The contribution of genetic and environmental factors to phenotypic variance observed during the perinatal period can be estimated on the basis of differences within the pairs of twins. This type of research, the so-called twin studies, is based on the assumption that monozygotic (MZ) twins are genetically identical and dizygotic (DZ) twins are related to the same degree as any other siblings. Appropriate use of this method requires not only information on zygosity type but most of all on chorionicity, as these two parameters are vital for determining the effects of intrauterine environment on twin development. However, a question arises whether the intrauterine environment can be considered homogeneous for all twins?
According to some researchers, the intrauterine environment can be considered similar only in the case of DZ twins and MZ twins from dichorionic (DC) and diamniotic pregnancies, as only these fetuses develop in separate chorions and amnions (Bergman & Sawicki, Reference Bergman and Sawicki1988; Gielen et al., Reference Gielen, Lindsey, Derom, Smeets, Souren, Paulussen and Nijhuis2008; Loos et al., Reference Loos, Derom, Derom and Vlietinck2005, Touwslager et al., Reference Touwslager, Gielen, Derom, Mulder, Gerver, Zimmermann and Zeegers2011; Vlietinck et al., Reference Vlietinck, Derom, Neale, Maes, van Loon, Derom and Thiery1989; van Baal & Boomsma, Reference van Baal and Boomsma1998). In turn, fetuses from monochorionic (MC) — either di- or mono-amniotic — pregnancies develop under different intrauterine conditions and therefore are at risk of many complications (Baldwin, Reference Baldwin1994; Benirschke, Reference Benirschke1990; Cordero et al., Reference Cordero, Franco, Joy and O'Shaughnessy2005, Reference Cordero, Franco and Joy2006; De Paepe, Reference De Paepe2015; Dias et al., Reference Dias, Mahsud-Dornan, Bhide, Papageorghiou and Thilaganathan2010; Hack et al., Reference Hack, Derks, Elias, Franx, Roos, Voerman and Visser2008; Haverkamp et al., Reference Haverkamp, Lex, Hanisch, Fahnenstich and Zerres2001; Rossi & Prefumo, Reference Rossi and Prefumo2013; Zhao et al., Reference Zhao, Lipa, Wielgos, Cohen, Middeldorp, Oepkes and Lopriore2016). The most common complication is the formation of connecting blood vessels (anastomoses), which may result in acute or chronic twin-to-twin transfusion syndrome (TTTS).
Placental anastomoses observed during the course of TTTS (also referred to as feto-fetal transfusion syndrome) allow blood to pass from one fetus (the so-called donor) to the other (the so-called recipient). Resultant hemodynamic imbalance leads to various abnormalities (Bermudez et al., Reference Bermudez, Becerra, Bornick, Allen, Arroyo and Quintero2002; Lewi, Reference Lewi2010; Ropacka et al., Reference Ropacka, Markwitz, Keith and Bręborowicz2001; Zanardini et al., Reference Zanardini, Prefumo, Fichera, Botteri and Frusca2014; Zhao et al., Reference Zhao, Lipa, Wielgos, Cohen, Middeldorp, Oepkes and Lopriore2016), such as hypertension, polycythemia, and hypervolemia in the recipient, and hypotonia, hypovolemia, and anemia in the donor (Malinowski & Ropacka, Reference Malinowski, Ropacka, Bręborowicz, Malinowski and Ronin-Walknowska2003; Yinon et al., Reference Yinon, Ben Meir, Berezowsky, Weisz, Schiff, Mazaki-Tovi and Lipitz2014; Zhao et al., Reference Zhao, Slaghekke, Middeldorp, Duan, Oepkes and Lopriore2014). Moreover, TTTS is reflected by enhanced growth of the recipient and delayed development of the donor, which eventually results in growth discordance (Fick et al., Reference Fick, Feldstein, Norton, Wassel Fyr, Caughey and Machin2006). Discordant growth, manifesting as differences in fetal body weights and abdominal circumferences, is a characteristic feature of TTTS.
Previous studies showed that intrapair differences in MZ twins result solely from the influence of unique intrauterine factors (Race et al., Reference Race, Townsend and Hughes2006; Sokol et al., Reference Sokol, Moore, Rose, Williams, Reed and Christian1995). This may either promote growth discordance (e.g., in the case of TTTS, according to Ropacka, Reference Ropacka, Bręborowicz, Malinowski and Ronin-Walknowska2003, observed in up to 15–20% of MC twins) or prevent it, if transfer of blood between co-twins is not associated with TTTS. TTTS may result in considerable birth-weight discordance (Blickstein, Reference Blickstein1990; Brennan et al., Reference Brennan, Diwan, Rosen and Bellon1982; Fick et al., Reference Fick, Feldstein, Norton, Wassel Fyr, Caughey and Machin2006; Foley et al., Reference Foley, Neale and Kendler2000). Formation of anastomoses between vascular systems of DZ twins with fused placentas is of extremely rare evidence (ca. 1.4%; Lage et al., Reference Lage, Vanmarter and Mikhail1989; Robertson & Neer, Reference Robertson and Neer1983) and leads to blood chimerism (Baldwin, Reference Baldwin1994; Malinowski & Ropacka, Reference Malinowski, Ropacka, Bręborowicz, Malinowski and Ronin-Walknowska2003). Consequently, it is still unclear whether shared chorion and placenta present in most MZ twins (70%) provide them with more similar environmental conditions than in the case of DZ twins, which always develop in separate chorions, and according to Bulmer (Reference Bulmer1970), have separate placentas in 58% of the cases.
The aim of this study was to determine the effects of intrauterine environment on the magnitude of intrapair differences in the somatic traits of MZ and DZ twins.
We verified previous opinions regarding conditions of twin intrauterine development, comparing the degree of intrapair discordance of somatic traits in MZ and DZ twins presenting with different types of fetal membranes. Only a few comparisons of this kind have been carried out, using sufficiently numerous and clinically selected twin material, and there remains a lack of reliable opinions on the subject (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). The lack of such analyses reflects difficulties in access to sufficiently large research material, of which a principal limiting factor is the relatively low rate of twin births. In Poland, twin pregnancy occurs once per 80 births. MZ twins represent 30% of all delivered twins, and only 30% of MZ twins develop in separate chorions and amnions.
Consequently, this study involving sufficiently large and representative material may provide an evidence for such conclusions.
In pursuing the goal of our research, we aimed to prove the veracity of the following research hypotheses:
-
1. The number of chorions in twin pregnancies is not a significant factor influencing the size of intrapair differences in the somatic traits in twins. TTTS can be considered such a factor.
-
2. Material on DZ and MZ twins from MC or DC pregnancies but without TTTS ensures comparability of intrauterine environmental conditions and can be used to estimate the share of genetic and environmental factors in the variability of phenotypic traits in the perinatal period.
Methods
The studied material, including 1,263 pairs of twins live-born at various stages of fetal life between the 22nd and 41st week of gestation, was collected from the Department of Perinatology and Gynaecology, Poznan University of Medical Sciences, between 2003 and 2012. Individuals with clinical evidence of congenital anomalies or mechanical injuries, as well as neonates whose data were likely unreliable, were excluded from further analyses.
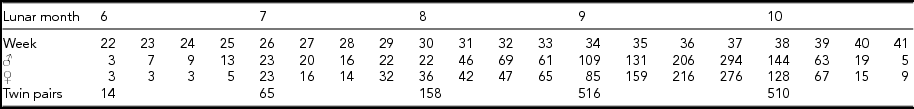
The calendar age of the neonates was determined on the basis of maternal information on the first day of the last menstrual period. The number of male and female neonates born in consecutive weeks of gestation and numbers of twin pairs from consecutive lunar months is presented in Table 1.
TABLE 1 Number of Male and Female Newborns from Twin Pregnancies Born at Various Gestational Weeks (22–41) and the Number of Twin Pairs Born in Various Lunar Months
The general status of each neonate was determined immediately after birth and expressed as an Apgar score, being a complex functional measure of the respiratory, cardiovascular, and central nervous systems. Apgar scores were interpreted according to widely accepted criteria: 10–8 points—normal, 7.5–6 points—intermediate, 5.5–4 points—fairly low, and 3.5–0 points—critically low. The study included only the twins whose general status at birth corresponded to an Apgar score >4 points. Stillborn neonates were excluded from the analysis.
Morphological development of each twin was assessed on the basis of six somatic traits: (1) body weight, (2) total body length, (3) crown-rump length, (4) shoulder width, (5) circumference of the head, and (6) circumference of the chest. Definitions of all these traits and methods of their measurement were consistent with conventional methodology proposed by Martin (Reference Martin1988).
The chorionicity was described in the first half of the pregnancy by ultrasound scan and in some cases also immediately after the birth based on the analysis of the placenta.
The zygosity of 821 pairs of same-gender twins was determined at the Laboratory of Molecular Genetics in Poznan. Biological material was 1 mL of umbilical blood collected in EDTA tubes (10 mL of 10% EDTA per 1 mL of the blood). The material was obtained immediately after birth to avoid exposure of twins to additional medical procedures. This is particularly important in the case of multiple pregnancies, which usually result in more complicated labor, lower birth weights, and worse physical condition of neonates than singleton pregnancies. Special attention was paid to material collection, appropriate labeling of each sample, storage, and isolation of DNA with the aid of techniques providing high throughput of the process and high degree of DNA purity. DNA was always isolated from lysate of umbilical blood lymphocytes, and purified by salting out of proteins. Such processed material was subjected to analysis of DNA polymorphism by means of hybridization with a molecular probe and with an aid of PCR. The hybridization studies included analysis of single locus systems (SLS): D7S21 (7p22) and D12S11 (12q24.3) with MS43A and MS31 probes, and simultaneous analysis of multi-locus systems (MLSs) with a (GTG)5 probe. PCR was used to analyze short tandem repeat (STR) polymorphisms within the following genes: pancreatic phospholipase (HUMPLA2A1, 12q23), cytochrome P450 (HUMCYARO, 15q21.1), von Willebrand factor (HUMvWF, 12p13), thyroid peroxidase (HUMTPOX, 2p23), and tyrosine hydroxylase (HUMTH01, 11p15.5). The analysis included loci located on five different chromosomes.
All the procedures were approved by the Local Ethics Committee of the Medical University in Poznan.
The study used the following methods of mathematical statistics:
-
1. Student's t test to determine the significance of differences between the two variables;
-
2. Fisher's test to verify the homogeneity of variance;
-
3. one-dimension and two-factor analysis of ANOVA variance for somatic traits in twins, standardized by gender and gestational age in order to reveal the diversity of the dependent variables, distinguished between the chosen categories of twins;
-
4. post-hoc test to determine the categories between which twins differences for variable data were statistically significant;
-
5. a graphic interpretation of the obtained results was also performed in the form of the graphs shown in Figures 1–10. The 95% confidence interval for the mean defines the range of values around the mean, which with 95% probability contains the true value of the mean. The size of the confidence interval depends on the sample size and variability of the given characteristic. An increase in the number of observations and less data dispersion causes narrowing of the confidence interval. When confidence intervals for the two means do not overlap, it means that they differ significantly.

FIGURE 1 Intrapair differences in the standardized body weight of MZ and DZ twins from consecutive lunar months. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 2 Intrapair differences in the standardized total body length of MZ and DZ twins from consecutive lunar months. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 3 Intrapair differences in the standardized crown-rump length of MZ and DZ twins from consecutive lunar months. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 4 Intrapair differences in the standardized head circumference of MZ and DZ twins from consecutive lunar months. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 5 Intrapair differences in the standardized chest circumference of MZ and DZ twins from consecutive lunar months. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 6 Intrapair differences in the standardized body weight for four groups of twins from consecutive lunar months, stratified according to their zygosity and chorionicity. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 7 Intrapair differences in the standardized total body length for four groups of twins from consecutive lunar months, stratified according to their zygosity and chorionicity. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 8 Intrapair differences in the standardized crown-rump length for four groups of twins from consecutive lunar months, stratified according to their zygosity and chorionicity. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 9 Intrapair differences in the standardized head circumference for four groups of twins from consecutive lunar months, stratified according to their zygosity and chorionicity. Vertical bars represent 95% confidence intervals for a mean.

FIGURE 10 Intrapair differences in the standardized chest circumference for four groups of twins from consecutive lunar months, stratified according to their zygosity and chorionicity. Vertical bars represent 95% confidence intervals for a mean.
Results
During the first stage of the intrapair discordance analysis, we conducted a Student t test to compare the magnitude of intrapair differences in each of the analyzed somatic traits of MZ and DZ twins. The test showed that MZ and DZ twins differed significantly in the magnitude of intrapair differences in all analyzed traits except for body weight (Table 2).
TABLE 2 Results of Student t Test Conducted to Compare Mean Magnitude of Intrapair Differences in the Standardized Somatic Traits of Monozygotic and Dizygotic Twins
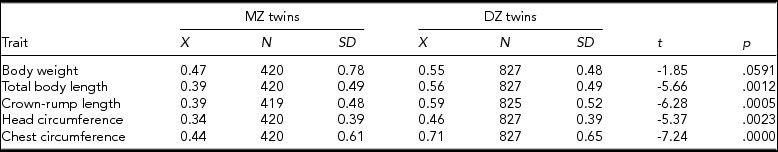
p values are significant at p ≤ .05.
The Fisher test did not provide any evidence to reject the hypothesis on the homogeneity of variance in all analyzed traits of MZ and DZ twins except for body weight. The value of the standard deviation for body weight of MZ twins, pointing to a considerable heterogeneity of intrapair differences in this parameter, was significantly higher than in the case of DZ twins (p value for F statistic was lower than 0.01 and therefore the criterion of variance homogeneity was not satisfied). This substantiated the use of the unequal variance in the Student t test. This test did not provide any evidence (p = .06) to reject the hypothesis on the homogeneity of the two groups of MZ and DZ twins. Therefore, MZ twins did not differ significantly from DZ twins in terms of their mean intrapair differences in body weight.
Moreover, we analyzed the magnitude of intrapair differences in the somatic traits of MZ and DZ twins from consecutive lunar months. Classification according to a lunar month was enforced by a small number (n < 20) of twins from some weeks of gestation. The results of the analysis are depicted in Figures 1–5.
As shown in the figures, during the whole prenatal period, intrapair discordance in all analyzed somatic traits except for body weight was larger in DZ twins than in MZ twins, but this difference was statistically significant only for terminal period of fetal ontogenesis, that is, for lunar months 9 and 10. In turn, the differences in the magnitude of intrapair discordance in body weight, apparently larger in MZ twins for lunar months 7 and 8, and in DZ twins for lunar months 9 and 10, were not statistically significant during the whole fetal ontogenesis.
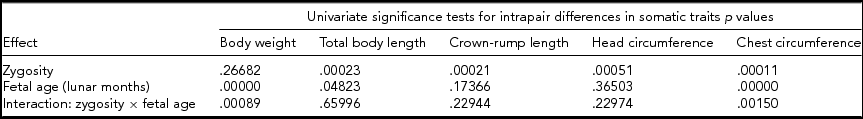
We conducted a univariate two-way analysis of variance (ANOVA) to study the effects of zygosity and fetal age on the magnitude of intrapair differences in the somatic traits of twins (Table 3). The p values for univariate tests suggested that zygosity exerted no significant effect on body weight, and fetal age did not modulate significantly either crown-rump length or circumference of the head. In contrast, both these factors exerted significant effects on total body length and circumference of the chest. In the case of the latter parameter, however, their effects were non-additive, that is, did not sum, and were additionally modulated due to interaction between the analyzed factors.
TABLE 3 Results of Univariate Two-Way Analysis of Variance (ANOVA) for Intrapair Differences in Standardized Somatic Traits
Y is a somatic trait influenced by factors α and β:
Y = μ + α + β + (αβ) + e
where μ: global mean value;
α: effect of zygosity factor;
β: effect of fetal age factor;
αβ: effect of interaction, i.e., zygosity × fetal age; and
e: error.
MZ twins may develop under different intrauterine environmental conditions, which to some extent depends on the time elapsed between fertilization and division of a single zygote into two genetically identical embryonic structures. Such twins may develop within two separate chorions or share the same chorion.
Potential discordance in the intrauterine environments of MZ twins enforced stratification of our research material into four groups: (1) MZ-MC-TTTS—MZ twins from MC pregnancy with TTTS, (2) MZ-MC—MZ twins from MC pregnancy without TTTS, (3) MZ-DC—MZ twins from DC pregnancy, and (4) DZ twins. These groups were compared in terms of the magnitude of intrapair differences in the standardized somatic traits (Table 4).
TABLE 4 Descriptive Statistics for Absolute Magnitude of Intrapair Differences in the Standardized Somatic Traits of Twins Stratified According to Their Zygosity and Chorionicity
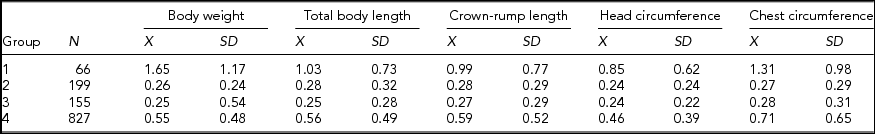
Group 1 = MZ-MC-TTTS, Group 2 = MZ-MC without TTTS, Group 3 = MZ-DC, and Group 4 = DZ.
MZ twins from MC pregnancies with TTTS were characterized by the largest intrapair differences in all analyzed traits, especially body weight and circumference of the chest (Table 4).
Analysis of variance, the results of which are presented in Table 5, confirmed that intrauterine environmental conditions exerted significant effects on the magnitude of intrapair differences in the analyzed traits. The least significant difference (LSD) test identified the groups of twins that differed significantly in terms of the magnitude of intrapair differences. MZ twins from MC pregnancies without TTTS and MZ twins from DC pregnancies turned out to be the only groups that did not differ significantly in terms of the magnitude of intrapair differences in the analyzed traits (Table 6).
TABLE 5 Results of the Analysis of Variance for a Relationship Between ‘Twin Category According to Zygosity and Chorionicity’ Factor and Selected Dependent Variables (Absolute Magnitude of Intrapair Differences in Standardized Somatic Traits)
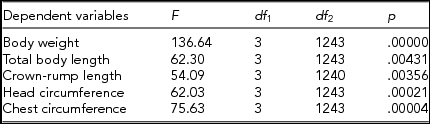
p values are significant at p ≤ .05.
TABLE 6 Significance of Differences in the Magnitude of Intrapair Discordance in Standardized Somatic Traits in the Subsets of Twins Differing in Terms of Their Zygosity and Chorionicity

**significant at p ≤ .01.
To determine the effects of intrauterine environment and fetal age in lunar months on the magnitude of intrapair differences, we conducted a univariate two-way ANOVA. The analysis showed that both the abovementioned factors exerted significant effects on body weight and circumference of the chest. These non-additive effects were additionally modulated by an interaction between the two factors. The magnitude of intrapair differences in the remaining traits was modulated solely by intrauterine environment (Table 7).
TABLE 7 Results of Univariate Two-Way Analysis of Variance (ANOVA) for Intrapair Differences in Standardized Somatic Traits

#According to zygosity and chorionicity.
Moreover, we analyzed the effects on intrauterine environmental conditions on the magnitude of intrapair differences in the studied somatic traits in consecutive lunar months. The results of this analysis are depicted in Figures 6–10.
As shown in the figures, irrespective of the analyzed trait, the intrapair differences were the largest in the case of MZ twins from MC pregnancies with TTTS. The twins from this subset differed significantly from the remaining groups for nearly the whole period of fetal ontogenesis. DZ twins were the group presenting with the second largest intrapair differences in the analyzed traits. At the end of pregnancy, that is, in lunar months 9 and 10, the magnitude of intrapair differences in all traits of twins from this group was significantly greater than in MZ twins from both MC and DC pregnancies. Irrespective of the analyzed period, the least evident, statistically insignificant intrapair differences in the studied traits were documented in the case of MZ twins from MC pregnancies without TTTS and twins from DC pregnancies.
The results of previous studies of fetal membrane biology imply that MZ twins and DZ twins developing within separate chorions and amnions are the only groups being comparable in terms of their intrauterine environments (Bergman & Sawicki, Reference Bergman and Sawicki1988; Loos et al., Reference Loos, Derom, Derom and Vlietinck2005; van Baal & Boomsma, Reference van Baal and Boomsma1998). The only difference in the intrauterine environments of these twins may pertain to the number of placentas, as they may either develop in two separate placentas or share a common placenta. To verify whether this factor influenced the magnitude of intrapair differences in somatic traits, we compared the subsets of MZ twins from DC pregnancies and DZ twins differing in terms of the placental numbers (Table 8).
TABLE 8 Descriptive Statistics for Absolute Magnitude of Intrapair Differences in the Standardized Somatic Traits of Twins Stratified According to Their Zygosity and Number of Placentas
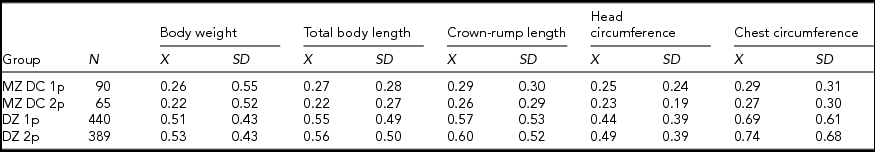
Analysis of variance revealed statistically significant differences (p ≤ .01) in the magnitude of intrapair discordance in the analyzed traits of twins from various subsets (Table 9), and the LSD test documented relationships between specific subgroups of twins. The only significant differences were observed between group 1 and groups 3 and 4, as well as between group 2 and groups 3 and 4 (Table 10). The LSD test confirmed that the number of placentas did not exert significant effect on the magnitude of intrapair differences in the analyzed somatic traits of twins.
TABLE 9 Results of the Analysis of Variance for a Relationship Between Zygosity (MZ-DC vs. DZ), Number of Placentas (1 vs. 2) and Selected Dependent Variables (Absolute Magnitude of Intrapair Differences in Standardized Somatic Traits)
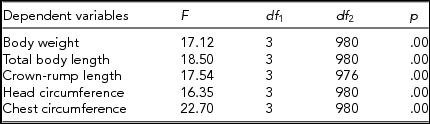
p values are significant at p ≤ .05.
TABLE 10 Significance of Differences in the Magnitude of Intrapair Discordance in Standardized Somatic Traits in the Subsets of Twins Differing in Terms of Their Zygosity (MZ- DC vs. DZ) and Placental Number (1 vs. 2)

**significant at p ≤ .01.
Discussion
One of the methods to determine the share of genetic and environmental factors in the phenotypic variability of anthropometric studies is twin studies. However, the twins need to develop under the same intrauterine environmental conditions if the analysis of intrapair differences in their somatic traits is used for this purpose. Published studies analyzing the role of fetal membrane type as a determinant of intrapair variance in the somatic traits of MZ and DZ twins are sparse, at least when the research on an appropriately selected clinical material is concerned (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). Furthermore, the results of the few published studies dealing with the problem in question are inconclusive.
Marceau et al. (Reference Marceau, McMaster, Smith, Daams, van Beijsterveldt, Boomsma and Knopik2016) in their last article reviewed the literature on chorionicity in twin pregnancies in reference to a number of the resulting traits in humans and formulated the following question: To what extent do the differences in chorions in identical twins affect the process of estimating the heredity? In summary, Marceau et al. (Reference Marceau, McMaster, Smith, Daams, van Beijsterveldt, Boomsma and Knopik2016) found that concerns about estimating the heritability based on the classic twin design, which is founded on the assumption of an identical environment, are not justified given the prenatal environment. However, many authors consistently believe that the estimates of heritability are underestimated as for the birth weight, when chorionicity is not taken into account.
According to Race et al. (Reference Race, Townsend and Hughes2006), chorion type is an important variable that may influence prenatal environment of MZ twin pairs, contributing to potential intrapair differences in growth and development. The aim of the study conducted within the pairs of MZ twins with known chorion type was to verify whether MC twin pairs show more birth-weight discordance than DC twin pairs due to greater intrapair differences in their intrauterine environment. As a result of the studies, it was stated that large discrepancies in birth weight more often occur in MC twin pairs than in DC twin pairs.
Research on the effects of chorionicity on a wide range of phenotypes that resulted in view of a greater similarity of DC-MZ twins than MC-MZ twins has been conducted by different authors (Fagard et al., Reference Fagard, Loos, Beunen, Derom and Vlietinck2003; Gutknecht et al., Reference Gutknecht, Spitz and Carlier1999; Hur, Reference Hur2007, Hur & Shin, Reference Hur and Shin2008; Jacobs et al., Reference Jacobs, Van Gestel, Derom, Thiery, Vernon, Derom and Vlietinck2001; Loos et al., Reference Loos, Derom, Derom and Vlietinck2005; Race et al., Reference Race, Townsend and Hughes2006; Reed et al., Reference Reed, Pfefferbaum, Sullivan and Carmelli2002; Sokol et al., Reference Sokol, Moore, Rose, Williams, Reed and Christian1995); however, they were most often characterized by small sample sizes and heterogeneity with respect to the age of the twins.
Fick et al. (Reference Fick, Feldstein, Norton, Wassel Fyr, Caughey and Machin2006) found 20% birth-weight discordance in their prospective study of a MC twin cohort from northern California. Since TTTS has been diagnosed in 8% of twin pairs from the birthweight-discordant subset, the authors pointed to unequal placental sharing as a significant risk factor for birth-weight discordance in diamniotic MC twins. Similarly, Chang (Reference Chang2008), Cleary-Goldman and D'Alton (Reference Cleary-Goldman and D'Alton2008), and Nikkels et al. (Reference Nikkels, Hack and van Gemert2008) perceived an uneven placenta distribution as one of the main causes of non-compliance in fetal growth in MZ twins. Also, according to De Paepe et al. (Reference De Paepe, Shapiro, Young and Luks2010), uneven placental sharing and velamentous cord insertion are two major placental determinants of selective birth-weight discordance in diamniotic MC twins. In turn, the research by Melmed et al. (Reference Melmed, Polonsky, Larsen and Kronenberg2012) emphasizes the important role of shared placenta in MC twins as an endocrine organ that leads to greater similarities in MC twins than in DC twins.
The results of a recent Dutch study, which included a group of more than 9,000 twin pairs, imply that intrauterine environment, measured by chorion status, has limited contribution to the similarities in physical and behavioral development of MZ twins. This conclusion was formulated on the basis of a comparative analysis of intrapair differences in 66 traits of MC and DC twins (van Beijsterveldt et al., Reference van Beijsterveldt, Overbeek, Rozendaal, McMaster, Glasner, Bartels and Boomsma2016). Van Beijsterveldt et al. stated that ‘the impact of the intrauterine environment on foetal correlation between MZ twins measured by separating the type of the chorion is small and limited to only few phenotypes’ (p. 311). The results of the studies by Mukherjee et al. (Reference Mukherjee, Kang, Wolfe, Hertzberg, Smith, Lin and Gilmore2009) also showed no effect of chorionicity on intrapair differentiation for head circumference in newborns from MC and DC twin pregnancies.
Sebire et al. (Reference Sebire, D'Ercole, Soares, Nayar and Nicolaides1998) analyzed 123 MC and 416 DC twin pregnancies and found no significant intrapair differences in fetal size at birth. However, they stated that this observation might have been biased due to the small sample size, and postulated further research on a larger material.
The results of our present study, involving a representative sample of 1,263 twin pairs, provide the basis for unbiased conclusions about the participation of genetic and environmental factors in phenotypic variability of the anthropometric traits.
MZ twins from MC pregnancies complicated by TTTS showed the largest intrapair differences in all analyzed traits, especially body weight and circumference of the chest. These differences resulted from growth discordance associated with one-way transfer of the blood from one fetus to another via arteriovenous anastomoses developed during the course of chronic TTTS (Dias et al., Reference Dias, Mahsud-Dornan, Bhide, Papageorghiou and Thilaganathan2010; Rossi & Prefumo, Reference Rossi and Prefumo2013; Zhao et al., Reference Zhao, Lipa, Wielgos, Cohen, Middeldorp, Oepkes and Lopriore2016). According to Malinowski and Ropacka (Reference Malinowski, Ropacka, Bręborowicz, Malinowski and Ronin-Walknowska2003), one characteristic feature of this syndrome is considerable intrapair discordance in fetal weights, chest and abdominal circumferences.
Our research shows that the intrauterine environment factor differentiating somatic features of twins is not the number of chorions but the TTTS syndrome. MZ twins from MC pregnancies without TTTS did not differ significantly in intrapair differences in observed characteristics from the MZ twins from DC pregnancies. The advantage of these studies in affecting the reliability of the results is the large number of carefully and clinically selected research material. The lack of clinically documented knowledge about the presence of the TTTS is very often the reason why some claim that the intrauterine environment differentiates the MC twins.
In conclusion, our analysis showed that DZ twins and MZ twins from MC or DC pregnancies without TTTS develop under comparable intrauterine conditions and therefore can be used as a model to study the contribution of genetic and environmental factors to phenotypic variance. The intrauterine environment contributes to intrapair discordance solely in the case of MZ twins from MC pregnancies complicated with TTTS (i.e., approximately 10–20% of all MZ twins), and this effect is usually stronger than in DZ twins. In turn, the intrauterine environment does not modulate the magnitude of intrapair differences in the somatic traits of MZ twins from MC pregnancies without TTTS and MZ twins from DC pregnancies.
Acknowledgments
This work was supported from the statutory funds of the University School of Physical Education in Poznan.
Disclosure of Interests
None.
Details of Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






















