Alanine aminotransferase (ALT) is one of the most commonly used liver enzymes in clinical practice and is used to aid in the diagnosis and management of patients with liver disease, ranging from viral hepatitis to fatty liver (Lazo et al., Reference Lazo, Selvin and Clark2008; Valenti et al., Reference Valenti, Pelusi, Bianco, Ceriotti, Berzuini, Iogna Prat, Trotti, Malvestiti, D’Ambrosio, Lampertico, Colli, Colombo, Tsochatzis, Fraquelli and Prati2021). Elevated ALT levels are an independent predictor of mortality from liver disease in the general population. In addition, ALT is also significantly associated with the risk of metabolic syndrome, diabetes and cardiovascular disease (Goessling et al., Reference Goessling, Massaro, Vasan, D’Agostino, Ellison and Fox2008; Sattar et al., Reference Sattar, Scherbakova, Ford, O’Reilly, Stanley, Forrest, Macfarlane, Packard, Cobbe and Shepherd2004; Song et al., Reference Song, Yun and Park2008). Therefore, investigating the factors that influence serum ALT concentration is of great significance to human health.
Previous studies have found that some environmental factors may be related to serum ALT levels in humans; for example, obesity, waist circumference and hip circumference (Ali et al., Reference Ali, Sumon, Fariha, Asaduzzaman, Kathak, Molla, Mou, Barman, Hasan, Miah and Islam2021; Danielsson et al., Reference Danielsson, Nissinen, Jula, Salomaa, Männistö, Lundqvist, Perola and Åberg2021), BMI, physical activity, alcohol consumption, smoking (Park et al., Reference Park, Lim, Oh, Cho, Bae, Yun, Kim and Shin2013; Prati et al., Reference Prati, Taioli, Zanella, Della Torre, Butelli, Del Vecchio, Vianello, Zanuso, Mozzi, Milani, Conte, Colombo and Sirchia2002), and lead exposure (Lee et al., Reference Lee, Cho, Jeong, Park, Shin, Kim and Kim2020). In addition, genetic factors have also been found to correlate with ALT levels. Concentrations of ALT are highly heritable (22%−55%; Bathum et al., Reference Bathum, Petersen, Rosholm, Hyltoft Petersen, Vaupel and Christensen2001; Makkonen et al., Reference Makkonen, Pietiläinen, Rissanen, Kaprio and Yki-Järvinen2009; Rahmioglu et al., Reference Rahmioglu, Andrew, Cherkas, Surdulescu, Swaminathan, Spector and Ahmadi2009; van Beek et al., Reference van Beek, de Moor, de Geus, Lubke, Vink, Willemsen and Boomsma2013; Whitfield et al., Reference Whitfield, Zhu, Nestler, Heath and Martin2002). However, ALT heritability has not been reported for the Chinese population. In this study, we assessed the relative contributions of genetic and environmental factors of inter-individual variation in serum ALT levels among monozygotic (MZ) and dizygotic (DZ) adult Chinese twins.
Methods
Study Participants
This study was based on the Qingdao Twin Registry (QTR), which was established in 1998 at the Qingdao Center for Disease Control and Prevention (Qingdao CDC) as the first population-based twins registry in China (Duan et al., Reference Duan, Ning, Zhang, Wang, Zhang, Tan, Tian and Pang2013; Li et al., Reference Li, Gao, Yu, Lv, Cao, Zhan, Wang, Wu and Hu2013). Twins were recruited by Qingdao CDC in residential communities throughout the municipality of Qingdao by using the residency register, medical data and media advertisements. Complete pairs of middle-aged and elderly twins were recruited in 2012 and 2013. Twins who were unconscious, reluctant, or unable to cooperate, as well as incomplete twin pairs, were excluded from the study. Furthermore, twins with no ALT data or twins with hepatitis (the disease data was obtained through the questionnaire) were excluded from the samples in pairs.
This research adhered to the Helsinki Declaration and was approved by the Regional Ethics Committee of the Institutional Review Committee of the Qingdao CDC. All participants have signed informed consent for this project.
Information Collection
Questionnaires and health examination were completed by participants at the local service center of the Qingdao CDC or at community hospital or clinics. Basic personal information such as age and gender and the data about alcohol consumption (How much alcohol do you drink on average per day now?) were obtained through a questionnaire. Data for body mass index were calculated (weight [kg]/height [m]2) by measured data: height and weight.
For blood samples, all participants drew 10 ml of venous blood after an overnight fast. Within 30 minutes, the serum and plasma were separated from the blood cells and stored at -80 °C. The serum ALT concentration was tested by Hitachi 7600 (Japan), a semiautomatic analyzer.
Zygosity Identification
Twins with different gender or blood type were identified as DZ. The zygosity of other twins with same gender and blood type was determined using satellite DNA genotyping. When all 15 short tandem repeats (STRs) were identical, the twins were classified as MZ; otherwise, they were classified as DZ. The accuracy of zygosity assignment was 99.9% (Becker et al., Reference Becker, Busjahn, Faulhaber, Bähring, Robertson, Schuster and Luft1997; Tomsey et al., Reference Tomsey, Kurtz, Kist, Hockensmith and Call2001).
Statistical Analysis
Raw data were double-entered using Epidata 3.0 software. For data preparation and statistical description, SPSS 22.0 was utilized. Considering the skewed distributions of serum ALT level, rank transformation (a data normal transformation method) was conducted for ALT level data. In the following, we refer to the normal ALT level data obtained by rank transformation as normal-ALT. The normal-ALT data conformed to the normal distribution (p = .20).
To quantify intraclass coefficients (ICCs) per zygosity, a Pearson correlation was computed. ICCs were computed using the function twinlm of mets package in R. When the half of the ICC of MZ twins (r MZ) was lower than the ICC of DZ twins (r DZ), the ACE model was considered; otherwise, the ADE model was selected.
The Mx program was utilized for genetic analysis. In the conventional twin design, the phenotypic variance was separated into three categories: additive genetic (A), shared environmental variables (C)/dominant genetic (D), and unique environmental (E) factors. We utilized standard structural equation modeling (SEM) approaches to estimate the A, C/D, and E components, adjusting for age, gender, BMI, and alcohol use, as these variables are related to liver function and may influence the serum ALT level.
We applied a likelihood ratio test to compare the performance of the complete ACE (or ADE) model and its nested model, that is, the AE model. In accordance with the parsimonious principle, as measured by the Akaike information criterion (AIC) value, the model with the best fit was selected (Vrieze, Reference Vrieze2012). When the optimal structural equation model was completed, we further calculated the statistical power of the additive genetic (A) path using the R package umx. The significance level α was set at .05.
Results
The current study contained 369 sets of twins (233 MZ and 136 DZ) ranging in age from 40 to 80. The median (interquartile range [IQR]) age of all twins was 50 (46, 57) years, and the median (IQR) of ALT level was 18 (12, 27) upper/lower (U/L). For MZ twins, the median (IQR) age was 51 (46, 57) and the median (IQR) ALT level was 19 (12, 27) U/L. For DZ twins, the median (IQR) age was 49 (45, 56) and the median (IQR) ALT level was 18 (12, 27) U/L. More detailed information about age, BMI, and ALT level between different zygosity twins is shown in Table 1.
Table 1. Descriptive statistics for twins

Note: MZ, monozygotic; DZ, dizygotic; IQR, interquartile range; ALT, abnormal alanine aminotransferase; U/L, upper/lower; normal-ALT, ALT data were processed by rank transformation.
In Figure 1, scatter plots of normal-ALT in MZ twins and DZ twins revealed a considerable stronger dependence in MZ twins, suggesting hereditary effect of the trait. The ICCs of normal-ALT of MZ twins (r MZ) and DZ twins (r DZ) were .64 (95% CI [.56, .7]1) and .42 (95% CI [.28, .55]). The correlation coefficient of MZ twins was higher than that of DZ twins, indicating that serum ALT level is significantly influenced by genetic factors.
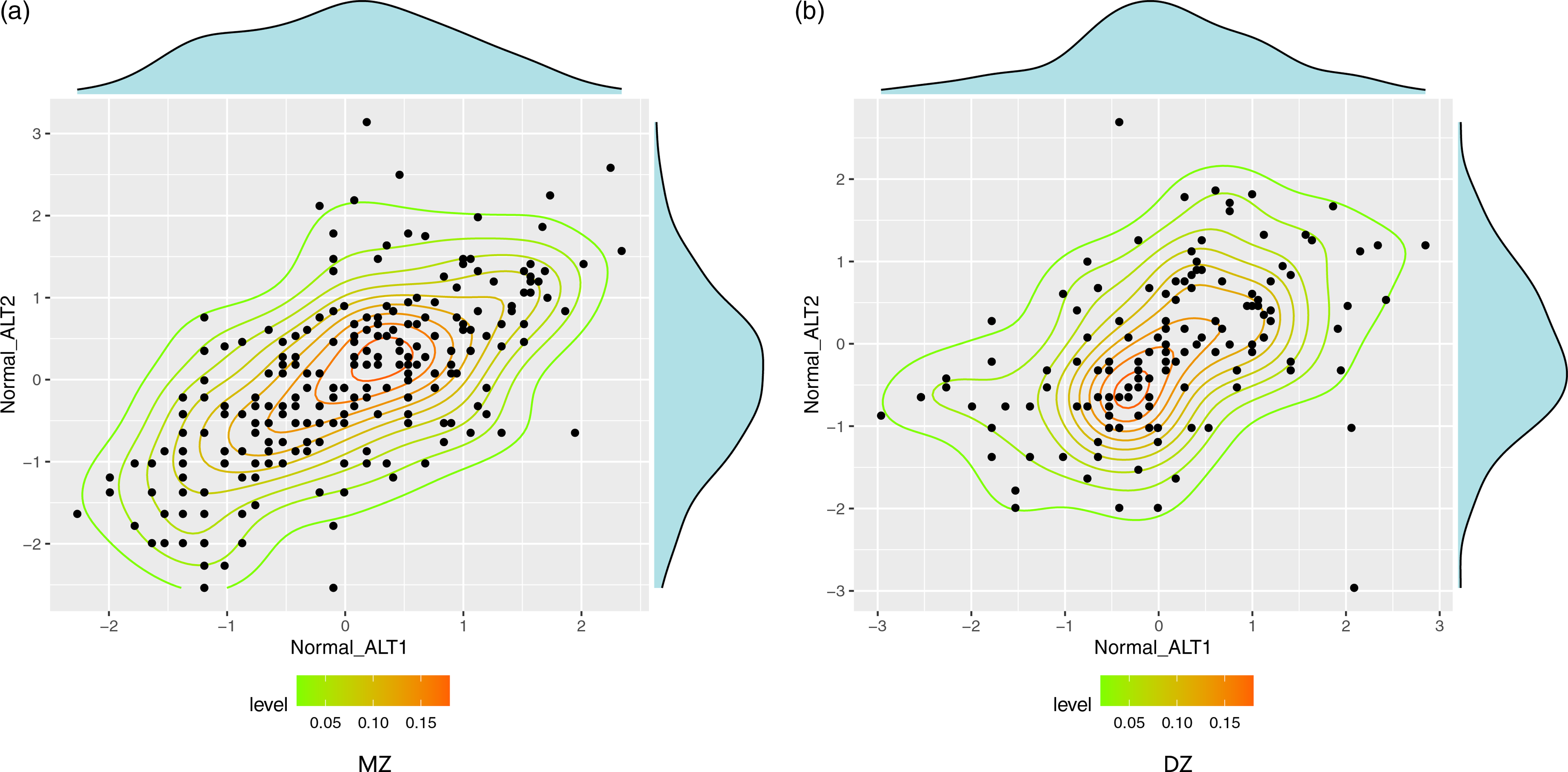
Fig. 1. Scatter plot of normal-ALT in MZ and DZ twins. Note: MZ, monozygotic; DZ, dizygotic; ALT, abnormal alanine aminotransferase; normal-ALT, ALT data were processed by rank transformation.
Because half of r MZ was less than r DZ, we fitted the full ACE and its nested models (AE, CE) to normal-ALT. After adjusting for all covariates (age, gender, BMI, alcohol consumption), we evaluated the variance components and heritability estimates. Table 2 contains the outcomes of the model fit. The difference between the AE model and the full model was not significant (p = .39), and the AE model had lower AIC than the full model (419.84 vs. 421.10). Thus, according to the parsimonious principle, the AE model was considered to be the best-fitting model. After adjusting for age, gender, BMI, and alcohol consumption, the results of the AE model demonstrated that additive genetic parameters (A) accounted for .65 (95% CI [.57, .71]) and unique environmental or residual parameters (E) accounted for .35 (95% CI [.29, .44]) respectively. Shared environmental influences were not significant. A power analysis revealed that when the twin sample size was 369 pairs, the power of the SEM A path was .99 (α = .05).
Table 2. Results of model-fitting analysis for serum ALT level in Chinese population
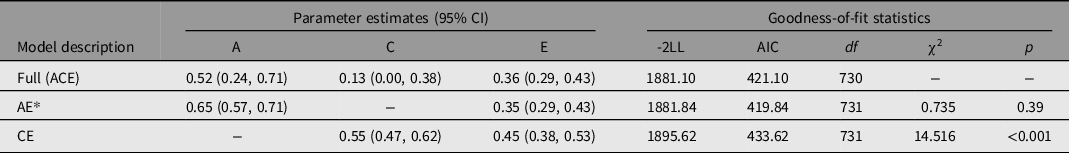
Note: Models were adjusted by age, gender, BMI, and alcohol consumption. -2LL, -2 times log-likelihood of data; df, degrees of freedom; AIC, Akaike’s Information Criterion; A, additive genetic influence; C, shared environmental influence; E, nonshared environment and measurement error.
*The best-fitting model.
Discussion
A twin study is a well-established method in genetic epidemiology to explore the relative effects of genetic and environmental factors on phenotypes. To our knowledge, this study is the first to report heritability of ALT in Chinese population. The results of the ICCs and SEM demonstrated strong evidence of genetic influence on ALT level. Serum ALT level showed heritability of 65% after taking into account age, gender, BMI, and alcohol consumption among Chinese population. The power test result of the A path in our best-fitting model (AE model) was .99 (α = .05). This high enough power means that the sample size of our study was adequate and our result was stable. In addition, our analysis revealed that approximately 35% of the variation in serum ALT levels could be attributed to environmental factors.
Previous studies have explored the genetic influence of ALT variants. A study of adult twins in the UK showed that 32% of the total variation in ALT level was accounted for by additive genetic factors in an ACE model after adjusting for age, gender, BMI, and relative alcohol use (Rahmioglu et al., Reference Rahmioglu, Andrew, Cherkas, Surdulescu, Swaminathan, Spector and Ahmadi2009). A Finland study (Makkonen et al., Reference Makkonen, Pietiläinen, Rissanen, Kaprio and Yki-Järvinen2009) and a Danish study (Bathum et al., Reference Bathum, Petersen, Rosholm, Hyltoft Petersen, Vaupel and Christensen2001) found that heritability of ALT level was 55% and 33% respectively, and the genetic effects due to dominance. An Australian twin study indicated that the heritability of ALT was 48% according to the AE model (Whitfield et al., Reference Whitfield, Zhu, Nestler, Heath and Martin2002). Moreover, according to a study conducted by the Netherlands Twin Register Biobank, ALT heritability was 40% for males and 22% for females; the best-fitting model was the AE model, which was consistent with our SEM result (van Beek et al., Reference van Beek, de Moor, de Geus, Lubke, Vink, Willemsen and Boomsma2013). Compared to the above studies, our study obtained a higher heritability of ALT. Since our results have enough test power, we speculate that these differences may be due to differences in ethnicity, since ethnicity is often the main reason why phenotypic heritability can be different.
The genetic pathways underlying ALT level variation have begun to be elucidated by genomewide association (GWA) studies. GWA studies of European whites, Indian Asians (Chambers et al., Reference Chambers, Zhang, Sehmi, Li, Wass, Van der Harst, Holm, Sanna, Kavousi, Baumeister, Coin, Deng, Gieger, Heard-Costa, Hottenga, Kühnel, Kumar, Lagou, Liang and Kooner2011), Mexican Americans (Young et al., Reference Young, Palmer, Fingerlin, Langefeld, Norris, Wang, Xiang, Guo, Williams, Chen, Taylor, Rotter, Raffel, Goodarzi, Watanabe and Wagenknecht2019), and the Japanese population (Kamatani et al., Reference Kamatani, Matsuda, Okada, Kubo, Hosono, Daigo, Nakamura and Kamatani2010) have identified dozens of loci and genes associated with concentrations of liver enzymes, including ALT. Among those, PNPLA3 and SAMM50 were found in the results of several GWA studies, which suggests that these two genes may be strongly related to human ALT levels. However, genetic studies about ALT concentration on the Chinese population are limited. Because the subjects of the above studies have different genes and live in different environments than Chinese people, a GWA study of ALT levels needs to be conducted on Chinese people living in China to learn more about how ALT levels change and how Chinese liver health works.
Our study was indeed constrained by its small sample size. Nevertheless, our power analysis revealed that our heritability result has sufficient power (0.99). Second, as it did in all studies employing the classical twin methodology, the equal-environment assumption, upon which the classical twin methodology is based, may have affected our estimates of heritability.
In conclusion, our findings on Chinese twins suggest that genetic factors influence variation in serum ALT concentration, a marker of liver injury. The heritability was 65%. The influence of genetics on ALT levels was due to additive genetic effects. As our study suggests that ALT is highly hereditary, it may help to develop effective prevention and control measures for liver disease in the Chinese population. Moreover, considering the limited genetic study of ALT in Chinese, our study also suggested a positive message for GWA studies.
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Acknowledgments
This study utilized data from the Qingdao Twin Registry (QTR). We would like to thank twins who participated in this study. And we acknowledge the staff at the Qingdao Center for Disease Control and Prevention (Qingdao CDC).
Author contributions
J.L., X.K., T.Z., W.W., and D.Z. conceived and designed the study; C.X., H.D., prepared the data; J.L., X.T., and W.W. analyzed the data; J.L., X.K. drafted the manuscript; D.Z. reviewed the manuscript.
Funding
None.
Conflict of interest
None.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






