It is now well established that moderate-to-high levels of physical activity (PA) are positively associated with a variety of psychological and biological health markers (Physical Activity Guidelines Advisory Committee, 2018) in addition to increases in longevity (Bellavia et al., Reference Bellavia, Bottai, Wolk and Orsini2013; Yates et al., Reference Yates, Djousse, Kurth, Buring and Gaziano2008). The evidence summarized in recent reviews (de Vilhena e Santos et al., Reference de Vilhena e Santos, Katzmarzyk, Seabra and Maia2012; Lightfoot et al., Reference Lightfoot, De Geus, Booth, Bray, den Hoed, Kaprio and Bouchard2017; Lin et al., Reference Lin, Eaton, Manson and Liu2017) indicated that biological/genetic mechanisms explain a significant fraction of the variance in PA. It has been estimated from twin data that genetic factors explain from 0% (Joosen et al., Reference Joosen, Gielen, Vlietinck and Westerterp2005) to 85% of the total variance of population PA levels (Stubbe et al., Reference Stubbe, Boomsma and De Geus2005); on the other hand, a smaller range of estimates was obtained from familial (nuclear or extended pedigree) data, ranging from 9% (Mitchell et al., Reference Mitchell, Rainwater, Hsueh, Kennedy, Stern and Maccluer2003) to 57% (Butte et al., Reference Butte, Cai, Cole and Comuzzie2006).
Sport participation is a specific form of leisure-time PA characterized by systematic training schedules and competitions. Sport participation may elicit moderate-to-high levels of energy expenditure (Patience et al., Reference Patience, Kilpatrick, Sun, Flory and Watterson2013), and may contribute to the likelihood that children meet recommended levels of PA (Katzmarzyk & Malina, Reference Katzmarzyk and Malina1998). Further, sport participation has the potential to positively affect health (Geidne et al., Reference Geidne, Quennerstedt and Eriksson2013) and, given the known longevity of former athletes, increase life expectancy (Clarke et al., Reference Clarke, Walter, Hayen, Mallon, Heijmans and Studdert2012; Coate & Sun, Reference Coate and Sun2013). Apart from research dealing with sport excellence and its enhancing factors in high-level athletes (Bouchard & Hoffman, Reference Bouchard and Hoffman2011), there is limited knowledge about the magnitude of genetic factors governing sport participation in the general population. Available twin data showed that the heritability (h 2) of sport participation ranges from 45% (Koopmans et al., Reference Koopmans, Lorenzo, Boomsma, Goldbout, Faire and Berg1994) to ~83% (Beunen & Thomis, Reference Beunen and Thomis1999; Maia et al., Reference Maia, Thomis and Beunen2002). In nuclear family studies, h 2 is estimated lower than in twin research and ranges from 0% (Perusse et al., Reference Perusse, Tremblay, Leblanc and Bouchard1989) to 60% (Butte et al., Reference Butte, Cai, Cole and Comuzzie2006).
Few studies have addressed the issue of consistency of genetic effects on PA and sport participation across age in children. Stubbe et al. (Reference Stubbe, Boomsma and De Geus2005), using data from various twin surveys conducted in the Netherlands, showed that common environmental factors are essentially responsible for sport participation from 13 to 16 years, whereas from 17 to 20 years, a marked shift is present, with genetic factors largely explaining individual differences in sport participation. Using a similar twin sample and the same data analysis strategy, van der Aa et al. (Reference van der Aa, De Geus, van Beijsterveldt, Boomsma and Bartels2010) analyzed questionnaire-based exercise behaviors across three age groups (13–14, 15–16, and 17–19 year olds). The variation in sport participation was largely explained by genetic factors (72–85%) across the three age groups, while environmental influences common to the twin pair (46%) were only evident in girls aged 13–14 years.
To date, we were only able to identify one study that examined the stability of genetic and environment effects in adolescence, across six different surveys (aged 7–18 years) in exercise behavior during leisure time (Huppertz et al., Reference Huppertz, Bartels, de Zeeuw, van Beijsterveldt, Hudziak, Willemsen and de Geus2016). Using a twin sample, the authors demonstrated that genetic effects tended to increase across age, and shared environmental effects tended to decrease. To the best of our knowledge, no other study has previously examined the stability of genetic factors in leisure-time PA and sport participation using nuclear families during adolescence.
To the extent that the partition of the phenotypic variability among groups of individuals forming defined family structures (e.g., siblings, parent-offspring, and spouses) allows for the quantification of the stability of genetic effects between and within a particular family structure, the present study aims to investigate how consistent genetic factors are at explaining the variance, particularly as inferred from heritability estimates (h 2) in the leisure-time physical activity index (LTPAI) and the sport participation index (SPI) from early to late adolescence using nuclear family data.
Material and Methods
Sample
Between 2006 and 2009, the Portuguese Healthy Families Study (from the Portuguese Estudo das Famílias Portuguesas Saudáveis) enrolled families from the north and central mainland regions of Portugal, as well as the Azores and Madeira islands. Recruitment occurred mainly through public schools, both preparatory and secondary, where 10- to 19-year-old students, who had at least one brother or sister, were asked to become involved in a research project dealing with active and healthy living. Participation was voluntary. In phase 1, only physical activity (PA) was assessed and 3,378 families offered to participate (12,385 subjects). Given that Portuguese families with three or more children over 10 years of age are very scarce in the Portuguese population (Rosa & Chitas, Reference Rosa and Chitas2010), our sample was based on nuclear families with two siblings. Furthermore, we excluded children with physical handicaps, psychological disorders, and chronic diseases that may impair their ability to be physically active or to participate in sports. The families were divided into two groups based on the age of both siblings: 10–14 years and 15–19 years. Families who had siblings in more than one age group were not considered for the analysis. This project was approved by the Faculty of Sport, University of Porto, and governmental school authorities. Informed consent was obtained from parents or legal guardians, and assent from all children and adolescents.
Measures
Anthropometry
The height and weight of all adolescents were measured using the protocols of Lohman et al. (Reference Lohman, Roche and Martorell1988). Height was measured to the nearest 1 mm with a portable stadiometer (Siber Hegner GPM), and weight with a Seca scale (Model 762) with a precision of 0.1 kg. For parents, a questionnaire was sent home where they self-reported their height and weight, as well as their professional occupation. Body mass index (BMI = weight [kg]/height [m2]) was computed for all subjects; they were classified as normal weight, overweight, or obese according to the World Health Organization (WHO, 2000) reference categories for adults and Cole et al.’s (Reference Cole, Bellizzi, Flegal and Dietz2000) reference categories for children and adolescents.
Physical activity assessment
The Baecke questionnaire (Baecke et al., Reference Baecke, Burema and Frijters1982) was used to assess PA. This has been consistently shown to be a reliable and valid instrument compared with accelerometry (Miller et al., Reference Miller, Freedson and Kline1994), doubly-labeled water (Philippaerts et al., Reference Philippaerts, Westerterp and Lefevre1999), and other questionnaires (Pereira et al., Reference Pereira, FitzerGerald, Gregg, Joswiak, Ryan, Suminski and Zmuda1997). The Portuguese version of this questionnaire has been widely used in youth (Antunes et al., Reference Antunes, Maia, Stasinopoulos, Gouveia, Thomis, Lefevre and Freitas2015; Seabra et al., Reference Seabra, Mendonca, Thomis, Malina and Maia2007), twin (Maia et al., Reference Maia, Thomis and Beunen2002), and sibling studies (Pereira et al., Reference Pereira, Todd Katzmarzyk, Gomes, Souza, Chaves, Dos Santos and Maia2017; Reference Pereira, Katzmarzyk, Gomes, Souza, Chaves, Santos and Maia2018), as well as in family studies (Maia et al., Reference Maia, Gomes, Tregouet and Katzmarzyk2014; Santos et al., Reference Santos, Katzmarzyk, Diego, Blangero, Souza, Freitas and Maia2014). The Baecke questionnaire comprises 16 questions that allow the extraction of three component scores: (1) work/school, (2) leisure time, and (3) sport participation. In addition, a total PA index was derived, which is the unweighted sum of the three component indexes. Each component/domain consists of a set of questions scored mostly on a five-point Likert scale, where higher scores indicate higher PA levels. In the present study, only two component scores were used: the LTPAI and the SPI. The LTPAI questions are related to modes of transportation to work or school, time spent watching TV, and walking and cycling, whereas the SPI incorporates questions related to type of sport, duration and frequency of practice, and sweating during sport practice. The two indexes range from 1 to 5. Children and adolescents answered the questionnaire following standard instructions from a team member in their physical education classes, while parents completed the questionnaire at home. Furthermore, all research team members were available to answer any questions from parents.
Parental occupations
Classification of parental occupation was used as a proxy for socioeconomic status (SES). Occupations were categorized into nine groups (1 = highest SES to 9 = lowest SES) according to the Portuguese National Occupations Classification (Instituto do Emprego e Formação Profissional, 2001). The categories are as follows: 1 = central administration/politicians and executive directors, 2 = specialists of intellectual and scientific activities, 3 = technicians and intermediate-level jobs, 4 = back-office jobs, 5 = security and individual services, 6 = farmer and qualified farm, fish, and forest workers, 7 = industry and qualified building jobs, 8 = machine and equipment operators, and 9 = non-qualified jobs. For all analyses, we clustered the responses into three groups: 0–3 (high SES); 4–6 (medium SES); and 7–9 (low SES).
Statistical analysis
Exploratory analysis and descriptive statistics were computed in SPSS 20, ignoring the sibling relationship; this only trivially biases the reported standard deviations. Variance components and heritability were estimated by maximum likelihood assuming normality, as implemented in the S.A.G.E. (S.A.G.E. — Statistical Analysis for Genetic Epidemiology, 2016) program ASSOC. A linear regression incorporates the covariates age, age2, sex, BMI, and SES, and to allow for each family structure, the correlations of the residuals from this linear regression are expressed as functions of estimated variance components. To better approximate normality, each individual's random component, expressed as the sum of shared components and a residual chosen to keep the total variance (σ2T) constant (Elston et al., Reference Elston, George and Severtson1992), undergoes an estimated power transformation; all parameters, including the power parameter, are simultaneously estimated and their standard errors are determined by numerical double differentiation of the likelihood surface. Apart from an individual-specific random component (assumed to be due to measurement error + random environment), the model contains three additional random components that can be shared among two or more relatives: additive polygenic (σ2p), marital (σ2M), and the excess sibling component (σ2S) over and above the additive polygenic component. This last component is thus an environmental component common to siblings, confounded with any non-additive polygenic component, which we assume to be trivially small. Explicitly, we can write the linear mixed model as follows:
where, for the ith individual, yi (SPI or LTPAI) is the quantitative measure, c1i, c2i . . . are the covariates (age, age2, sex, BMI, and SES) taken as fixed effects, pi is the random additive polygenic effect, mi is the random marital effect (i.e., an effect common to spouses), si is the random excess sibling effect (i.e., an effect common to siblings not accounted for by additive polygenic inheritance), and εi is the individual-specific random effect.
With the total variance as the denominator, the numerator of heritability is the polygenic variance (h 2 = σ2p/σT2). Similarly, with the same denominator, the numerators for the residual full sibling, parent–offspring, and marital correlations are, respectively, the full-sibling variance plus half the polygenic variance (σ2S + 1/2σP2), half the polygenic variance (1/2σ2P), and the marital component of variance (σ2M). The environmental correlation is calculated using as denominator the total variance minus the polygenic variance, and as numerator the variance of the excess sibling effect, that is, the sibling environmental correlation is (σ2S)/(σT2 − σ2P), on the assumption that any non-additive polygenic effect can be ignored. (Note that it is an intraclass correlation because there can be more than two siblings in a sibship and we assume that after regressing out covariates, all the siblings in a sibship have the same mean.) Details of how the transformation — used to obtain a better fit to normality — is performed are given in Bochud (Reference Bochud and Elston2017). Note that the actual variance components are estimated on the transformed scale and so are not provided in the tables. To test for differences in heritability estimates for the SPI and LTPAI calculated from two independent samples (younger vs. older adolescents), an approximate large sample test was used in which the difference between the two h 2 estimates was divided by the square root of the sum of their squared standard errors, and the result referred to a standard normal distribution. Analogously, we can test whether any other parameter is different between the younger and older adolescent samples.
Sex-specific familial correlations and their asymptotic standard errors were also estimated on the raw data as well as adjusted for covariates, based on theory developed by Keen and Elston (Reference Keen and Elston2003) and Mathew et al. (Reference Mathew, Song and Elston2011) using the FCOR module of S.A.G.E. (2016).
Results
Table 1 presents descriptive statistics for all family members. On average, fathers are slightly older than mothers, and siblings show similar ages by gender. As expected, fathers and sons are, on average, taller and heavier than mothers and daughters, respectively. The prevalence of overweight and obesity is similar between sons and daughters, but the prevalence was higher in fathers compared to mothers. On average, fathers are slightly less active than mothers in their LTPAI, but have a slightly higher SPI. Furthermore, siblings are more physically active than their parents, and sons more active than daughters.
TABLE 1 Sample Descriptive Statistics of All Family Members (n = Number of Individuals)
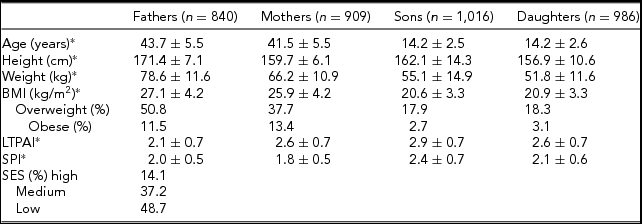
*Mean ± standard deviation.
Table 2 shows parameter estimates obtained using the S.A.G.E. program ASSOC for LTPAI after adjusting for covariates, namely residual heritability, residual correlations among family members (siblings, parent–offspring, and spouses), and environmental correlations in the two age groups (10–14 and 15–19 years old). The heritability estimates are very significant (p < 10−6), but moderate in their effect size, h 2 = 0.297 ± 0.064 at 10–14 years and h 2 = 0.322 ± 0.063 at 15–19 years. Sibling residual correlations are higher in the younger age group (0.386 ± 0.035 vs. 0.294 ± 0.044) and greater than parent–offspring correlations (0.149 ± 0.032, 0.161 ± 0.031), and slightly larger than marital correlations (0.312 ± 0.047, 0.264 ± 0.048). Environmental correlations are low, but higher at 10–14 years old (0.338 ± 0.049 vs. 0.195 ± 0.066). The large sample tests showed no significant difference between the two age groups, in particular for the LTPAI h 2 estimates (z = 0.278, p = .77).
TABLE 2 Heritability, Residual Familial Correlations, and Environmental Correlations for Leisure-Time Physical Activity Index (LTPAI) of Families Whose Siblings’ Age is Within 10–14 or 15–19 Years of Age
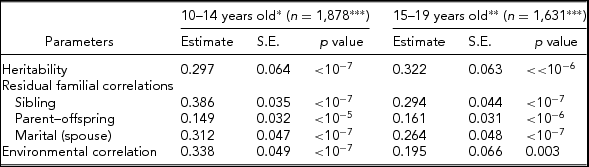
*Significant covariates included: sex and age.
**Significant covariates included= age, sex, and BMI.
***Total number of pedigree members.
Table 3 provides results for the SPI. The heritability estimates are very significant (p < 10−7), but moderate in size: h 2 = 0.413 ± 0.068 at 10–14 years, and h 2 = 0.428 ± 0.068 at 15–19 years. The sibling residual correlation is slightly higher in the younger age group (0.410 ± 0.037 vs. 0.316 ± 0.041), larger than the parent–offspring correlation in both age groups (0.206 ± 0.034, 0.214 ± 0.034), and slightly larger than the marital correlation in the younger age group (0.361 ± 0.048) but slightly smaller in the older age group (0.408 ± 0.047). Environmental correlations are low, but higher at 10–14 years old (0.332 ± 0.061 vs. 0.178 ± 0.070). No significant difference between the two age groups was given by the approximate large sample test, in particular for the SPI h 2 estimates (z = 0.156, p = .79).
TABLE 3 Heritability, Residual Familial Correlations, and Environmental Correlations for Sport Participation Activity Index (SPI) of Families Whose Siblings’ Age is Within 10–14 or 15–19 Years of Age
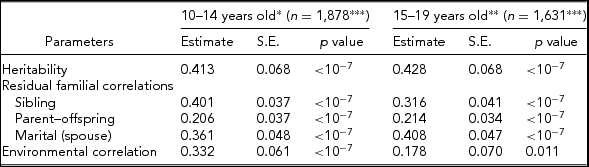
*Significant covariates included = age, sex, and BMI.
**Significant covariates included = age and BMI.
***Total number of pedigree members.
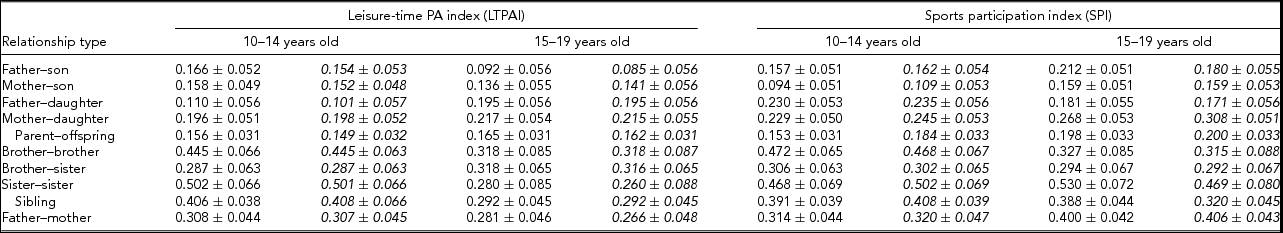
Familial correlations for all possible sex-specific types using the raw data, as well as adjusted for covariates, are presented in Table 4 and were obtained from the S.A.G.E. program FCOR. Correlations between father or mother and siblings are lower than between siblings for the two phenotypes (LTPAI and SPI). Further, correlations are of similar size in families with siblings aged 10–14 and 15–19 years of age. These values remain similar when correlations were calculated using residuals from models adjusted for covariates.
TABLE 4 Correlations (±S.E.) for Leisure-Time Physical Activity Index (LTPI) and for Sports Participation Index (SPI) of Families Whose Siblings’ Age is Within 10–14 or 15–19 Years of Age. Italicized Values are Correlations from the Residuals of the Models Estimating Heritability, i.e., Adjusted for Covariates
Discussion
This study examined how consistent genetic factors are for the LTPAI and SPI from early to late adolescence. Results showed moderate LTPAI and SPI heritabilities in both age groups (10–14 and 15–19 yrs). Further, no significant difference was observed in h 2 estimates in the two age groups, suggesting that the portion of the total variance explained by genes remains consistent across age in adolescence; however, the sets of genes involved remain unidentified. Recent reports have identified some putative effects of specific candidate genes on PA and physical exercise levels, as well as sport participation in different populations (de Geus et al., Reference de Geus, Bartels, Kaprio, Lightfoot and Thomis2014; de Vilhena e Santos et al., Reference de Vilhena e Santos, Katzmarzyk, Seabra and Maia2012; Lightfoot, Reference Lightfoot2011). However, the results are unclear, and large-scale replication is needed before any confirmation of these findings can be achieved. For example, in a review by de Vilhena e Santos et al. (Reference de Vilhena e Santos, Katzmarzyk, Seabra and Maia2012), the authors reported genome-wide linkage data with markers near different physical activity-related genes — EDNRB, MC4R, UCP1, FABP2, CASR, and SLC9A9. However, no marker was present in more than one study. On the other hand, a review by Lightfoot (Reference Lightfoot2011) revealed two candidate genes that showed consistent associations in the regulation of PA: dopamine receptor 1 (DRD1) and helixloop helix 2 (NHLH2). Although the dopamine gene is apparently involved in the regulation of PA, specifically its links to locomotion (Yao et al., Reference Yao, Walker and MacKenzie2013), the precise mechanism of how DRD1 regulates the manifold PA expressions is not yet fully known (Lightfoot, Reference Lightfoot2011). NHLH2 has a potential functional relationship with PA through its effect on β-endorphin production as well as an interaction with melanocortin-4 receptor (Good et al., Reference Good, Coyle and Fox2008). However, we were not able to find a study that investigated changes in the effects of these candidate genes in different PA manifestations across age.
Notwithstanding what is stated above, available cross-sectional family studies using the Baecke questionnaire have reported different h 2 estimates ranging from 0.17 to 0.63 in the LTPAI, and from 0.19 to 0.68 in the SPI (Choh et al., Reference Choh, Demerath, Lee, Williams, Towne, Siervogel and Czerwinski2009; Eriksson et al., Reference Eriksson, Rasmussen and Tynelius2006; Maia et al., Reference Maia, Thomis and Beunen2002; Mustelin et al., Reference Mustelin, Joutsi, Latvala, Pietilainen, Rissanen and Kaprio2012; Seabra et al., Reference Seabra, Mendonca, Goring, Thomis and Maia2008). Similarly, previous studies focusing on the effect of age on h 2 also showed varied results. For example, Stubbe et al. (Reference Stubbe, Boomsma and De Geus2005) reported that SPI h 2 estimates increased with age from 0 in two younger age groups (13–14 and 15–16 yrs) to 0.85 in an older age group (19–20 yrs). One possible explanation for this shift in results may be associated with the age period, that is, when youth finish high school and cease to have mandatory involvement in PA through physical education classes, the additive effects of genetic influences and familial environment emerge. On the other hand, and based on adult data, Aaltonen et al. (Reference Aaltonen, Ortega-Alonso, Kujala and Kaprio2010) examined changes in the genetic and environmental influences on the LTPAI in twins over a 6-year follow-up; they showed that genetic influences declined from baseline to follow-up and specific environmental influences increased from baseline to follow-up. Notwithstanding the use of different methodological strategies among studies that make comparisons difficult, there appears to be a clear fluctuation of the effect sizes of environmental factors in PA across age. For example, previous studies indicated that key life periods have been associated with changes in PA and that PA tends to decline with age (Sallis, Reference Sallis2000), especially during adolescence (Dumith et al., Reference Dumith, Gigante, Domingues and Kohl2011). This PA change may occur as a result of the interaction between individual characteristics and the environment in which the individual lives, learns, works, and plays. For example, D'Haese et al. (Reference D'Haese, Cardon, De Bourdeaudhuij, Deforche, De Meester and Van Dyck2016), using longitudinal data, examined changes in individual and social environmental characteristics relative to PA changes and reported that most individual and social factors became less positive toward PA after the transition to secondary school. Van Der Horst et al. (Reference Van Der Horst, Paw, Twisk and Van Mechelen2007) suggested in their systematic review that PA correlates for both children and adolescents were sex, self-efficacy, and family/parental support. However, attitude, goal orientation/motivation, physical education, and peer support were mostly linked with PA during adolescence. Similarly, the systematic review of Wendel-Vos et al. (Reference Wendel-Vos, Droomers, Kremers, Brug and van Lenthe2007) on correlates of PA among adults revealed that socio-cultural environmental, social support, and having a companion for PA were mostly important in different PA types and intensities.
Residual full-sibling correlations after allowing for covariates tend to decrease across age groups for both the LTPAI (from p = .386 to p = .294, 10–14 and 15–19 age groups, respectively) and the SPI (from p =.410 to p = .316, 10–14 and 15–19 age groups, respectively). These results may be explained by changes in unmeasured environment factors shared among the siblings. Usually during infancy and pre-adolescence, siblings tend to share common environmental factors, namely their neighborhood and recreational environments, as well as school and common friends (Jago et al., Reference Jago, Macdonald-Wallis, Solomon-Moore, Thompson, Lawlor and Sebire2017; Maitland et al., Reference Maitland, Stratton, Foster, Braham and Rosenberg2013; Sullivan et al., Reference Sullivan, Broyles, Barreira, Chaput, Fogelholm and Hu2017); yet, during adolescence and early adulthood, there is growing evidence suggesting that peer relationships (e.g., friends and couples; Barnett et al., Reference Barnett, Guell and Ogilvie2013; Chung et al., Reference Chung, Ersig and McCarthy2017) and intrinsic motivation (Teixeira et al., Reference Teixeira, Carraça, Markland, Silva and Ryan2012) are more likely contributors to unshared environmental factors on PA. This is in line with the observation that environmental correlations in the younger group are higher than in the older group in both the LTPAI and the SPI. It is now acknowledged that across the life course — that is, as a person grows and becomes more independent — that she/he tends to create her/his own environment, influenced by all the significant others who are considered important. Additionally, we also showed that parent–offspring correlations are low and that there are practically no changes in their values across age, for both the LTPA and the SPI. Unfortunately, we were not able to identify a study that investigated changes in parent–offspring resemblance across age to make any comparisons. However, previous studies also reported low correlations between parents and their siblings, ranging from 0 to 0.19 (Horimoto et al., Reference Horimoto, Giolo, Oliveira, Alvim, Soler, de Andrade and Pereira2011; Jacobi et al., Reference Jacobi, Caille, Borys, Lommez, Couet and Charles2011; Maia et al., Reference Maia, Gomes, Tregouet and Katzmarzyk2014). These results may also suggest that, notwithstanding the transmission of genetic factors that may impact PA levels in offspring, there are probably generation-specific environmental factors promoting higher resemblance among siblings rather than across generations (parents to offspring; De Moor et al., Reference De Moor, Willemsen, Rebollo-Mesa, Stubbe, De Geus and Boomsma2011).
In the present study, the LTPA marital residual correlations were 0.312 ± 0.032 for the 10–14 year-old sibling sample and 0.264 ± 0.048 for those 15–19 years old, whereas for the SPI, the correlations were 0.361 ± 0.048 and 0.408 ± 0.047, respectively. Available spousal correlations from Canada (Simonen et al., Reference Simonen, Perusse, Rankinen, Rice, Rao and Bouchard2002), the United States (Mitchell et al., Reference Mitchell, Rainwater, Hsueh, Kennedy, Stern and Maccluer2003), France (Jacobi et al., Reference Jacobi, Caille, Borys, Lommez, Couet and Charles2011), and Brazil (Horimoto et al., Reference Horimoto, Giolo, Oliveira, Alvim, Soler, de Andrade and Pereira2011) varied substantially from 0.02 (95% CI [0.00, 0.15]) with pedometer weekdays’ information in French couples, to 0.43 ± 0.06 in Canadian spouses using their past-year PA information. Although these spouse correlation discrepancies may be due to different family sampling strategies, diverse covariate adjustment, different statistical techniques used to compute correlations, and the phenotypic expression and instruments used, none of these studies tested the possible processes by which spouses correlate in their PA behaviors. In addition, spousal support, attitudes, number of marriage years, and lifestyle choices of spouses can also play important roles (Barnett et al., Reference Barnett, Guell and Ogilvie2013; Falba & Sindelar, Reference Falba and Sindelar2008). Although we do not have any information regarding spouses’ number of marriage years in our family data, and no such reference was made in the previous reports, we think that the mutual interaction-influence effect might explain these Portuguese results given their sharing of cultural assets, health beliefs, background behaviors, and their multifaceted experiences as couples. Further, in this study, although an increase in marriage years is implied by siblings’ age categories (10–14 vs. 15–19), the correlations were similar.
This study is not without limitations. First, the use of questionnaires to obtain information about PA, sport, or physical exercise levels is susceptible to errors. Yet, self-report instruments are very often used, especially when sample sizes are relatively large as is the case of twin and nuclear family studies. Additionally, the Baecke questionnaire is frequently used in Portuguese and Brazilian studies with valid and reliable results (Santos et al., Reference Santos, Katzmarzyk, Diego, Blangero, Souza, Freitas and Maia2014; Seabra et al., Reference Seabra, Mendonca, Thomis, Malina and Maia2007; Silva et al., Reference Silva, Fernandes, Ohara, Collings, Souza, Tomeleri and Cyrino2016). Second, we do not have longitudinal data that investigated chronometric time effects in genetic and environmental links with PA manifold manifestations. Third, no direct measures of environmental factors are available so that direct effects could be properly analyzed and interpreted. This limitation is present in all available studies. Finally, we cannot generalize our results to other populations because of existing environmental and possibly genetic differences.
In conclusion, we showed that LTPA and SPI heritability remains stable across age, from early to late adolescence. This observed familial resemblance is the net result of combined environmental influences that are shared within a family, as well as their genetic endowments. In adolescence, shared environment plays an important role on individual differences in LTPA or SPI. However, the influence of unique environment tends to increase from adolescence to young adulthood. Irrespective of the importance of these findings, there is a need to investigate the complex and dynamic relationships that emerge within and across families followed longitudinally in their varied contexts to more precisely identify the genetic and environment roles in various physical activity manifestations. These are of utmost importance to achieve more efficient health-enhanced behavioral changes and for preventive medicine.
Acknowledgments
We express our gratitude to all participants in The Portuguese Healthy Families Study. PK is supported, in part, by the Marie Edana Corcoran Endowed Chair in Pediatric Obesity and Diabetes.
Authors’ contributions
JM designed the study and supervised the data collection. JM, SP, and TG organized the data set, cleaned the data, and did the descriptive statistics. JM and RE did the core statistical analysis. SP, PK, RE, TG, and JM wrote the document.
Disclosure of Interests
None of the authors have any conflicts of interest to report.






