Fatigue is a highly prevalent trait with multidimensional symptoms. The broad symptom spectrum is associated with quantifiable difficulties, resulting in fatigue classifications based on arbitrarily defined durations and severities. Commonly utilized classifications include prolonged fatigue, chronic fatigue (CF), idiopathic chronic fatigue (ICF), and chronic fatigue syndrome (CFS). Prolonged fatigue is classified as self-reported persistent or relapsing fatigue experienced for at least one month (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994), and has an estimated population prevalence of 6.16–28.00%. (Eveng Ård et al., Reference Eveng Ård, Jacks, Pedersen and Sullivan2005; Hamaguchi et al., Reference Hamaguchi, Kawahito, Takeda, Kato and Kojima2011; Jason et al., Reference Jason, Richman, Rademaker, Jordan, Plioplys, Taylor and Plioplys1999; Kim et al., Reference Kim, Shin and Won2005; Njoku et al., Reference Njoku, Leonard and Torres-Harding2007). CF is classified as self-reported persistent or relapsing fatigue experienced for at least six months (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994), and has an estimated population prevalence of 2.00–12.20% (Bierl et al., Reference Bierl, Nisenbaum, Hoaglin, Randall, Jones, Unger and Reeves2004; Cho et al., Reference Cho, Menezes, Hotopf, Bhugra and Wessely2009; Eveng Ård et al., Reference Eveng Ård, Jacks, Pedersen and Sullivan2005; Friedberg et al., Reference Friedberg, Tintle, Clark and Bromet2015; Hamaguchi et al., Reference Hamaguchi, Kawahito, Takeda, Kato and Kojima2011; Jason et al., Reference Jason, Taylor, Wagner, Holden, Ferrari, Plioplys and Papernik1995, Reference Jason, Richman, Rademaker, Jordan, Plioplys, Taylor and Plioplys1999; Kim et al., Reference Kim, Shin and Won2005; Loge et al., Reference Loge, Ekeberg and Kaasa1998; Njoku et al., Reference Njoku, Leonard and Torres-Harding2007; Patel et al., Reference Patel, Kirkwood, Weiss, Pednekar, Fernandes, Pereira and Mabey2005; Steele et al., Reference Steele, Dobbins, Fukuda, Reyes, Randall, Koppelman and Reeves1998; Wessely et al., Reference Wessely, Chalder, Hirsch, Pawlikowska, Wallace and Wright1995, Reference Wessely, Chalder, Hirsch, Wallace and Wright1997; Wong & Fielding, Reference Wong and Fielding2010). ICF is classified as clinically evaluated, medically unexplained CF, with insufficient symptom presentation for diagnosis with CFS (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994), and has an estimated population prevalence of 1.00–9.00% (Hamaguchi et al., Reference Hamaguchi, Kawahito, Takeda, Kato and Kojima2011; Kim et al., Reference Kim, Shin and Won2005; Wessely et al., Reference Wessely, Chalder, Hirsch, Wallace and Wright1997).
The original CFS classification was published in 1988 by the Centres for Disease Control (Holmes et al., Reference Holmes, Kaplan, Gantz, Komaroff, Schonberger, Straus and Brus1988). This CFS classification required the presence of new onset unexplained CF and either six or more symptom criteria (mild fever or chills, sore throat, painful lymph nodes, muscle weakness, muscle discomfort or myalgia, post-exertional fatigue, headaches, migratory arthralgia, neuropsychologic complaints, sleep disturbance, and acute onset) and two physical criteria (low-grade fever, non-exudative pharyngitis, and palpable or tender lymph nodes), or at least eight of the symptom criteria. In 1994, the Centres for Disease Control published a revision to the CFS classification that has become the standard definition utilized worldwide (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994). The 1994 CFS classification requires clinically evaluated, medically unexplained CF, with four or more physical symptoms (sore throat, tender lymph nodes, headaches, cognitive difficulties, unrefreshing sleep, multijoint pain, muscle pain, and post-exertional malaise) experienced over a 6-month period that have not pre-dated the fatigue (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994). The population prevalence of CFS has been estimated at 0.07–2.60% (Cho et al., Reference Cho, Menezes, Hotopf, Bhugra and Wessely2009; Hamaguchi et al., Reference Hamaguchi, Kawahito, Takeda, Kato and Kojima2011; Jason et al., Reference Jason, Richman, Rademaker, Jordan, Plioplys, Taylor and Plioplys1999; Kawakami et al., Reference Kawakami, Iwata, Fujihara and Kitamura1998; Kim et al., Reference Kim, Shin and Won2005; Lindal et al., Reference Lindal, Stefansson and Bergmann2002; Nacul et al., Reference Nacul, Lacerda, Pheby, Campion, Molokhia, Fayyaz and Drachler2011; Njoku et al., Reference Njoku, Leonard and Torres-Harding2007; Reyes et al., Reference Reyes, Nisenbaum, Hoaglin, Unger, Emmons, Randall and Reeves2003; Vincent et al., Reference Vincent, Brimmer, Whipple, Jones, Boneva, Lahr and Reeves2012; Wessely et al., Reference Wessely, Chalder, Hirsch, Pawlikowska, Wallace and Wright1995, Reference Wessely, Chalder, Hirsch, Wallace and Wright1997).
Familial studies of fatigue have mainly focused on CFS. In 1991, Bell et al. (Reference Bell, Cookfair, Bell, Reese and Cooper1991) showed that children (aged 6–17) with CFS (based on the original CFS classification) were significantly more likely to have family members with CFS symptoms than asymptomatic controls (relative risks [RR] = 48.60, 95% CI [9.43, 587.22]), although the degree of relatedness investigated by the authors is unclear. In 2001, Walsh et al. (Reference Walsh, Zainal, Middleton and Paykel2001) showed that first-degree relatives of CFS cases (with a mean age of 37.6 years) have an increased risk of prolonged fatigue (RR = 2.18, 95% CI [0.88, 3.48]) and CFS (RR = 9.22, 95% CI [7.84, 10.60]). Additionally, Buchwald et al. (Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001) showed that monozygotic (MZ) twin pairs have higher concordance rates compared to dizygotic (DZ) twin pairs for CF and ICF (within a cohort with a mean age of 46 years). In 2006, adolescents, aged 12–18, with CFS and their mothers were shown to have shared symptom complexes that were not exhibited by their fathers (van de Putte et al., Reference van de Putte, van Doornen, Engelbert, Kuis, Kimpen and Uiterwaal2006). Finally, in 2011, Albright et al. (Reference Albright, Light, Light, Bateman and Cannon-Albright2011) showed that CFS cases’ first- (RR = 2.70, 95% CI [1.56, 4.66]), second- (RR = 2.34, 95% CI [1.32, 4.19]), and third- degree relatives (RR = 1.93, 95% CI [1.21, 3.07]) had an increased risk of CFS compared to controls. These family studies indicate genetic and common environmental factors likely contribute to CFS.
Univariate twin studies have been utilized to estimate the contribution of additive genetic (also known as narrow-sense heritability [h 2]), common environmental, and unique environmental factors to the variation observed in the population of interfering fatigue (tiredness or fatigue experienced for at least five days), abnormal tiredness, prolonged fatigue, CF, ICF, and CFS, in adults (see Table 1 for a summary) (Buchwald et al., Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001; Schur et al., Reference Schur, Afari, Goldberg, Buchwald and Sullivan2007; Sullivan et al., Reference Sullivan, Kovalenko, York, Prescott and Kendler2003, Reference Sullivan, Evengard, Jacks and Pedersen2005). Interfering fatigue has an estimated genetic heritability of 6% in males and 26% in females (Sullivan et al., Reference Sullivan, Kovalenko, York, Prescott and Kendler2003). Similarly, abnormal tiredness has an estimated genetic heritability of 30% in males and 26% in females (Sullivan et al., Reference Sullivan, Evengard, Jacks and Pedersen2005). Prolonged fatigue has an estimated genetic heritability of 34–51% in males and 18–27% in females (Schur et al., Reference Schur, Afari, Goldberg, Buchwald and Sullivan2007; Sullivan et al., Reference Sullivan, Evengard, Jacks and Pedersen2005). CF has an estimated genetic heritability of 30–47% in males and 12–32% in females (Buchwald et al., Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001; Schur et al., Reference Schur, Afari, Goldberg, Buchwald and Sullivan2007; Sullivan et al., Reference Sullivan, Evengard, Jacks and Pedersen2005). Finally, ICF and CFS both have an estimated genetic heritability of 51% in females (Buchwald et al., Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001; Schur et al., Reference Schur, Afari, Goldberg, Buchwald and Sullivan2007). Notably, the heritability estimates for males and females were similar within the Swedish cohort, which had an age range of 42–64 years. Meanwhile, the American cohorts with mean ages of 32.4 years and approximately 35 years have greater differences in genetic heritability estimates between the sexes.
TABLE 1 Previously Published Variance Estimates (With Their 95% Confidence Intervals) for Varying Fatigue Classifications, in Adults, From Univariate Structural Equation Modeling

A = additive genetic component, C = common environmental component, E = unique environmental component.
To date, only two studies have been conducted that included children or adolescents and utilized univariate twin modeling to investigate the contribution of genetic and environmental factors in fatigue phenotypes. The first study investigated the heritability of short-duration fatigue (fatigue experienced for at least one week) and prolonged fatigue within children (aged 5–17) from South Wales (Farmer et al., Reference Farmer, Scourfield, Martin, Cardno and McGuffin1999). However, sex-specific effects were not investigated and confidence intervals were not reported for the heritability estimates. Nonetheless, short-duration fatigue had an estimated additive genetic, common environmental, and unique environmental contribution of 42%, 38%, and 20%, respectively. Similarly, prolonged fatigue had an estimated additive genetic, common environmental, and unique environmental contribution of 54%, 19%, and 26%, respectively. Meanwhile, the heritability of fatigue severity (a continuous scale of the 11 core fatigue items and 2 muscle pain items of the Chalder Fatigue Questionnaire; Chalder et al., Reference Chalder, Berelowitz, Pawlikowska, Watts, Wessely, Wright and Wallace1993) and abnormal fatigue (assessed by the 11 core fatigue items of the Chalder Fatigue Questionnaire) was investigated in a Sri Lankan population of adolescents and adults (aged ≥15; Ball et al., Reference Ball, Sumathipala, Siribaddana, Kovas, Glozier, McGuffin and Hotopf2010). Fatigue severity had an estimated additive genetic and unique environmental contribution of 30% (95% CI [24, 35]) and 70% (95% CI [65, 76]), respectively. Similarly, abnormal fatigue had an estimated additive genetic and unique environmental contribution of 39% (95% CI [29, 49]) and 61% (95% CI [51, 71]), respectively.
Additional studies have utilized multivariate twin modeling to investigate the contribution of shared genetic and environmental factors to numerous traits that are comorbid or hypothesized to be associated with fatigue. The traits investigated within previous multivariate studies include various fatigue definitions (i.e., fatigue symptoms, short-duration fatigue, abnormal fatigue, fatigue, prolonged fatigue, and CF) and major depressive disorder, insomnia, psychological distress, anxiety, depression, psychological symptoms, somatic symptoms, generalized anxiety disorder, disability pension due to neurotic diagnoses, headaches, irritable bowel syndrome, chronic widespread pain, immune responsiveness, and the immunological factors IL-4, IFN-γ, and sCD23. The heritability of the various fatigue measures ranged from 7% to 60%, and a number of these studies reported significant evidence for shared genetic factors between fatigue and other traits; in particular, strong genetic correlations (r g) were observed between prolonged fatigue and depression (r g = 0.53), CF and depression or anxiety (r g = 0.60), fatigue and psychological distress (r g = 0.67), and fatigue and immune responsiveness (r g = 0.76) (Ball et al., Reference Ball, Siribaddana, Sumathipala, Kovas, Glozier, Rijsdijk and Hotopf2011; Fowler et al., Reference Fowler, Rice, Thapar and Farmer2006; Hickie, Bennett et al., Reference Hickie, Bennett, Lloyd, Heath and Martin1999; Hickie, Kirk et al., Reference Hickie, Kirk and Martin1999; Hickie et al., Reference Hickie, Bansal, Kirk, Lloyd and Martin2001; Hur et al., Reference Hur, Burri and Spector2012; Kato et al., Reference Kato, Sullivan, Evengard and Pedersen2009; Narusyte et al., Reference Narusyte, Ropponen, Alexanderson and Svedberg2016).
Given the large variation in both the definition of fatigue and estimates of heritability produced from a relatively small number of univariate twin studies (conducted in Swedish and American cohorts), the current study aimed to investigate the heritability of fatigue experienced over the past few weeks in a cohort of Australian twin pairs. While previously published family studies have focused on CFS, we assessed the familiality of fatigue experienced over a shorter time period.
Materials and Methods
Study Cohort and Fatigue Classification
The present study utilized data from the over 50’s (aged) study conducted by the genetic epidemiology group within the QIMR Berghofer Medical Research Institute (QIMRB), between 1993 and 1996. The study invited 2,281 twin pairs, aged over 50, from the Australian Twin Registry to complete a 16-page mailed Health and Lifestyle Questionnaire (Bucholz et al., Reference Bucholz, Heath, Madden, Slutske, Statham, Dunne, Martin, Gomberg, Hegedus and Zucker1998; Mosing et al., Reference Mosing, Medland, McRae, Landers, Wright and Martin2012). Informed written consent was obtained from each participant, and the study was approved by the Human Research Ethics Committee (HREC) of QIMRB.
The fatigue classification utilized throughout this study was assessed by the Schedule of Fatigue and Anergia (SOFA; Hickie et al., Reference Hickie, Hooker, Hadzi-Pavlovic, Bennett and Wilson1996). Ten questions are contained in the SOFA; however, a shorter eight-item version was used in the Health and Lifestyle Questionnaire, due to two items being replicated within the General Health Questionnaire (GHQ; Goldberg & Blackwell, Reference Goldberg and Blackwell1970) that was also administered to the participants. Responses to the eight SOFA and two GHQ items were used to assess fatigue within the cohort, as previously detailed (Corfield et al., Reference Corfield, Martin and Nyholt2016). Individuals were classified as fatigued if they reported three or more of the 10 fatigue symptoms (muscle pain at rest, post-exertional muscle pain, post-exertional muscle fatigue, post-exertional fatigue, hypersomnia, insomnia, poor concentration, speech problems, poor memory, and headaches) over the past few weeks.
Statistical Analysis
Familial clustering of fatigue was investigated by calculating RR, measured by the prevalence ratio, with their 95% CI in complete MZ and DZ twin pairs. RR were calculated relative to non-fatigued individuals. Within MZ and same-sex DZ twin pairs, RR were calculated by averaging over using twin 1 or twin 2 as the proband.
Tetrachoric correlations were calculated for fatigue within MZ and DZ twin pairs and singleton twins using the polycor package in R (R Core Team, 2014). The tetrachoric correlation assumes that underlying the observed binary distribution of affection status, there exists a continuous, normally distributed latent (non-observable) liability (Kendler, Reference Kendler1993). That is, the tetrachoric correlation is an estimate of the correlation between two latent variables, where each latent variable is assumed to have a bivariate normal distribution. Comparison of the correlations between MZ and DZ twins was used to provide information on the importance of genetic and environmental factors contributing to the heritability of fatigue. Correlations that are larger in MZ compared to DZ twins indicate that the phenotype has a genetic contribution, while correlations that are similar in MZ and DZ twins indicate that the environmental factors explain the majority of variation in the phenotype.
Structural equation modeling (SEM), including the threshold model, was utilized to investigate the heritability of fatigue. The threshold model posits that distinct traits represent a single, normally distributed, severity continuum. A single threshold was used to separate non-fatigued and fatigued individuals. SEM was used to estimate the contribution of additive genetic (A), non-additive (dominance) genetic (D), common environmental (C), and unique environmental (E) variance components (Neale & Cardon, Reference Neale and Cardon1992). Adjustments for (linear) age and sex effects were included in the model. Significance of the variance components was assessed by comparing the fit of the full model (ACE/ADE) to the nested models (AE, CE, and E) where the effect was dropped, using OpenMx in R (Boker et al., Reference Boker, Neale, Maes, Wilde, Spiegel, Brick and Fox2011). Additionally, sex-limitation modeling was conducted to determine whether sex-specific effects contribute to the heritability of fatigue. Initially, a non-scalar sex-limitation model was fitted that included variance components for females (i.e., Af, Cf, and Ef) and males (i.e., Am, Cm, and Em), as well as an additional additive genetic component specific to males (A′m). Restricted non-scalar sex-limitation modeling was then conducted, whereby A′m was removed. The goodness-of-fit parameters used to assess the differences in the twin models were the likelihood-ratio chi-square test (χ 2) and the p value. Additionally, model fit was compared utilizing Akaike's Information Criteria (AIC), with the lowest AIC indicating the most parsimonious model (Akaike, Reference Akaike1973, Reference Akaike1974).
Tetrachoric correlations and SEM were estimated using full information maximum likelihood (FIML), whereby both complete twin pairs and incomplete twin pairs (singleton twins) were included in the analyses. The inclusion of singleton twins provides more accurate estimation of the thresholds and may correct for participation bias.
Results
Within the over 50’s study, 473 twin pairs and 555 singleton twins returned incomplete responses to the SOFA and GHQ questionnaire items utilized to assess fatigue within the present study and were therefore excluded. The remaining 1,253 complete twin pairs and 555 singleton twins with fatigue data comprised the cohort utilized within the present study. The study cohort contained 660 MZ twin pairs (504 female–female and 156 male–male twin pairs) and 190 MZ singleton twins (109 females and 81 males) with a mean age of 61.3 ± 8.9 (range = 50–92), and 593 DZ twin pairs (272 female–female, 76 male–male, 137 female–male, and 108 male–female twin pairs) and 365 DZ singleton twins (260 females and 105 males) with a mean age of 61.0 ± 8.5 (range = 50–94). The prevalence of fatigue defined as above was 30.7% (31.7% of females and 28.3% of males).
An increased risk of fatigue in co-twins of fatigued probands was observed, indicating a significant familial contribution. Strong evidence for a genetic contribution to fatigue is provided by the higher RR observed in MZ compared to DZ twin pairs (Table 2). In particular, the risk of fatigue in co-twins of fatigued probands was 2.20 (95% CI [1.77, 2.75]) in MZ twin pairs compared to 1.32 (95% CI [1.01, 1.73]) in DZ twin pairs (applicable to first-degree relatives in the general population). Analysis of familial clustering within males and females indicated a similar pattern of risks.
TABLE 2 Relative Riska of Fatigue Within Complete Monozygotic (MZ), Same-Sex Dizygotic (DZss), and Opposite-Sex Dizygotic (DZos) Twin Pairs
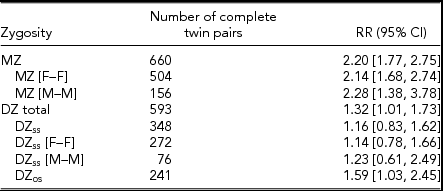
F = female, M = male.
aRelative risks and 95% confidence intervals (CI) were calculated with respect to non-depressed or non-fatigued status in twin 1. Same-sex twin pair tables were made symmetrical by averaging over using twin 1 or twin 2 as the proband.
The tetrachoric correlations for fatigue were approximately three times larger in MZ compared to DZ twin pairs (Table 3). Overall, the observed MZ > DZ correlations indicate that additive genetic factors contribute to the variation in fatigue.
TABLE 3 Tetrachoric Correlations (r) With Their 95% Confidence Intervals (CI) for Fatigue According to Zygosity

F = female, M = male.
Initially, full univariate ACE and ADE models were fitted; however, systematic dropping of A, C, and D effects was used to determine whether the effect of the individual variance components were significant (Table 4). Dropping C (i.e., AE model) from the ACE model did not worsen the model fit. However, dropping A (i.e., CE model) or both A and C (i.e., E model) was significant (p = 4.76 × 10−3 and 8.91 × 10−11, respectively)—indicating A is an important source of variance in the heritability of fatigue. Meanwhile, dropping D (i.e., AE model), from the ADE model, did not worsen the model fit. However, dropping both A and D (i.e., E model) was significant (p = 6.46 × 10−11)—indicating genetic factors play an essential role in the heritability of fatigue. Therefore, the AE model was selected as the most parsimonious model based on fit statistics. No differences in threshold distributions were observed within twin pairs and singleton twins, or across zygosity and sex groups.
TABLE 4 Fit Statistics and Variance Estimates (With Their 95% Confidence Intervals) From Univariate Structural Equation Modeling
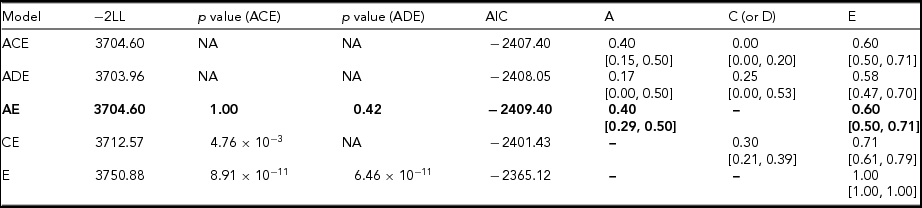
−2LL = minus two log-likelihood, A = additive genetic component, C = common environmental component, E = unique environmental component. The best-fitting model is indicated in bold. p value compares −2LL for the full ACE or ADE model to the reduced (AE, CE, DE, and E) models.
Additive genetic factors were estimated to explain approximately 40% of the heritability of fatigue. No significant evidence for sex-specific genetic effects was observed within the cohort. The results of the non-scalar sex-limitation modeling indicated that the restricted model was the most parsimonious (AIC = −2405.41) with similar heritability estimates for fatigue in males, at 41% (95% CI [18, 62]; E = 59%, 95% CI [38, 82]), compared to females, at 40% (95% CI [27, 52]; E = 60%, 95% CI [48, 73]).
Discussion
The findings from the present study indicate that fatigue in older adults is familial and has a genetic contribution with no significant sex-specific effects.
The familial clustering analysis revealed that co-twins of fatigued probands were at an increased risk of fatigue. These results indicate that the familial contribution of fatigue is not specific to CFS, although in 2001 the first-degree relatives of CFS cases were shown to have an increased risk of prolonged fatigue and MZ twin pairs were shown to have higher concordance rates than DZ twin pairs for CF and ICF (Buchwald et al., Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001; Walsh et al., Reference Walsh, Zainal, Middleton and Paykel2001). However, to our knowledge, this is the first study to characterize the familial clustering of fatigue experienced for less than 6 months. The higher risk observed in MZ twin pairs compared to DZ twin pairs indicates genetic factors likely contribute to the etiology of fatigue. These results are reflective of the conclusions drawn from previous family studies of CF, ICF, and CFS (Buchwald et al., Reference Buchwald, Herrell, Ashton, Belcourt, Schmaling, Sullivan and Goldberg2001; van de Putte et al., Reference van de Putte, van Doornen, Engelbert, Kuis, Kimpen and Uiterwaal2006). However, the similar pattern of risks observed within males and females indicates that the underlying etiology of fatigue is likely independent of sex. This finding opposes the results of van de Putte et al. (Reference van de Putte, van Doornen, Engelbert, Kuis, Kimpen and Uiterwaal2006), who found an increase of CFS symptoms in mothers of children with CFS, but not fathers. Indicating the etiology of fatigue may differ with age.
The differences in genetic heritability between males and females identified within previous twin studies are larger in the cohorts comprised of younger adults compared to cohorts of older cohorts. In contrast, results from the present study indicate that the underlying etiology of fatigue is independent of sex in older adults. Sex-limitation modeling revealed males (h 2 = 41%, 95% CI [18, 62]) and females (h 2 = 40% (95% CI [27, 52]) had very similar heritability estimates. Furthermore, based on fit statistics, the most parsimonious model was the univariate AE twin model, which did not include sex-specific effects. These results support the suggestion of Sullivan et al. (Reference Sullivan, Evengard, Jacks and Pedersen2005) that females and males have similar genetic and environmental contributions for varying fatigue classifications, despite the higher prevalence of fatigue in females. In comparison, Schur et al. (Reference Schur, Afari, Goldberg, Buchwald and Sullivan2007) suggested that further investigations are required to understand the differences in fatigue etiology between the sexes. Based on our results and previous findings, we suggest that a further investigation into the heritability of fatigue across the lifespan is required.
A possible limitation of our study is the utilization of self-report rather than interview-based data. However, considering prolonged fatigue and CF classifications are based on self-report, the utilization of questionnaire-based data is valid. Additionally, this prevented confounding within the study by healthcare-seeking behavior, due to the population-based structure of the cohort. Another potential limitation of the study was the utilization of a non-standard fatigue duration due to the ambiguity of the questionnaire, which assessed fatigue symptoms experienced ‘over the past few weeks’. However, considering the SOFA was designed to assess CFS symptoms, fatigue is representative of a spectrum, and previous studies have looked at similar fatigue definitions—our findings still offer valid insights into the underlying etiology of fatigue.
In summary, we have shown that fatigue experienced over the past few weeks is familial, with additive genetic factors explaining a substantial proportion of its variance in older adults. Future research aimed at identifying the specific genes and risk loci associated with fatigue (e.g., via genome-wide association studies), will increase our understanding of its underlying biological mechanisms.
Acknowledgements
We would like to thank the twins for their cooperation and study staff for data collection. Mr. George Landers’, of Chania, Crete, generous donations funded this research. Elizabeth C. Corfield was supported by an Australian Postgraduate Award (APA) from the Australian Government. Dale R. Nyholt was supported by an Australian National Health and Medical Research Council (NHMRC) Research Fellowship (Application ID 613674).
Disclosure of Interests
None.
Details of Ethical Approval
Informed written consent was obtained from each participant, and the study was approved by the Human Research Ethics Committee (HREC) of the QIMR Berghofer Medical Research Institute (QIMRB).






