Height and body mass index (BMI, kg/m2) are among the most intensively studied traits in human genetics and public health. The first genetic study on height was published in the late 19th century (Galton, Reference Galton1886) and on BMI in the early 20th century (Davenport, Reference Davenport1923), both utilizing information on familial resemblance. These anthropometric traits were also among the first traits studied in humans when new scientific innovations in molecular genetics became available (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). With the current global obesity epidemic coexisting with severe undernutrition affecting growth in some populations (NCD Risk Factor Collaboration, 2016a) and well-known associations of growth and adult height with several health indicators (Silventoinen, Reference Silventoinen, Komlos and Kelly2015), questions about the roles of genetic and environmental factors in variations of these traits in rapidly changing environments are important.
In order to delineate conditions modifying the heritability of height and BMI, genotype-by-exposure (G × E) studies that include large, diverse samples are needed. To address G × E, there have been some previous studies comparing the heritability of height (Silventoinen et al., Reference Silventoinen, Sammalisto, Perola, Boomsma, Cornes, Davis and Kaprio2003) and BMI (Hur et al., Reference Hur, Kaprio, Iacono, Boomsma, McGue, Silventoinen and Mitchell2008; Schousboe et al., Reference Schousboe, Willemsen, Kyvik, Mortensen, Boomsma, Cornes and Harris2003) between countries or over time periods when mean height (Silventoinen et al., Reference Silventoinen, Kaprio, Lahelma and Koskenvuo2000) and BMI have increased (Rokholm, Silventoinen, Angquist et al., Reference Rokholm, Silventoinen, Angquist, Skytthe, Kyvik and Sørensen2011; Rokholm, Silventoinen, Tynelius et al., Reference Rokholm, Silventoinen, Tynelius, Gamborg, Sørensen and Rasmussen2011). In these studies, a significant difference in heritability indicates the modification of gene expression by environment (i.e., for G × E interaction; Boomsma & Martin, Reference Boomsma, Martin, D’haenen, den Boer and Wilner2002). There is also evidence that the heritability of BMI may change with age, based on both literature-based meta-analyses (Elks et al., Reference Elks, den Hoed, Zhao, Sharp, Wareham, Loos and Ong2012; Silventoinen et al., Reference Silventoinen, Rokholm, Kaprio and Sørensen2010) and pooled individual data (Dubois et al., Reference Dubois, Ohm Kyvik, Girard, Tatone-Tokuda, Perusse, Hjelmborg and Martin2012). Studies investigating factors affecting heritability require large sample sizes and broad ranges of exposures to obtain a comprehensive understanding of this variation. As our environment rapidly changes, with large differences within and between countries in rates of obesity and lifestyle factors, pooling twin data from different environments is critically important to analyze the dependence of heritability on environments.
Objectives of the Project
To obtain an accurate answer to the question about how the heritability of height and BMI vary over age and sex as well as time and space, the COllaborative project of the Development of Anthropometrical measures in Twins (CODATwins) project was started in June 2013. The idea behind the CODATwins project was to bring together all globally available twin data on height and weight. Additionally, year of birth, sex, zygosity and age at measurement were collected, as well as data on birth weight and length, birth order, gestational age, ethnicity, own and parental education, and own smoking status to analyze how these factors are related to height and BMI, including the genetic architecture of these traits. The project is open for all twin cohorts that have collected data on height and weight from monozygotic (MZ) and dizygotic (DZ) twins. Twin cohorts were identified from several sources. The most important was the previous special issue of Twin Research and Human Genetics on twin registers (Hur & Craig, Reference Hur and Craig2013), which was complemented with other sources and personal contacts. The first invitation letter was sent in September 2013 to the potential collaborators. Follow-up letters were sent in October 2013, January 2014 and September 2014. The first version of the harmonized database used in the first scientific papers was ready in January 2015 (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Honda, Aaltonen, Yokoyama and Kaprio2015). However, we have received several new cohorts and updates after this first paper. The current version of the CODATwins database includes a vast majority of height and weight measures known to have been collected from twins with information on zygosity.
The initial objective of the project was to analyze the heritability of height and BMI across different cultures and geographic regions. As the project progressed, we also analyzed how birth-related factors were associated with later physical development and education, differences in the physical development of same-sex and opposite-sex DZ twins, and associations between smoking and BMI. Throughout the project, we classified the twin cohorts to three cultural–geographic regions based on average BMI levels: East Asia, having the lowest BMI; Europe, having intermediate BMI; and North America and Australia having the highest BMI (NCD Risk Factor Collaboration, 2016a). These regions reflect different social and nutritional environments, including different obesogenic levels, which may affect not only the heritability of BMI, but also birth size and later height.
Current Status of the Database
Figure 1 presents the geographic diversity of the 54 individual twin projects who have contributed data to the CODATwins database. The collaborators come from 24 countries. They mainly represent Europe, North America, Australia and, to a lesser extent, East Asia; individual twin cohorts come from South Asia, Middle East, Africa and South America.
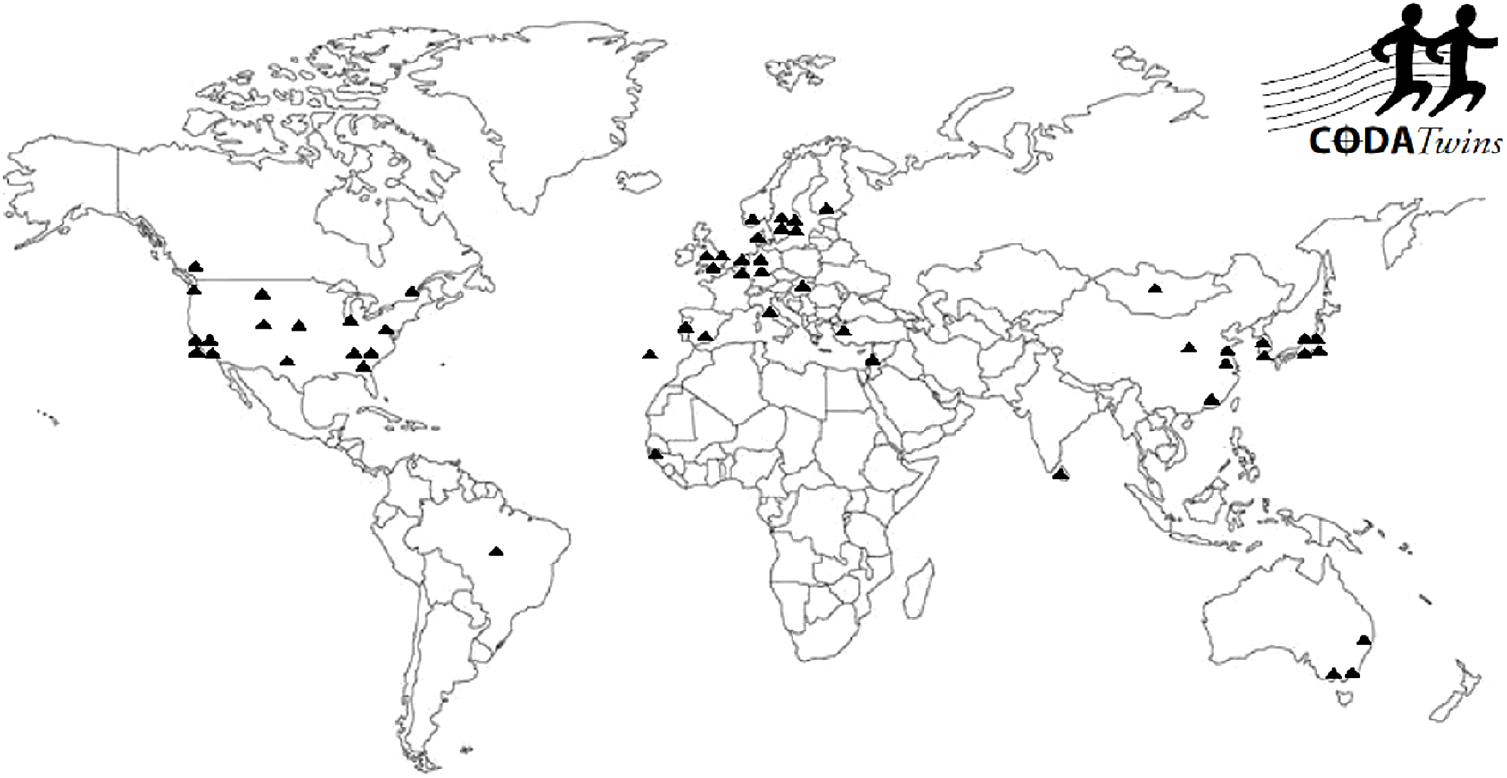
Fig. 1. Geographic distribution of the CODATwins collaborators.
Table 1 presents the basic characteristics of the CODATwins database by country. The footnote indicates that there are 58 twin cohorts in the CODATwins database. Note that one study project can include more than one cohort. Together, the database includes nearly a half million twin individuals, including nearly a quarter million complete twin pairs. Half of the twins are females, but this overall proportion conceals variation in the sex ratios between twin cohorts. In many cohorts, including adult twins, there are somewhat more women than men because of more active participation and lower mortality in women than men. However, we have three male-only cohorts, including army veterans and conscripts, equalizing the overall sex ratio in the whole database. A larger proportion of twins are same-sex DZ twins (39%), compared to opposite-sex DZ twins (22%). This is mainly because some of the cohorts have collected data only on same-sex twin pairs, but also partly because of lower participation rates of opposite-sex compared to same-sex DZ twins in adult cohorts.
Table 1. The current status of the CODATwins database by country
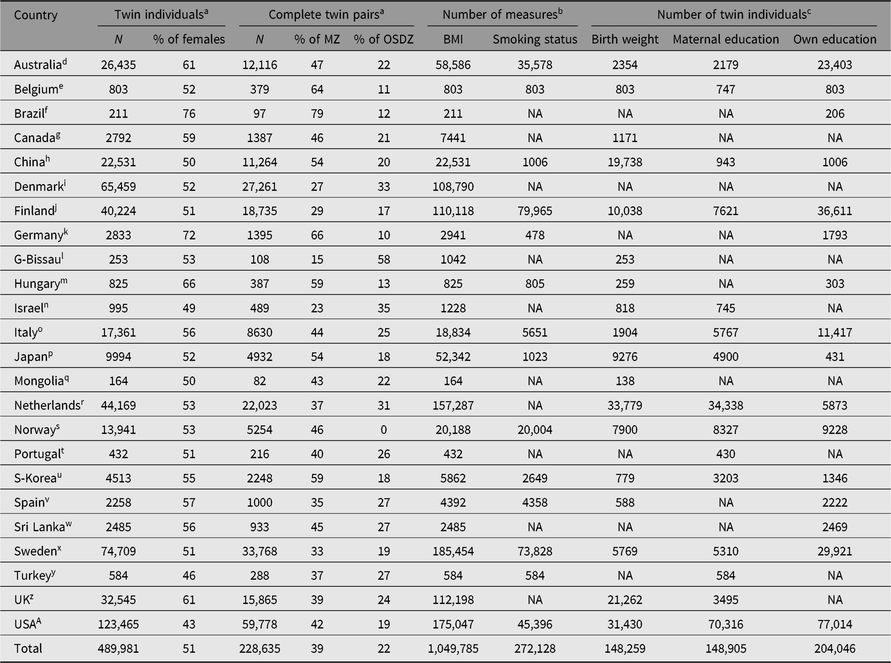
Note: MZ = monozygotic twins; OSDZ = opposite-sex dizygotic twins; BMI = body mass index; NA = not available.
a Only twins including information on height, weight, age at the time of measurement, sex and zygosity are included.
b Longitudinal measures available for part of twins.
c Only one measure available for each twin.
d Australian Twin Registry, Peri/Postnatal Epigenetic Twins Study (PETS), Queensland Twin Register.
e East Flanders Prospective Twin Survey.
f Brazilian Twin Registry.
g Quebec Newborn Twin Study, University of British Columbia Twin Project.
h Chinese National Twin Cohort Study, Guangzhou Twin Eye Study, Qingdao Twin Registry (Children), Qingdao Twin Registry (Adults).
i Danish Twin Cohort.
j Finnish Older Twin Cohort, FinnTwin12, FinnTwin16.
k Berlin Twin Register Health (TwiSt), Bielefeld Longitudinal Study of Adult Twins.
l Guinea Bissau Twin Study.
m Hungarian Twin Registry.
n Longitudinal Israeli Study of Twins.
o Italian Twin Registry.
p Japanese Twin Registry, Ochanomizu University Twin Project, Osaka University Aged Twin Registry, West Japan Twins and Higher Order Multiple Births Registry.
q Mongolian Twin Registry.
r Netherlands Twin cohort (children), Netherlands Twin cohort (adults).
s Norwegian Twin Registry.
t Portugal Twin Cohort, Madeira Twin Family Study.
u Korean Twin-Family Register, South Korea Twin Registry.
v Murcia Twin Registry.
w Sri Lanka Twin Registry.
x Child and Adolescent Twin Study in Sweden (CATSS), TCHAD-Study, Swedish Twin Cohorts, Swedish Young Male Twins Study.
y Turkish Twin Study.
z Gemini Study, Genesis 12–19 study, Twins Early Developmental Study, TwinsUK.
A Boston University Twin Project, California Twin Program, Carolina African American Twin Study of Aging, Colorado Twin Registry, Michigan Twins Project, Mid-Atlantic Twin Registry, Minnesota Twin Family Study, Minnesota Twin Registry, NAS-NRC Twin Cohort, SRI-International, Texas Twin Project, University of Southern California Twin Study, University of Washington Twin Registry, Vietnam Era Twin Registry.
There are about 1 million height and weight measures after 6 months of age from the 489,981 twins available in the database. For around half of twins (N = 252,624), we had only baseline data available, and for those having follow-up data, 112,691 twins had only one follow-up measure whereas 5695 had nine or more follow-up measures. The majority of measures are based on self-reports (63%) or parental reports (20%), and only a minority are measured values (17%). For nearly 150,000 twin individuals, we had additional information on birth weight, but only a minority of these was measured birth weight (7%) and the majority was parentally reported (78%) or self-reported (15%). For slightly less than half of these twin individuals, we have additional information on birth length (41%) and gestational age (44%). However, it is noteworthy that especially in the parental reports of height or length and weight, the reliability of the data probably varies between the cohorts. In some cohorts, parents were able to use the records of measures provided by medical doctors or registered nurses during child health checkups, whereas in others they needed to rely on their own estimates and recall.
We also collected information on own smoking and own and parental education, but these measures were not mandatory for participation in the project and thus were available in only some of the cohorts, and in some cohorts were not available for all participants. Longitudinal information on smoking was collected if available, providing about a quarter of million assessments of smoking status. For own and parental education, only one measure was collected, preferring the most recent one (i.e., the highest attained educational level measure). We have information on maternal education for nearly 150,000 twin individuals, and for the large majority of these (96%), we have additional information on paternal education. Information on own education is available for about 200,000 twin individuals. However, because parental education is mainly available for children and own education for adults, we have only 63,138 twin individuals with all three educational measures.
Major Findings From Studies on Anthropometric Traits
We started this project by detailing the growth development of twins. DZ twins were consistently taller and in childhood and adolescence especially, they also had somewhat higher BMI than MZ twins. Slightly higher BMI variance was found for DZ twins than for MZ twins in childhood, but this difference disappeared in adolescence; for height, no zygosity differences in the variances were seen (Jelenkovic et al., Reference Jelenkovic, Yokoyama, Sund, Honda, Bogl, Aaltonen and Silventoinen2015). First-born twins were heavier in infancy and also slightly taller than second-born twins over childhood and adolescence, but this difference disappeared in adulthood (Yokoyama et al., Reference Yokoyama, Jelenkovic, Sund, Sung, Hopper, Ooki and Silventoinen2016). Thus, in further genetic modeling, we used different means of BMI and height for MZ and DZ twins. We also randomized the birth order within twin pairs since information on birth order was available for only 39% of the twins.
At birth, genetic factors explained a smaller proportion of the variation of weight, length and ponderal index (PI, kg/m3) than shared and unique environmental factors; the heritability estimates, however, somewhat increased when the results were adjusted for gestational age (Yokoyama et al., Reference Yokoyama, Jelenkovic, Hur, Sund, Fagnani, Stazi and Silventoinen2018). The heritability estimates of height increased from early childhood until adulthood because of decreasing shared environmental variation (Jelenkovic, Sund et al., Reference Jelenkovic, Sund, Hur, Yokoyama, Hjelmborg, Möller and Silventoinen2016). For BMI, the heritability estimates first decreased from infancy to early childhood and then increased being between 0.7 and 0.8 at most ages during late childhood and adolescence; these differences were due to shared environmental variation being highest in early childhood (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016). The heritability of BMI was highest in early adulthood and then decreased until old age due to increasing unique environmental variation (Silventoinen, Jelenkovic, Sund et al., Reference Silventoinen, Jelenkovic, Latvala, Sund, Yokoyama, Ullemar and Kaprio2017).
For height, we did not find systematic differences in the heritability estimates or the variances between the geographic and cultural regions in childhood and adolescence (Jelenkovic, Sund et al., Reference Jelenkovic, Sund, Hur, Yokoyama, Hjelmborg, Möller and Silventoinen2016) or in adulthood (Jelenkovic, Hur et al., Reference Jelenkovic, Hur, Sund, Yokoyama, Siribaddana, Hotopf and Silventoinen2016). The variances and heritability estimates of adult height were also roughly similar across birth cohorts from the late 19th to late 20th centuries (Jelenkovic, Hur et al., Reference Jelenkovic, Hur, Sund, Yokoyama, Siribaddana, Hotopf and Silventoinen2016). For BMI in childhood and adolescence, we found that the variation was highest in North America and Australia and lowest in East Asia, thus also corresponding to the mean BMI differences between the regions (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016). Similar differences between the cultural and geographic regions were also found in the variances of adult BMI. The adult BMI variance increased from the 1940s to the 2010s along with increasing mean BMI. The genetic and environmental variances, however, showed about the same differences leading to only minor and inconsistent differences in the heritability estimates of BMI over time or between the cultural and geographic regions (Silventoinen, Jelenkovic, Sund et al., Reference Silventoinen, Jelenkovic, Sund, Yokoyama, Hur, Cozen and Kaprio2017). The heritability estimates of height and BMI were about the same in males and females. However, we found sex-specific genetic effects already in childhood, increasing during adolescence and being highest in adulthood for both height (Jelenkovic, Hur et al., Reference Jelenkovic, Hur, Sund, Yokoyama, Siribaddana, Hotopf and Silventoinen2016; Jelenkovic, Sund et al., Reference Jelenkovic, Sund, Hur, Yokoyama, Hjelmborg, Möller and Silventoinen2016) and BMI (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016; Silventoinen, Jelenkovic, Sund et al., Reference Silventoinen, Jelenkovic, Sund, Yokoyama, Hur, Cozen and Kaprio2017).
We utilized the discordant twin pair design to analyze the effect of differences in intrauterine environment for the twins on later physical development. Within twin pairs, a lighter and shorter twin at birth was also shorter over childhood and adolescence than the co-twin, and this effect was still seen in adulthood (Jelenkovic, Yokoyama et al., Reference Jelenkovic, Yokoyama, Sund, Hur, Harris, Brandt, Nilsen, Ooki, Ullemar and Silventoinen2018). BMI showed similar effects, and a smaller co-twin at birth had lower BMI from early childhood to adulthood; however, the effect sizes somewhat attenuated in adulthood (Jelenkovic et al., Reference Jelenkovic, Yokoyama, Sund, Pietiläinen, Hur, Willemsen and Silventoinen2017).
The comparison of opposite-sex and same-sex DZ twins is uniquely suited to study sex differences that may arise in utero through a putative masculinization process of female twins who have a male co-twin (compared to same-sex twin pairs) that may arise from being exposed in utero to sex hormones of the opposite sex. However, our data did not support these effects for anthropometric traits. When we studied the sex of the co-twin, we found that boys having a female co-twin had a slightly greater birth weight and longer gestational age than DZ boys having a same-sex co-twin; in girls, no differences were seen between opposite-sex and same-sex DZ twins (Jelenkovic, Sund et al., Reference Jelenkovic, Sund, Yokoyama, Hur, Ullemar, Almqvist and Silventoinen2018). In adulthood, both men and women having an opposite-sex co-twin were slightly taller than those DZ twins having a same-sex co-twin, whereas BMI showed no differences between these twin-type groups (Bogl et al., Reference Bogl, Jelenkovic, Vuoksimaa, Ahrenfeldt, Pietiläinen, Stazi and Kaprio2017).
Major Findings From Studies on Education and Smoking
We have harmonized the different educational classifications in the individual data sets by transforming them into educational years. In our first study, we found only minor differences between MZ and DZ twins in own or parental education (Silventoinen, Jelenkovic, Latvala et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2017). Because of large differences between countries and birth cohorts in educational levels, in further studies we have decided to focus on relative education (i.e., education years adjusted for birth year and twin cohort). We used the discordant twin pair design to analyze how differences in birth weight between co-twins, which may reflect differences in the intrauterine environment, can affect differences in education in adulthood. We found that the lighter co-twin at birth had shorter education than the heavier co-twin, but the differences were very small and somewhat inconsistent between birth cohorts and zygosities (Jelenkovic, Mikkonen et al., Reference Jelenkovic, Mikkonen, Martikainen, Latvala, Yokoyama, Sund and Silventoinen2018). In another study, we analyzed how parental education modifies the genetic and environmental variation of BMI from infancy to old age in the cultural–geographic regions (Silventoinen et al., Reference Silventoinen, Jelenkovic, Latvala, Yokoyama, Sund, Sugawara and Kaprio2019). We found that the mean BMI and the genetic variance of BMI were greater in those whose parents had low education when compared to the offspring of highly educated parents. These associations were strongest in North America and Australia and weakest or nonexistent in East Asia. These results suggest that the interplay between genetic predisposition, childhood social environment and macrosocial context is important for socioeconomic differences in BMI.
We wished to have smoking data made as widely available as possible and therefore collected information on the current smoking status of twins themselves and harmonized it into three categories: never smokers, current smokers and former smokers. In our first study utilizing these measures, we examined BMI in twin pairs discordant for smoking, contrasting never, current and former smokers (Piirtola et al., Reference Piirtola, Jelenkovic, Latvala, Sund, Honda, Inui, Watanabe, Tomizawa and Iwatani2018). As expected, the currently smoking twin had slightly lower BMI than the co-twin who had never smoked. Also as expected, the former smoking twins had a higher BMI than their current smoking co-twins. However, when comparing twins from MZ pairs discordant for former smoking and never smoking, we found only small differences, which suggests that the net effect of smoking initiation and subsequent quitting on weight trajectory is minor.
Further Study Plans
There are numerous opportunities for further studies. Previous studies have presented literature-based meta-analyses of educational attainment (Branigan et al., Reference Branigan, McCallum and Freese2013; de Zeeuw et al., Reference de Zeeuw, de Geus and Boomsma2015), but our database offers possibilities to study in much more detail how the genetic and environmental variation of educational years have changed over birth cohorts and vary between countries. We also plan to analyze how parental education modifies the genetic architecture of height and birth weight by using the same approach as in the previous study on parental education and BMI (Silventoinen et al., Reference Silventoinen, Jelenkovic, Latvala, Yokoyama, Sund, Sugawara and Kaprio2019). Further, we can analyze the associations of height and BMI with own education. By using the discordant twin pair design, as previously used by Piirtola et al. (Reference Piirtola, Jelenkovic, Latvala, Sund, Honda, Inui, Watanabe, Tomizawa and Iwatani2018), it is possible to analyze whether the association between smoking and education is causal or due to common genetic or common environmental factors.
We have not yet addressed longitudinal associations. Since around half of the participating twin cohorts have longitudinal measures of height and weight, the CODATwins database offers good opportunities for this type of research. We can analyze how genetic factors affect the tracking of height and BMI over childhood and weight change in adulthood. Finally, thus far, most studies, including ours, have ignored the well-known skewness of BMI distribution, which may well be associated with both the increasing mean and variance of BMI (Pak et al., Reference Pak, Ferreira and Colson2016). New methods to analyze the skewness of BMI distribution utilizing twin data are now available (Tsang et al., Reference Tsang, Duncan, Dinescu and Turkheimer2018), and the CODATwins database would allow for analyzing, in detail, the differences in the skewness of BMI distribution between different ages, measurement years and cultural–geographic regions.
Discussion
The CODATwins project shows that conducting a large-scale international collaborative project of existing twin cohorts is feasible. Such large data sets can provide reliable answers to research questions that have not been resolved using small or moderate size cohorts. In addition, they can answer new research questions impossible to analyze in any single cohort, which rarely span multiple determinants such as age from birth to old age, time period and sufficient geocultural diversity. We have been able to collect an international database and answer several new research questions related to the genetic and environmental determinants of height and relative weight.
Our main results concern the heritability estimates of height and relative weight. We found that the heritability estimates of both height and relative weight (BMI or PI) varied considerably by age (Jelenkovic, Hur et al., Reference Jelenkovic, Hur, Sund, Yokoyama, Siribaddana, Hotopf and Silventoinen2016; Jelenkovic, Sund et al., Reference Jelenkovic, Sund, Hur, Yokoyama, Hjelmborg, Möller and Silventoinen2016; Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016; Silventoinen, Jelenkovic, Sund et al., Reference Silventoinen, Jelenkovic, Sund, Yokoyama, Hur, Cozen and Kaprio2017; Yokoyama et al., Reference Yokoyama, Jelenkovic, Hur, Sund, Fagnani, Stazi and Silventoinen2018). Thus, it is likely that in some previous literature-based meta-analyses on the heritability of BMI (Elks et al., Reference Elks, den Hoed, Zhao, Sharp, Wareham, Loos and Ong2012; Min et al., Reference Min, Chiu and Wang2013) the age ranges of original studies may have been too broad to capture this complexity, and the differences in the reported heritability estimates may reflect age differences between the cohorts. This further emphasizes the need for pooled analyses instead of relying only on meta-analyses of published results. On the other hand, we found only little evidence that the macroenvironment modifies the heritability estimates of height or BMI. Height has increased all over the world during the 20th century (NCD Risk Factor Collaboration, 2016b), and it could be speculated that this has affected the genetic architecture of height as environmental stress has diminished. However, we found that the heritability estimates of adult height and also total genetic and environmental variances were very similar between the cohorts born from the late 19th to late 20th centuries despite considerable differences in mean height across these environments (Jelenkovic, Hur et al., Reference Jelenkovic, Hur, Sund, Yokoyama, Siribaddana, Hotopf and Silventoinen2016). On the other hand, we found that the variance of BMI was higher in more obesogenic environments measured by both the cultural and geographic regions with different mean BMI levels as well as when analyzing BMI measures from the 1940s to the 2010s, over which time the mean BMI has increased (Silventoinen et al., Reference Silventoinen, Jelenkovic, Sund, Hur, Yokoyama, Honda and Kaprio2016; Silventoinen, Jelenkovic, Sund et al., Reference Silventoinen, Jelenkovic, Sund, Yokoyama, Hur, Cozen and Kaprio2017). However, even for BMI, we did not find any systematic differences in the heritability estimates across the measurement years or the cultural–geographic regions. This suggests that the heritability estimates of height and BMI are robust for differences in the macroenvironment. In the case of height, this seems to be because total variation is not sensitive to the change of environment. In contrast, BMI variation increased along with increasing mean BMI, but this was due to increases in both the genetic and environmental variations. This suggests that factors affecting increasing mean BMI may operate partly by amplifying the effect of genes on BMI variation.
In our studies not focusing on heritability, we found results both supporting and contradicting previous studies. Using the twin design, we demonstrated that intrauterine conditions affecting smaller birth size are associated with shorter height and lower BMI from early childhood to adulthood (Jelenkovic et al., Reference Jelenkovic, Yokoyama, Sund, Pietiläinen, Hur, Willemsen and Silventoinen2017; Jelenkovic, Yokoyama et al., Reference Jelenkovic, Yokoyama, Sund, Hur, Harris, Brandt, Nilsen, Ooki, Ullemar and Silventoinen2018), which are consistent with prior studies. On the other hand, our findings that birth weight was only weakly associated with adult education in discordant twin pairs (Jelenkovic, Mikkonen et al., Reference Jelenkovic, Mikkonen, Martikainen, Latvala, Yokoyama, Sund and Silventoinen2018) and that males and females having opposite-sex co-twins showed no consistent differences as compared to those having same-sex co-twins in height and BMI (Bogl et al., Reference Bogl, Jelenkovic, Vuoksimaa, Ahrenfeldt, Pietiläinen, Stazi and Kaprio2017) are not consistent with previous hypotheses. Because there is a well-known tendency to publish positive results (Thornton & Lee, Reference Thornton and Lee2000), these types of large collaborative studies are important to validly test hypotheses and estimate effect sizes not inflated by publication bias.
This project provides a good estimation of the total number of twins in different cohorts potentially available for further collaborative studies, and thus demonstrates the opportunities, as well as certain limitations, of the currently available twin data. Height and weight are among the most commonly collected traits, and we are aware of only a few twin cohorts that are not part of the database. Thus, the total number of participants in any twin study in the world may not be much higher than the half million twin individuals assembled in the CODATwins database. Less than one fifth of the height and weight values were based on direct measures, with the rest being self- or parentally reported. When studying physiological traits requiring clinical examination, such as blood pressure or cholesterol level, the number of participants available is likely to be much smaller. However, in the future, the linkage of twin cohorts to population-based biobanks together with health-care databases is an avenue to address more detailed biomedical and clinical research questions. This approach has already been exploited to estimate heritability by using an extended family design (Polubriaginof et al., Reference Polubriaginof, Vanguri, Quinnies, Belbin, Yahi, Salmasian and Tatonetti2018). The current twin data disproportionately represent Western populations and, to a lesser extent, East Asian populations. Thus, new data collections would be very important, especially in the geographic regions currently having only limited twin data available.
In conclusion, the CODATwins project demonstrates the scientific value of an international collaboration that aims to pool individual phenotypic data sets. This allowed the analyses of macroenvironmental effects on genetic and environmental variations and resulted in a tremendous increase in statistical power. A similar approach could be used to study many other traits not yet included in the CODATwins project. This would lead to new knowledge and make the maximal use of data already collected.
Acknowledgments
This study was conducted within the CODATwins project (Academy of Finland #266592). K Silventoinen is supported by Osaka University’s International Joint Research Promotion Program. This research was facilitated through access to Twins Research Australia, a national resource supported by a Centre of Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council. The Boston University Twin Project is funded by grants (#R01 HD068435 #R01 MH062375) from the National Institutes of Health to K. Saudino. Paulo Ferreira is funded by a National Medical Research Council Research Fellowship. California Twin Program was supported by The California Tobacco-Related Disease Research Program (7RT-0134H, 8RT-0107H, 6RT-0354H) and the National Institutes of Health (1R01ESO15150-01). The Carolina African American Twin Study of Aging (CAATSA) was funded by a grant from the National Institute on Aging (grant 1RO1-AG13662-01A2) to K. E. Whitfield. The CATSS-Study is supported by the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no 340-2013-5867, grants provided by the Stockholm County Council (ALF-projects), the Swedish Heart-Lung Foundation and the Swedish Asthma and Allergy Association’s Research Foundation. Chinese National Twin Registry is funded by Special Fund for Health Scientific Research in the Public Welfare (Project No: 201502006), China. Colorado Twin Registry is funded by NIDA-funded center grant DA011015, & Longitudinal Twin Study HD10333; Author Huibregtse is supported by 5T32DA017637 and 5T32AG052371. Danish Twin Registry is supported by the National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology and Innovation, The Research Council for Health and Disease, the Velux Foundation and the US National Institute of Health (P01 AG08761). Since its origin, the East Flanders Prospective Survey has been partly supported by grants from the Fund of Scientific Research, Flanders and Twins, a nonprofit Association for Scientific Research in Multiple Births (Belgium). Data collection and analyses in Finnish twin cohorts have been supported by ENGAGE — European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement number 201413, National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145 and AA-09203 to R J Rose, the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278 and 264146 to J Kaprio). Gemini was supported by a grant from Cancer Research UK (C1418/A7974). Waves 1–3 of Genesis 12–19 were funded by the W T Grant Foundation, the University of London Central Research fund and a Medical Research Council Training Fellowship (G81/343) and Career Development Award (G120/635) to Thalia C. Eley. Wave 4 was supported by grants from the Economic and Social Research Council (RES-000-22-2206) and the Institute of Social Psychiatry (06/07–11) to Alice M. Gregory, who was also supported at that time by a Leverhulme Research Fellowship (RF/2/RFG/2008/0145). Wave 5 was supported by funding to Alice M. Gregory from Goldsmiths, University of London. T. C. Eley is partly funded by a program grant from the UK Medical Research Council (MR/M021475/1). This study presents independent research [partly] funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The Minnesota Twin Registry (MTR) acknowledges support from NIH grant R01AG053217. Guangzhou Twin Eye Study is supported by National Natural Science Foundation of China (grant #81125007). Anthropometric measurements of the Hungarian twins were supported by Medexpert Ltd., Budapest, Hungary. Korean Twin-Family Register was supported by the Global Research Network Program of the National Research Foundation (NRF 2011-220-E00006). Longitudinal Israeli Study of Twins was funded by the Starting Grant no. 240994 from the European Research Council (ERC) to Ariel Knafo. The Michigan State University Twin Registry has been supported by Michigan State University, as well as grants R01-MH081813, R01-MH0820-54, R01-MH092377-02, R21-MH070542-01, R03-MH63851-01 and 1R01-MH118848-01 from the National Institute of Mental Health (NIMH), R01-HD066040 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) and 11-SPG-2518 from the MSU Foundation. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, the NICHD or the National Institutes of Health. The Murcia Twin Registry is supported by Fundación Séneca, Regional Agency for Science and Technology, Murcia, Spain (08633/PHCS/08, 15302/PHCS/10 & 19479/PI/14) and Ministry of Science and Innovation, Spain (PSI2009-11560 & PSI2014-56680-R). The NAS–NRC Twin Registry acknowledges financial support from the National Institutes of Health grant number R21 AG039572. Netherlands Twin Register acknowledges the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants 904-61-090, 985-10-002, 912-10-020, 904-61-193,480-04-004, 463-06-001, 451-04-034, 400-05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192; VU University’s Institute for Health and Care Research (EMGO+); the European Research Council (ERC-230374), the Avera Institute, Sioux Falls, South Dakota (USA). Osaka University Aged Twin Registry is supported by grants from JSPS KAKENHI JP (23593419, 24792601, 26671010, 24590695, 26293128, 16K15385, 16K15978, 16K15989, 16H03261). PETS was supported by grants from the Australian National Health and Medical Research Council (grant numbers 437015 and 607358 to JC, and RS), the Bonnie Babes Foundation (grant number BBF20704 to JMC), the Financial Markets Foundation for Children (grant no. 032-2007 to JMC), and by the Victorian Government’s Operational Infrastructure Support Program. Madeira data comes from the following project: Genetic and environmental influences on physical activity, fitness and health: the Madeira family study Project reference: POCI/DES/56834/2004 Founded by the Portuguese agency for research (The Foundation for Science and Technology [FCT]). TwinsUK receives funding from the Wellcome Trust, Medical Research Council and European Union. TwinsUK and M. Mangino are supported by the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. The Quebec Newborn Twin Study acknowledges financial support from the Fonds Québécois de la Recherche sur la Société et la Culture, the Fonds de la Recherche en Santé du Québec, the Social Science and Humanities Research Council of Canada, the National Health Research Development Program, the Canadian Institutes for Health Research, Sainte-Justine Hospital’s Research Center, and the Canada Research Chair Program (Michel Boivin). South Korea Twin Registry is supported by National Research Foundation of Korea (NRF-371-2011-1 B00047). We acknowledge The Swedish Twin Registry for access to data. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no. 2017-00641. The Twins Early Development Study (TEDS) is supported by a program grant (G0901245) from the UK Medical Research Council and the work on obesity in TEDS is supported in part by a grant from the UK Biotechnology and Biological Sciences Research Council (31/D19086). Currently TEDS is supported by MRC grant ‘MR/M021475/1’. The Texas Twin Project is currently funded by grant R01HD083613 from the National Institutes of Health. S. Y. Öncel and F. Aliev are supported by Kırıkkale University Research Grant: KKU, 2009/43 and TUBITAK grant 114C117. The University of Southern California Twin Study is funded by a grant from the National Institute of Mental Health (R01 MH58354). Washington State Twin Registry (formerly the University of Washington Twin Registry) was supported in part by grant NIH RC2 HL103416 (D. Buchwald, PI). Vietnam Era Twin Study of Aging was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381 and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA. The West Japan Twins and Higher Order Multiple Births Registry was supported by Grant-in-Aid for Scientific Research (B) (grant number 15H05105) from the Japan Society for the Promotion of Science.




