Menarche is a complex physiological event in a woman’s life, marking the beginning of reproductive capability. Menarche is a late event in puberty, which is known to be triggered by the release of gonadotropin-releasing hormone from specialized neurons of the hypothalamus (DiVall & Radovick, Reference DiVall and Radovick2008). Age at menarche (AAM), the onset of menses, has been linked to many adverse health outcomes. For example, early AAM has been associated with depression (Kaltiala-Heino et al., Reference Kaltiala-Heino, Kosunen and Rimpela2003), conduct disorder (Burt et al., Reference Burt, McGue, DeMarte, Krueger and Iacono2006), eating disorders (Kaltiala-Heino et al., Reference Kaltiala-Heino, Rimpela, Rissanen and Rantanen2001), obesity (Ong et al., Reference Ong, Northstone, Wells, Rubin, Ness, Golding and Dunger2007), type 2 diabetes (He et al., Reference He, Zhang, Hunter, Hankinson, Buck Louis, Hediger and Hu2010), breast cancer (Collaborative Group on Hormonal Factors in Breast Cancer, 2012), cardiovascular diseases (Feng et al., Reference Feng, Hong, Wilker, Li, Zhang, Jin and Xu2008) and spontaneous abortion (Liestol, Reference Liestol1980). Late AAM has been related to an increased risk of osteoporotic fractures (Naves et al., Reference Naves, Diaz-Lopez, Gomez, Rodriguez-Rebollar and Cannata-Andia2005).
It has been very well documented that a secular decrease in AAM occurred in developed countries over the last two centuries. In Europe, the mean AAM has declined by about 2–3 months per decade (Cabanes et al., Reference Cabanes, Ascunce, Vidal, Ederra, Barcos, Erdozain, Lope and PollÆn2009; Talma et al., Reference Talma, Schönbeck, Van Dommelen, Bakker, Van Buuren and Hirasing2013; Wyshak & Frisch, Reference Wyshak and Frisch1982), compared with a decline in the USA of about 1–2 months per decade among European-American females and of about 4.7 months per decade among African-American females (Freedman et al., Reference Freedman, Khan, Serdula, Dietz, Srinivasan and Berenson2002; McDowell et al., Reference McDowell, Brody and Hughes2007). Although evidence is not consistent, the downward trend in AAM has levelled off in the USA and some European countries in recent decades (Cole, Reference Cole2000; Lee et al., Reference Lee, Guo and Kulin2001; Papadimitriou et al., Reference Papadimitriou, Fytanidis, Douros, Bakoula, Nicolaidou and Fretzayas2008).
Many researchers of AAM speculate that the secular decrease may be attributable to improved nutritional status and general health of adolescent females in the developed countries (Parent et al., Reference Parent, Teilmann, Juul, Skakkebaek, Toppari and Bourguignon2003). The mean AAM decreased more rapidly in South Korean females than in European or American counterparts, perhaps due to the speed of environmental changes that have occurred in South Korea in the past decades. Ahn et al. (Reference Ahn, Lim, Song, Seo, Lee, Kim and Lim2013) found that the mean AAM decreased by 0.72 years (approximately 8.71 months) per decade in a large nationally representative sample of South Korean females (N = 11,065) born between 1904 and 1995. Interestingly, a North Korean female refugee sample [mean (SD) age = 31.3 (6.2) years; N = 411] also showed a similar level of the downward trend (0.4 years per 5 years) in the mean AAM (Ku et al., Reference Ku, Kang, Kim, Kim, Jee, Suh and Kim2006). However, the mean AAM was much higher (16.0 ± 2.1 years) in North Korean refugees than in South Korean females of similar age. Given that North and South Koreans have the same ancestry, the mean difference in AAM between the two groups is likely due to environmental differences.
Twin studies have found that about 45% (Snieder et al., Reference Snieder, MacGregor and Spector1998) to 95% (Loesch et al., Reference Loesch, Huggins, Rogucka, Hoang and Hopper1995) of the variance in AAM is due to additive genetic influences, with the remaining variance being attributable to individual-specific environment plus measurement error. However, as almost all of these studies were conducted in Western countries, the heritability of AAM in East Asians remains largely unknown. Given the racial and ethnic differences in the mean AAM (Karapanou & Papadimitriou, Reference Karapanou and Papadimitriou2010) and differences in genetic backgrounds and nutritional and other health-related environmental conditions between East Asians and Europeans, it is important to examine whether or not the heritability of AAM in South Koreans differs from those found in the USA or European countries. This study aimed to investigate the heritability of AAM in South Koreans from the female twins born in South Korea between 1988 and 2001.
Methods
Sample and Measure
Participants were the female twins who participated in telephone surveys conducted by the South Korean Twin Registry (Hur et al., Reference Hur, Jeong, Chung, Shin and Song2013). Although the telephone surveys were given to twins over 11 years of age, twins aged 16 years or older were chosen for data analysis for the present study because it is known that most females reach menarche between the ages of 12 and 15 years (Parent et al., Reference Parent, Teilmann, Juul, Skakkebaek, Toppari and Bourguignon2003). In total, 1387 twins (71.5%) who met the age criteria were selected from those who responded to the telephone surveys. Of the 1387 twins, 17 (1.2%) were twins who either had not menstruated yet (n = 3; 0.2%) or could not remember their AAM (n = 14; 1.0%). Removing 17 from the sample resulted in 1370 twins (933 monozygotic [MZ] twins, 294 dizygotic [DZ] twins and 160 female members of the opposite-sex DZ twins). The age of the sample at the time of the interview ranged from 16 to 28 years with a mean of 19.3 (SD = 2.2) years. In terms of birth year, the oldest individual in this study sample was born in 1988 and the youngest in 2001. Twins under 20 years of age were recruited mostly from schools throughout South Korea, while those aged 20 years or older were from Facebook, twin clubs on the Internet and colleges throughout South Korea. The telephone surveys for twins included a question on menarche. Female twins were first asked whether they had their first menstruation. If they responded ‘yes’, they were then asked to indicate the year and the month in which it had occurred. Because many twins provided the year confidently but often skipped the month, the current report defined AAM by age in years.
Zygosity of the twins was assessed using a three-item zygosity questionnaire. When compared with DNA analysis, this approach has been shown to achieve over 90% accuracy (Ooki et al., Reference Ooki, Yamada, Asaka and Hayakawa1990). The number of MZ twins was much greater than that of DZ twins in the present sample, which likely reflected the low DZ twin birth rates in the South Korean population for the birth cohorts in the present study rather than sampling bias (Hur & Kwon, Reference Hur and Kwon2005; Hur & Song, Reference Hur and Song2009).
Statistical Analyses
Maximum likelihood twin correlation and model-fitting analyses were conducted using a raw data approach in Mx (Neale et al., Reference Neale, Boker, Xie and Maes2003). The total variance of AAM was decomposed into four components: additive genetic (A; rMZ = 1.0, rDZ = 0.5), nonadditive genetic (D; rMZ = 1.0, rDZ = 0.25), shared environment (C; rMZ = 1.0, rDZ = 1.0) and individual-specific environment plus measurement error (E; rMZ = 0, rDZ = 0). Two full models (ACE vs. ADE) were fit to the data separately to select the better-fitting one. The selected full model was compared with that of the nested submodels by using the log-likelihood ratio test to determine the best-fitting, most parsimonious model. In addition, Akaike’s information criterion (AIC = −2LL − 2df; Akaike, Reference Akaike1987) was used to evaluate alternative models when the models were not nested to each other. The model with lowest AIC was retained as the best-fitting model because lower values indicate a better balance between parsimony and goodness-of-fit. In twin correlational and model-fitting analysis, age was treated as a covariate.
Results
Descriptive Statistics
The mean AAM in the total sample was 12.49 (SD = 1.41) years, which was only slightly lower than the average of 12.7 years found in a nationally representative sample of South Korean adolescents born between 1983 and 2001 (Lee et al., Reference Lee, Kim, Oh, Lee and Park2016). Consistent with a large body of literature on AAM, the present twin sample showed a declining pattern of the mean AAM over the years (see Figure 1). However, it appeared that the decrease stopped in years 2000–2001. Note that the confidence interval around the mean was larger in 2000–2001 than in 1997–1999. The mean AAM was not significantly different between MZ and DZ twins (12.53 vs. 12.38 years) or between the first- and the second-born twins (12.36 vs. 12.38 years).
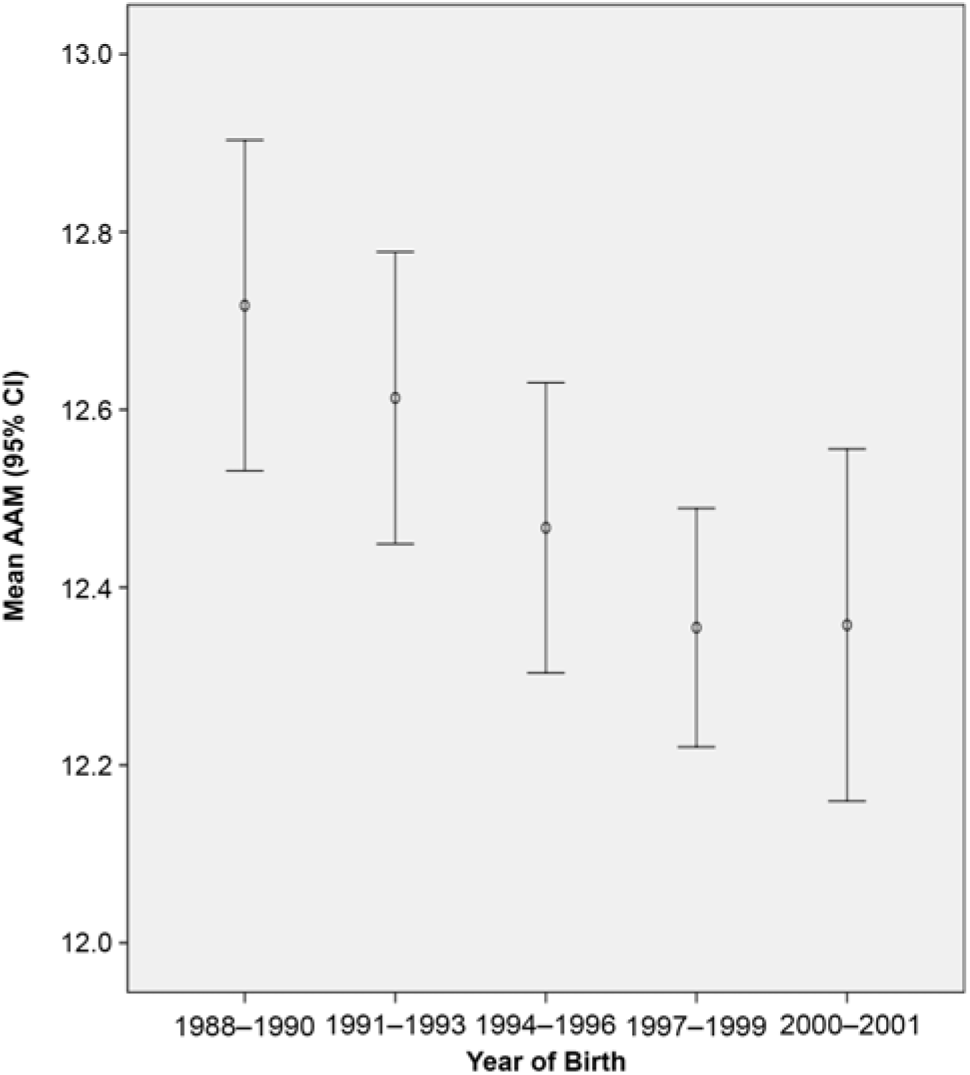
Fig. 1. Mean age at menarche (AAM) in years and their 95% CI during birth years 1988–2001 [s3].
Twin Correlations and Univariate Model Fitting in the Total Sample
Female members of the opposite-sex DZ twins were excluded from twin correlation and univariate model-fitting analysis. The maximum-likelihood MZ and DZ twin correlations were 0.72 [95% CI (0.67, 0.76)] and 0.35 [(95% CI (0.19, 0.50)], respectively. Table 1 shows the results of the model-fitting analysis. As indicated in Table 1, the ACE model was slightly better than the ADE model. Furthermore, nonadditive genetic effects in the ADE model were not significant. The final best-fitting model indicated that additive genetic and individual-specific environmental influences, including measurement error, were 72% [95% CI (67%, 76%)] and 28% [95% CI (24%, 33%)] respectively. Shared environmental effects were not significant.
Table 1. Results of univariate model-fitting analysis
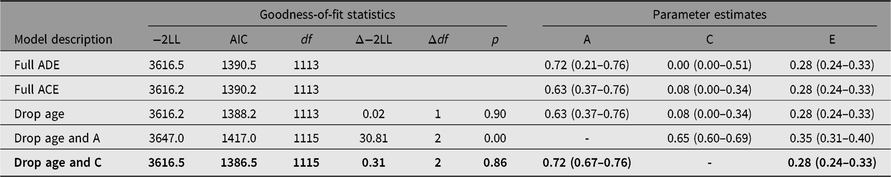
Note: −2LL = −2 log-likelihood. A = additive genetic effects, D = non-additive genetic effects, C = shared environmental effects, E = individual-specific environmental effects and measurement error. The fit indices of the submodels were compared with those of the full ACE model. The best-fitting model is indicated in bold.
Discussion
Early menarche is associated with the risk of a number of diseases. Therefore, it is important to understand the genetic and environmental variances as well as the secular decrease of the mean AAM. The present sample showed that the mean AAM decreased with increasing birth years, although it levelled off in birth years 2000–2001. Heritability of 72% found in the present study was within the range of the estimates reported from Western twin studies (Towne et al., Reference Towne, Czerwinski, Demerath, Blangero, Roche and Siervogel2005), and, especially, it was the same as that estimated from the combination of large samples of Australian, Dutch and UK twins (Anderson et al., Reference Anderson, Zhu, Falchi, Van den Berg, Treloar, Spector and Montgomery2008).
Recent genome-wide association studies (GWAS) have identified more than 100 candidate genes at loci associated with AAM in Caucasian females (Day et al., Reference Day, Thompson, Helgason, Chasman, Finucane, Sulem and Perry2017; Delahanty et al., Reference Delahanty, Beeghly-Fadiel, Long, Gao, Lu, Xiang and Shu2013; Elks et al., Reference Elks, Perry, Sulem, Chasman, Franceschini, Chunyan and Murray2010; Perry et al., Reference Perry, Day, Elks, Sulem, Thompson, Ferreira and Ong2014). These loci were in or near genes related to hormonal regulation, cellular development, body weight regulation, skeletal maturation and other biological functions (Elks et al., Reference Elks, Perry, Sulem, Chasman, Franceschini, Chunyan and Murray2010). However, the loci discovered to date explained at most about 7.4% of the population variance in AAM, corresponding to approximately 25% of the estimated heritability (Day et al., Reference Day, Thompson, Helgason, Chasman, Finucane, Sulem and Perry2017), suggesting that more GWAS are needed to determine genes for AAM. In the meantime, GWAS based on Asian females (Pyun et al., Reference Pyun, Kim, Cho, Koh, Lee, Shin and Kwack2014; Shi et al., Reference Shi, Zhang, Choi, Gao, Li, Lu and Shu2016; Tanikawa et al., Reference Tanikawa, Okada, Takahashi, Oda, Kamatani, Kubo, Nakamura and Matsuda2013), albeit from small samples, replicated some of the loci for AAM identified in European samples, suggesting the existence of shared genetic architecture for AAM across human populations. It would be of interest for future researchers to carry out a large-scale international collaboration of GWAS to better understand ethnic or racial similarities or differences in genetic mechanisms for AAM.
A few limitations of the present study should be acknowledged. First, as with many other studies of AAM, this study employed self-reported AAM. The use of self-report of AAM has been criticized for the presence of recall error (Karapanou & Papadimitriou, Reference Karapanou and Papadimitriou2010). However, given the ages of the twins at the time of assessment (16–28 years), there was a relatively short interval between the actual menarche and the time of report, which may have substantially reduced the recall error in the present study. Second, the sample used in the present study was relatively recent-born twins (1988–2001). Using a sample in the USA, Johnson et al. (Reference Johnson, Choh, Curran, Czerwinski, Bellis, Dyer and Demerath2013) demonstrated that shared genetic influences on menarche timing and BMI were amplified over the years 1928–1992. In the last century, rapid changes have occurred in nutritional and nonnutritional environments in South Korea. Thus, the heritability of AAM in elderly South Korean twins might be different from that reported in the present study.
Acknowledgments
The authors would like to thank twins who participated in the South Korean Twin Registry. This study was supported by the National Research Foundation of Korea grant NRF-371-2011-1 B00047 and the ‘Development of Health Prediction Technology based on Big Data’ (K18092) funded by the Ministry of Science and ICT (MSIT) of Korea given to the Korea Institute of Oriental Medicine.




