or Between the ‘Standard’ and the Solomon Techniques (P2)
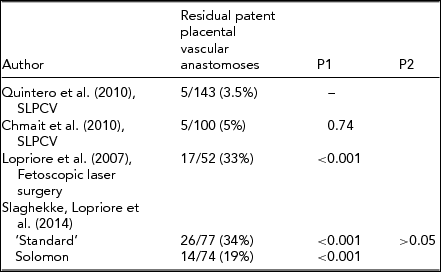
TABLE 2 Reported Incidence of Clinical Outcomes After Laser Therapy Reflecting Residual Patent Placental Vascular Anastomoses. Comparisons Made Relative to the Lowest Reported Rate
In view of the relatively high incidence of residual patent placental vascular anastomoses seen by some groups, some authors proposed ‘connecting the dots’ between photocoagulated areas on the surface of the placenta (Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011). The premise behind this idea was that by lasering areas between laser-ablated placental vascular anastomoses, such ‘blind lasering’ would capture ‘anastomoses’ that would otherwise be missed (i.e., ‘not visible’; Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006). Such authors believed that, in fact, not all of the placental vascular anastomoses can be identified endoscopically (Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006), and that therefore, ablating only the visible ones using the selective technique could miss vascular anastomoses and explain their high rate of residual patent placental vascular anastomoses. The resulting surgical technique of ‘connecting the dots’ was dubbed ‘the Solomon technique’ (Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011), in reference to the biblical passage where, in order to resolve a dispute between two alleging mothers of a child, King Solomon proposed to cut the baby in half (1 Kings 3:16–28, NIV). The analogy, therefore, is that by lasering the areas of the placenta between endoscopically identified and laser-ablated vascular anastomoses, the placenta would be ‘cut in half’. Recent studies suggest that indeed, relative to the surgical groups’ prior experience with the selective technique, the use of the Solomon technique was associated with improved perinatal outcomes (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013).
To test whether the Solomon technique could indeed reduce the rate of residual patent placental vascular anastomoses, an open-label randomized clinical trial was conducted in Europe (the Solomon trial) comparing the Solomon technique with the ‘standard’ technique (Slaghekke, Lopriore et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). The latter, although not directly mentioned, was intended to refer to the selective technique (SLPCV). Interestingly, the actual rate of persistent residual patent placental vascular anastomoses was no different between the two techniques (14/74, 19% vs. 23/77, 29.8%, Solomon vs. ‘standard’, respectively, p = .12). However, the study did show a decreased rate of PRTTTS (2/137, 1% vs. 9/135, 7%, Solomon vs. ‘standard’, respectively, p = .03) and of twin-anemia-polycythemia syndrome (TAPS; 4/137, 2.9% vs. 21/135, 15.5%, Solomon vs. ‘standard’, respectively, p = < .001). Although the rationale and primary outcome of the study (to reduce the rate of residual patent placental vascular anastomoses) was no different between the groups, the authors concluded that the Solomon technique was superior to the ‘standard’ technique. Two additional observational studies comparing the Solomon technique with the selective technique also appeared to show favorable results with the former technique (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013).
Table 1 shows the rate of residual patent placental vascular anastomoses reported by the different groups using either the Quintero selective (SLPCV) technique, or the ‘standard’ or the Solomon technique in the Solomon trial. As can be seen, the rate of residual patent placental vascular anastomoses is lowest using the Quintero SLPCV versus the ‘standard’ or Solomon techniques. Table 2 shows that the Quintero SLPCV technique is also associated with a lower rate of PRTTTS than that of the ‘standard’ technique in the Solomon trial and the ‘selective technique’ of other authors, and that the Solomon technique in all studies achieves the same rate of PRTTTS than that reported with Quintero SLPCV. Given that the Solomon technique is still associated with approximately 20% of residual patent placental vascular anastomoses, the initial rationale for the technique, that is, to reduce the high rate of residual patent placental vascular anastomoses, does not appear to hold. Furthermore, given that the proponents of the Solomon technique have also shown that most missed anastomoses are located in the margins of the placenta (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Klumper, Walther and Vandenbussche2006), lasering inexistent placental vascular anastomoses in otherwise healthy placental tissue between vascular anastomoses within the main body of the placenta is incongruent with the rationale (Table 3, Figure 1(a) and (b)). Altogether, the Solomon technique would seem to represent a step backwards in the ability to correctly identify all of the placental vascular anastomoses, by accepting the unproven theory of the presence of non-visible placental vascular anastomoses on the otherwise healthy appearing fetal surface of the placenta. Alternatively, we have shown that all of the placental vascular anastomoses can be clearly identified without having to ablate inexistent anastomoses in healthy placental areas. Stated differently, the use of the Solomon technique may simply represent an approach to achieving similar results as those that can be obtained with the performance of the Quintero SLPCV technique, rather than a true advantage over the SLPCV technique, at the expense of lasering healthy placental tissue.
TABLE 3 Principles and Results of the Use of the Solomon Technique to Identify and Ablate All Placental Vascular Anastomoses in Twin–Twin Transfusion Syndrome

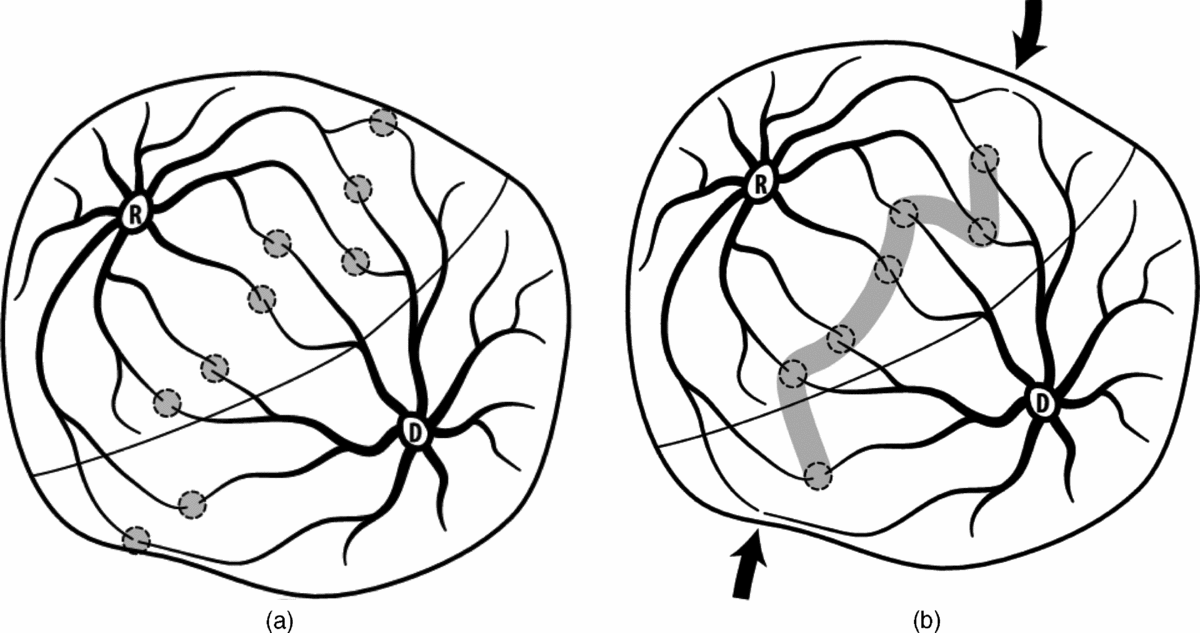
FIGURE 1 (a). Selective photocoagulation of communicating vessels (SLPCV). All of the anastomoses are photocoagulated, regardless of their location relative to the dividing membrane, while sparing non-anastomotic vessels. Rate of residual patent placental vascular anastomoses: 3.5–5%. (Chmait et al., Reference Chmait, Assaf and Benirschke2010; Kontopoulos et al., Reference Kontopoulos, Chmait, Baker, Llanes and Quintero2015). (b) Solomon modification of the SLPCV technique. The fetal surface of the placenta between endoscopically identified anastomoses is also lasered, to occlude ‘anastomoses’ not visible by the endoscope. Note that marginal anastomoses may be missed, as they are not between laser shots. Rate of residual patent vascular anastomoses: 20% (Slaghekke, Lopriore et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014).
Step Two: Ablation of the Placental Vascular Anastomoses
Assuming that there is a conceptual agreement on how to identify the anastomoses, the next step consists of being able to ablate them. Successful ablation of the placental vascular anastomoses assumes that the surgeon can adapt to the different clinical scenarios and overcome the various challenges that may be present in each case. Particular known surgical challenges include:
-
1. the location of the placenta (anterior, posterior);
-
2. interference with the visualization of the anastomoses by the donor twin that is ‘stuck’ along the vascular equator from severe oligohydramnios (unmovable donor);
-
3. the presence of discolored fluid within the sac of the recipient twin from prior procedures (amniocenteses) or from prior intra-amniotic bleeding;
-
4. triplet (or higher order multiple) gestations, whether monochorionic or not;
-
5. close proximity of the umbilical cord placental insertions;
-
6. large anastomotic vessels;
-
7. tangential access to the placenta (whether the placenta is anterior or posterior);
-
8. anastomoses located behind the entry of the trocar;
-
9. high maternal BMI.
The management of each of these challenges requires the use of special techniques or technology, including the use of fluid management systems, blunt probes, trocar assistance (Quintero et al., Reference Quintero, Chmait, Bornick and Kontopoulos2010), and special laser photocoagulation techniques, which are beyond the scope of this article (Quintero, Reference Quintero and Quintero2007). A recent Delphi study outlined the multitude of steps needed to perform the surgery. Most authors agreed on the basic surgical goal (the purpose of the Delphi survey), including the ablation of all of the placental vascular anastomoses along the vascular equator, without injuring healthy placenta or non-anastomotic vessels (Peeters et al., Reference Peeters, Akkermans, Westra, Lopriore, Middeldorp, Klumper and Oepkes2015). The question is: How often can these goals be accomplished while overcoming the various challenges mentioned above? One way to address this question is by noting both how often the placenta can be adequately assessed, as well as how often the vessels can be selectively ablated.
Adequate Placental Assessment
Adequate placental assessment refers to the ability of the surgeon to survey the entire vascular equator. For example, in a subanalysis of the Solomon trial, the authors reported that they were able to adequately assess the placenta in only 65 out of 74 patients in the Solomon group (87%) compared to also only 69 out of 77 (89%) in the ‘standard’ group (Slaghekke, Lewi et al., Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekoninck and Lopriore2014). The reasons behind the inability of the surgeons to adequately assess the placenta in more than 10% of the cases in each group were not stated. Obviously, if the placenta cannot be adequately assessed, this may result in missing anastomoses and thus an increased likelihood for adverse clinical outcomes, including PRTTTS. In contrast, our group has shown consistently the ability to assess the placenta adequately in over 99% of the patients (Crisan et al., Reference Crisan, Kontopoulos and Quintero2010).
Selective Ablation of the Anastomoses
Another surgical competency benchmark refers to the ability of the surgeon to selectively ablate the vascular anastomoses without including non-anastomotic vessels. A ‘selectivity index’ (SI) was proposed by Stirnemann et al. (Reference Stirnemann, Nasr, Quarello, Ortqvist, Nassar, Bernard and Ville2008), as SI = log (SC + 1)/(NSC + 1), where SC are the selectively coagulated vessels and NSC are the non-anastomotic vessels. NSC were also vessels that were considered presumed anastomoses, but that could not be followed to their terminal end and were nonetheless lasered. In their experience, most surgeries involved lasering both anastomotic and non-anastomotic vessels (Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011; Stirnemann et al., Reference Stirnemann, Nasr, Quarello, Ortqvist, Nassar, Bernard and Ville2008). A ‘high’ SI, defined as -0.25, was reported by the authors to correlate with improved postnatal survival at 28 days of at least one twin and both twins. Crisan et al. (Reference Crisan, Kontopoulos and Quintero2010) have shown that such an index is mathematically inaccurate and should not be used to assess the adequacy of the laser surgery. Instead, a simpler index consisting of a ratio between how often the surgery can be done using the Quintero selective technique versus non-selectively, is more representative and easier to understand:
![]() $( {{\rm{QSI}} = 100*\frac{{{\rm{SLPCV}}\, -\, {\rm{NSLPCV}}}}{{{\rm{SLPCV}}}}{\rm{\ }}} )$
, where QSI is the Quintero SI, SLPCV is a surgery performed selectively, NSLPCV is a surgery where at least one vessel was not clearly identified as an anastomosis but was lasered. For example, in that same article, the authors showed that they were able to perform a selective surgery in only 34% of cases (Stirnemann et al., Reference Stirnemann, Nasr, Quarello, Ortqvist, Nassar, Bernard and Ville2008). In another report of 123 patients, surgery could not be completed in five cases for a stuck twin obscuring the equator (2), poor visualization (2) and a large anastomotic vessel (1) (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Klumper, Walther and Vandenbussche2006). Obviously, the goal is to try to perform the surgery selectively as close to 100% of the time as possible (Chmait et al., Reference Chmait, Kontopoulos, Korst, Llanes, Petisco and Quintero2011; Crisan et al., Reference Crisan, Kontopoulos and Quintero2010; Kontopoulos et al., Reference Kontopoulos, Chmait, Baker, Llanes and Quintero2015).
$( {{\rm{QSI}} = 100*\frac{{{\rm{SLPCV}}\, -\, {\rm{NSLPCV}}}}{{{\rm{SLPCV}}}}{\rm{\ }}} )$
, where QSI is the Quintero SI, SLPCV is a surgery performed selectively, NSLPCV is a surgery where at least one vessel was not clearly identified as an anastomosis but was lasered. For example, in that same article, the authors showed that they were able to perform a selective surgery in only 34% of cases (Stirnemann et al., Reference Stirnemann, Nasr, Quarello, Ortqvist, Nassar, Bernard and Ville2008). In another report of 123 patients, surgery could not be completed in five cases for a stuck twin obscuring the equator (2), poor visualization (2) and a large anastomotic vessel (1) (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Klumper, Walther and Vandenbussche2006). Obviously, the goal is to try to perform the surgery selectively as close to 100% of the time as possible (Chmait et al., Reference Chmait, Kontopoulos, Korst, Llanes, Petisco and Quintero2011; Crisan et al., Reference Crisan, Kontopoulos and Quintero2010; Kontopoulos et al., Reference Kontopoulos, Chmait, Baker, Llanes and Quintero2015).
Accuracy of Laser Therapy
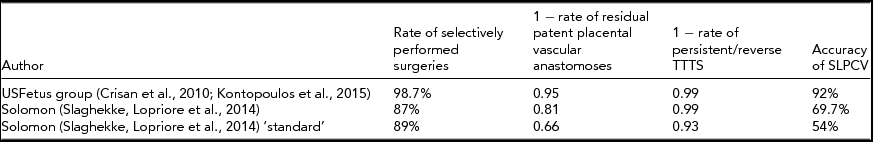
Theoretically, one could combine the rate of adequate placental assessment and of selective laser surgery with the rate of either residual patent placental vascular anastomoses (when available) and the rate of PRTTTS to determine how accurate the laser surgery is being performed at a given center or by a given surgeon. Accuracy of SLPCV could thus be defined as:
where AccSLPCV is the accuracy in performing SLPCV, QSI is the rate of Quintero selectively performed surgeries, RPPVA is the rate of residual patent placental vascular anastomoses (when available) and PRTTTS is the rate of PRTTTS. Table 4 shows such a theoretical calculation and its use to compare different reports.
TABLE 4 Accuracy of Laser Surgery for Twin–Twin Transfusion Syndrome
Should the Rate of TAPS be Included as a Benchmark for the Performance of Laser Therapy in TTTS?
The rate of TAPS has been included as a measure of failed laser therapy for TTTS, in addition to the rate of residual patent placental vascular anastomoses and persistent/reverse TTTS (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Chmait et al., Reference Chmait, Assaf and Benirschke2010; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013; Slaghekke, Lewi, et al., Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekoninck and Lopriore2014; Slaghekke, Lopriore, et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). The decision stems from the purported etiology of TAPS, which presumably results from the transfer of blood between two monochorionic twins through small placental vascular anastomoses in such a way that one fetus develops anemia and the other twin develops polycythemia, but without the net blood volume inequalities typical of TTTS (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Kanhai, Walther and Vandenbussche2007). Presumably, the syndrome occurs through small caliber AV anastomoses, in contrast to larger size vessels typically seen in TTTS. In actuality, such a theory is even less plausible than the contested theory of TTTS (in which all indirect and circumstantial evidence does point to placental vascular anastomoses as being the etiological factor for TTTS, and indirectly confirmed by resolution of the syndrome with occlusion of all placental vascular anastomoses). The proposed theory of TAPS is thus suspect for many reasons:
-
1. the original theory was based on the presence of residual patent placental vascular anastomoses. Given the high incidence of residual patent placental vascular anastomoses reported from such groups (20–33%), it is not possible to discern what role, if any, these anastomoses play in the etiology of TAPS.
-
2. while TAPS has been described in the presence of residual patent placental vascular anastomoses, they are not indispensable. We and others have recently reported on the presence of TAPS without apparent placental vascular anastomoses (Kontopoulos et al., Reference Kontopoulos, Chmait, Baker, Llanes and Quintero2015; Taniguchi et al., Reference Taniguchi, Sumie, Sugibayashi, Wada, Matsuoka and Sago2015).
-
3. contrary to TTTS, ablation of residual patent placental anastomoses in cases of TAPS does not necessarily eliminate the condition. Table 5 compares TTTS with TAPS relative to the role of placental vascular anastomoses.
TABLE 5 Role of Placental Vascular Anastomoses in the Etiology of Twin–Twin Transfusion Syndrome and Twin-Anemia-Polycythemia Syndrome
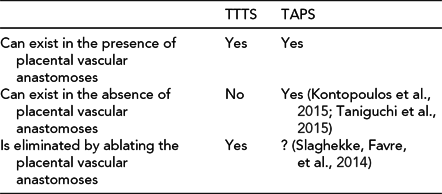
TTTS = twin–twin transfusion syndrome; TAPS = twin-anemia-polycythemia syndrome.
Therefore, at this point, the assumption that TAPS is the result of unsuccessful laser therapy constitutes a leap of faith in the understanding of both the etiology of TAPS and the role that laser therapy may play in the occurrence of this condition. In our opinion, the inclusion of TAPS as a benchmark of failed laser therapy should be reconsidered.
Functional Ablation of the Placental Vascular Anastomoses: The Sequential Technique
The development of the selective technique represents an important historical step in the surgical treatment of TTTS. SLPCV is indeed an anatomical surgical technique, which involves the identification and selective laser obliteration of the placental vascular anastomoses. However, intrauterine fetal demise of one of the two fetuses after SLPCV would still occur in approximately 9–29% of cases with this technique (Quintero et al., Reference Quintero, Morales, Mendoza, Allen, Kalter, Giannina and Angel1998; R. Quintero, C. Comas, Reference Quintero, Comas, Bornick, Allen and Kruger2000). Because TTTS presumably occurs from an excessive transfer of blood from the donor twin to the recipient twin, the sequence with which the anastomoses are obliterated during surgery could have prognostic implications. Indeed, if the anastomoses from the donor to the recipient are obliterated first, that would immediately stop the transfer of blood from this twin to the recipient. Furthermore, during this interval, however brief, the recipient twin would be transfusing the donor twin back. As a result, the donor twin, which is presumably hypotensive, would stop losing blood as soon as the laser process starts, while at the same time would start receiving additional blood back from the recipient twin. The photocoagulation of the vascular anastomoses from donor-to-recipient first, followed by from recipient-to-donor second was called the ‘sequential technique’ or SQLPCV. Using a sequential technique, our group showed a reduction in the rate of intrauterine fetal demise of the donor twin from 21% to 7%, and an increase in the double survival rate from 56% to 75% (Quintero et al., Reference Quintero, Ishii, Chmait, Bornick, Allen and Kontopoulos2007). Our group is currently assessing the merits of performing a sequential technique through a randomized clinical trial of the USFetus group (Chmait et al., Reference Chmait, Kontopoulos and Quintero2014). While a sequential technique may not necessarily be required in all cases, it could have an indication in patients where the condition of the donor twin would be most compromised.
Is There a Role for Umbilical Cord Occlusion in TTTS?
Interruption of the blood exchange between the fetuses can also be accomplished by occluding one of the two umbilical cords. This procedure should be contemplated only as a last resort to treat the syndrome. Unfortunately, the availability of bipolar photocoagulation and radiofrequency ablation has resulted in an unwarranted number of selective feticide in patients that otherwise could have perhaps been treated best with laser surgery (Chalouhi et al., Reference Chalouhi, Stirnemann, Salomon, Essaoui, Quibel and Ville2010; Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011). Umbilical cord occlusion should not be offered as an alternative to laser because of limitations of the surgeon or the center, unless the patient cannot be referred to another center capable of offering laser. Umbilical cord occlusion should be offered to patients with TTTS in which additional complicating circumstances may exist. This may be the case of patients with a severe congenital anomaly of one of the twins, or a moribund hydropic fetus. Since such cases are rare, the performance of selective feticide via umbilical cord occlusion in TTTS should be an exception, rather than the rule. Indeed, the counseling of our patients involves mentioning a survival rate of approximately 90% with a 5% risk of neurological damage if laser therapy is chosen, compared to 90% survival and a 5% risk of neurological damage to the surviving co-twin if umbilical cord occlusion is chosen. Therefore, since both survival and morbidity statistics are similar between the two procedures, but with umbilical cord occlusion one of the fetuses is denied the chance to survive, the justification is not there to offer feticide to an otherwise anatomically normal fetus.
Conclusion
It has been more than 20 years since laser therapy was first proposed for the treatment of TTTS. Significant strides have been made both in establishing the scientific merit of using laser to ablate the placental vascular anastomoses to treat the condition (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004) as well as in the various steps, techniques, and other technical aspects that allow such therapy. Selectively interrupting the placental vascular anastomoses without injuring healthy portions of the placenta using the Quintero selective technique, while obvious as a concept and as a surgical goal, has been associated with markedly different outcome results between centers. The Solomon technique has been proposed as a way to mitigate such differences, but has not shown to lower the high rate of residual patent placental vascular anastomoses that prompted its development. A properly performed Quintero SLPCV technique is associated with the highest rate of clinical success and with the lowest rate of failed therapy either by surgical pathology or clinical criteria. Further improvements in clinical outcomes with the use of the sequential technique, particularly for specific situations in which the donor may be at a unique disadvantage, could be expected and is being addressed in the ongoing randomized clinical trial conducted by our groups comparing SLPCV with SQLPCV. Selective feticide via umbilical cord occlusion should be the exception rather than the rule for severe cases of TTTS, and should not be performed to compensate for physician or surgical center limitations. Improvements in the surgical equipment and other ancillary technology, while difficult to pursue, should continue to remain among the objectives of caregivers in this field. The education of the next generation of surgeons using the wealth of information thus far gathered by the different centers should also be a main focus of all programs.








