Heart valves are thin membranes regulating blood flow by constant opening and closing. The four valves in the heart are between the atria and ventricules (called mitral and tricuspid valves), and in the arteries leaving the heart (called aortic and pulmonary valves). Regurgitation occurs when a valve malfunctions and allows some blood to flow in the wrong direction leading to valvular heart disease (Lancellotti et al., Reference Lancellotti, Tribouilloy, Hagendorff, Moura, Popescu, Agricola and Zamorano2010b). Valvular heart disease is a multifactorial disorder determined by both genetic and environmental factors. In clinical practice the most frequent cause of mitral and tricuspid regurgitation is ischemic heart disease, while the aortic valve is mostly affected by the degenerative calcification (Lancellotti et al., Reference Lancellotti, Moura, Pierard, Agricola, Popescu, Tribouilloy and Zamorano2010a, Reference Lancellotti, Tribouilloy, Hagendorff, Moura, Popescu, Agricola and Zamorano2010b). However, valve lesions defined dominantly by genetic factors are also known; for example, in Marfan syndrome, Ehlers–Danlos syndrome, and other connective tissue disorders (Boudoulas et al., Reference Boudoulas, Vavuranakis and Wooley1994; Grau et al., Reference Grau, Pirelli, Yu, Galloway and Ostrer2007). Deeper understanding of genetic or environmental influences in the development of functional and organic valve lesions is of high importance. Recent advances in echocardiography can help to accurately assess the pathomechanism and the severity of valvular disorders, while twin studies are used to evaluate the ratio of underlying genetic and environmental components. Investigation of a triplet set, including both monozygotic and dizygotic pairs, is a unique opportunity to highlight these issues.
Case Presentation
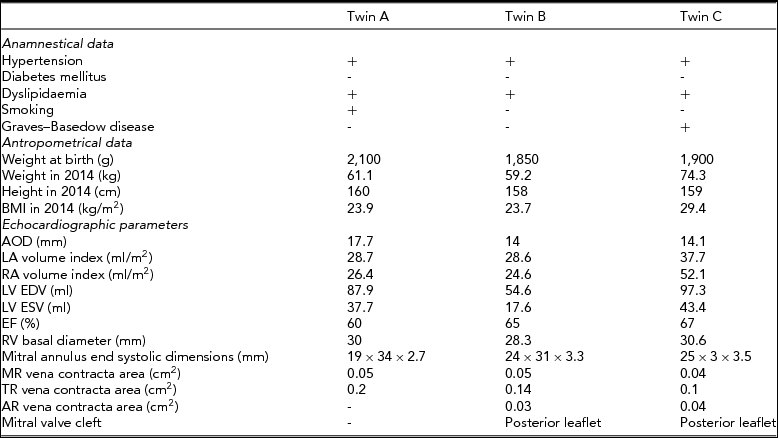
We report the case of a 45-year-old asymptomatic Hungarian female triplet. The first-born twin (twin A) was dizygotic with the others, the second- (twin B) and third-born twins (twin C) were monozygotic with each other. Zygosity was determinated by a standard multiple-choice questionnaire (Heath et al., Reference Heath, Nyholt, Neuman, Madden, Bucholz, Todd and Martin2003). The triplets were recruited by the Hungarian Twin Registry and examined as part of our twin study, using multiple cardiovascular imaging modalities (Littvay et al., Reference Littvay, Métneki, Tarnoki and Tarnoki2013). Twins provided their informed consent before entering the study. First, medical history and anthropometrical data were collected (Table 1). According to their medical records, all of the siblings had a separate amniotic sac. The birth weight of twin A was 2,100 g, twin B was 1,850 g, and twin C was 1,900 g. All the siblings were diagnosed and treated for hypertension since 2008, and dyslipidemia since 2003. Twin C was treated for an autoimmune disease of the thyroid gland named the Graves–Basedow disease since 2011. Only twin A was an active smoker since 1988; the other twins have never smoked.
TABLE 1 Clinical Characteristics and Echocardiographic Data

AOD: aortic annulus diameter; LA: left atrium; RA: right atrium; LV EDV: left ventricular end diastolic volume; LV ESV: left ventricular end systolic volume; EF: ejection fraction of left ventricule; RV: right ventricule; MR: mitral regurgitation; TR: tricuspidal regurgitation; AR: aortic regurgitation.
Transthoracic, two-dimensional echocardiographic examination (Philips iE33, S5-1 transducer) revealed multiple valvular heart lesions in each twin, but with a different pattern. Mild aortic, mild holosystolic mitral, and mild tricuspid regurgitation with central regurgitant jet were found during the examination of the monozygotic twin pair (twins B and C; Figure 1). Mild protosystolic mitral and mild tricuspidal regurgitation with central regurgitant jet were also noticed in twin A, but no aortic regurgitation could be detected. Transthoracic echocardiography showed normal dimensions of both ventricules and proximal part of the aorta, and normal left ventricular systolic function (Table 1). Left ventricular systolic function was characterized by ejection fraction, which represented the volumetric fraction of blood pumped out of the left ventricle with each heartbeat (Table 1).

FIGURE 1 Two-dimensional transthoracic color Doppler image of parasternal long axis view of Twin B showing mild aortic and mitral regurgitation (A image), and four-chamber view demonstrating central mitral and tricuspid regurgitant jet (B and C images).AR: aortic regurgitation; MR: mitral regurgitation; TR: tricuspidal regurgitation; Ao: aorta; LA: left atrium; LV: left ventricule; RA: right atrium; RV: right ventricule.
In order to reveal the pathomechanism and accurately quantify the severity of valve lesions, three-dimensional transesophageal echocardiography (Philips iE33, X7-2t transducer) was also performed. Three-dimensional echocardiographic imaging modalities are ideal for the assessment of anatomy and function of each of the individual components of the valve apparatus (Lang et al., Reference Lang, Badano, Tsang, Adams, Agricola, Buck and Zoghbi2012). The mitral valve consists of two valve leaflets connected by opposing anterolateral and posteromedial commissures, the mitral valve annulus, which forms a ring around the valve leaflets, and the highly variable chordae tendineae arrangement with two papillary muscles, which tether the valve leaflets to the left ventricle and prevent them from prolapsing into the left atrium (Figure 2). Dysfunction of any of these portions of the mitral valve apparatus can cause mitral regurgitation (Lancellotti et al., Reference Lancellotti, Moura, Pierard, Agricola, Popescu, Tribouilloy and Zamorano2010a). Both leaflets can be divided into three scallops: anterior A1, A2, and A3, and posterior P1, P2, P3 (Figure 3). Leaflet segmentation is particularly useful to precisely localize prolapsing segments and anatomic lesions of the mitral valve (Lang et al., Reference Lang, Badano, Tsang, Adams, Agricola, Buck and Zoghbi2012). Mitral valve prolapse is defined as an abnormal displacement of the mitral valve leaflets into the left atrium during ventricular systole and is one of the most common causes of mitral valve regurgitation (Lancellotti et al., Reference Lancellotti, Moura, Pierard, Agricola, Popescu, Tribouilloy and Zamorano2010a). Cleft of mitral valve leaflet is an anatomic lesion defined as a slit-like hole that extends to the mitral annulus and can be accompanied by mitral valve prolapse, atrial septal defect, counterclockwise rotation of the papillary muscles, and the presence of an accessory papillary muscle or mitral valve leaflet (Di Segni & Edwards, Reference Di Segni and Edwards1983). Indentation of mitral valve leaflet is a discontinuation in the leaflet that does not extend to the annulus. Both twins of the monozygotic pair (twins B and C) showed isolated posterior leaflet cleft between P2 and P3 scallops, resulting in mitral regurgitation, and indentation between P1 and P2 scallops (Figure 2.). These findings were absent in twin A: mitral leaflets were unimpaired and the central regurgitation jet was detected only in the protosystolic phase at the onset of coaptation. None of the twins had mitral valve prolapse.

FIGURE 2 Three-dimensional transesophageal image of the mitral valve as viewed from the left atrium demonstrating the posterior mitral leaflet cleft (arrow) in twin C. ALC: antero-lateral commissure (arrowhead); PMC: postero-medial commissure (arrowhead); *anterior mitral leaflet; **posterior mitral leaflet.

FIGURE 3 Three-dimensional morphologic analysis and model of the mitral valve in twin C. (A) The mitral annulus is manually defined in multiple rotational planes, (B) yielding a resultant three-dimensional contour superimposed on the en face view of the valve. (C) The mitral valve leaflets are then manually traced in multiple parallel planes, resulting in (D) a line of coaptation displayed on a color-coded, three-dimension-rendered valve surface. A: anterior; P: posterior; AL: anterolateral; PM: posteromedial; Ao: aorta; A1, A2, A3: scallops of the anterior mitral valve leaflet; P1, P2, P3: scallops of the posterior mitral valve leaflet.
The aortic valve consists of three cusps. Their association with the respective coronary artery identifies them as left, right, and non-coronary cusp. The most common congenital abnormality of the heart is the bicuspid aortic valve leading to aortic valve malfunction. In this condition, instead of three cusps, the aortic valve has two cusps (Lancellotti et al., Reference Lancellotti, Tribouilloy, Hagendorff, Moura, Popescu, Agricola and Zamorano2010b; Martin et al., Reference Martin, Ramachandran, Cripe, Hinton, Andelfinger, Tabangin and Benson2007). In our case, the three-dimensional transesophageal echocardiography proved that the aortic valve included three cusps in all of the twins. The pathomechanism of central mild aortic regurgitation found in the monozygotic twin pair was early stage aortosclerosis. Signs of early stage calcification were detected in the right aortic cusp in twin B and non-coronary aortic cusp in twin C. Calcification restricts the mobility of aortic valve and causes valve closure malfunction, leading to regurgitation (Lancellotti et al., Reference Lancellotti, Tribouilloy, Hagendorff, Moura, Popescu, Agricola and Zamorano2010b). The aortic valve in twin A was structurally and functionally normal. All leaflets of the tricuspid valve were structurally unimpaired.
Quantification of the severity of valve regurgitation is essential for therapeutic management. The vena contracta is a quantitative method to assess the severity of regurgitation, and is defined as the narrowest portion of the regurgitant jet downstream from the regurgitant orifice (Enriquez-Sarano et al., Reference Enriquez-Sarano, Avierinos, Messika-Zeitoun, Detaint, Capps, Nkomo and Tajik2005). In our case, vena contracta areas of valve regurgitations were measured using multiplanar reconstruction of the three-dimensional full-volume color Doppler data set in all of the twins (Table 1 and Figure 4). All valve lesions were classified as mild in all the twins. The number and location of the papillary muscles were normal and no atrial septal defect could be detected.

FIGURE 4. Quantification of mitral valve regurgitation in twin C using multiplanar reconstruction of the three-dimensional full-volume color Doppler dataset to assess the vena contracta area. A1: vena contracta area.
Discussion
Our report shows a unique case regarding a triplet with multivalvular disease. The monozygotic pair showed the same pattern and pathomechanism of valvular lesions: mild mitral, tricuspidal and aortic regurgitation, as well. Isolated cleft of the posterior mitral valve leaflet and early-stage aortosclerosis were explored in the background of valvular lesions in both monozygotic twins. Interestingly, the examination of twin A, who was dizygotic to twins B and C, revealed no aortic insufficiency, and the characteristics and pathomechanism of mitral valve regurgitation were different. Early-stage calcification of aortic cusps was found only in the monozygotic twins, despite the same cardiovascular risk factors being present in all the twins. This may lead us to the assumption that genetic factors might play a role in the development of aortic valve calcification. Limited information is available regarding genetic determinants of valvular calcification, which is an important precursor of clinical valvular disease. In a genomewide association study, Thanassoulis and coworkers (Reference Thanassoulis, Campbell, Owens, Smith, Smith, Peloso and Post2013) have identified a single-nucleotide polymorphism in the lipoprotein(a) locus that correlated significantly with aortic valve calcification.
Cleft of mitral valve represents a relatively uncommon congenital cause of mitral regurgitation. Acquired causes of clefts include infective endocarditis and trauma from surgical operation. Two-dimensional echocardiography facilitates the diagnosis of cleft mitral valve (Di Segni & Edwards, Reference Di Segni and Edwards1983; Wyss et al., Reference Wyss, Enseleit, van der Loo, Grünenfelder, Oechslin and Jenni2009); however, its evaluation by two-dimensional echocardiography can be difficult, and sometimes a patient with an isolated cleft may remain undiagnosed (Van Praagh et al., Reference Van Praagh, Porras, Oppido, Geva and Van Praagh2003). Wyss and coworkers (Reference Wyss, Enseleit, van der Loo, Grünenfelder, Oechslin and Jenni2009) reported that the prevalence of isolated cleft of the posterior mitral leaflet was 0.11% (n = 22 out of 19,320 two-dimensional transthoracic echocardiograms). In our case, two-dimensional transthoracic echocardiography did not reveal the mechanism of mitral regurgitation. Using the three-dimensional transesophageal echocardiography technique enabled us to identify the cleft of the posterior mitral valve leaflet. Assessment of the mechanism and severity of mitral valve regurgitation is of paramount importance for therapeutic management. Three-dimensional echocardiography has improved both morphological and functional assessment of valvular heart disease. It provides additional morphologic information of the components of mitral valve apparatus, which leads to better understanding of the mechanism of mitral regurgitation (Cai & Ahmad, Reference Cai and Ahmad2012; Lancellotti et al., Reference Lancellotti, Moura, Pierard, Agricola, Popescu, Tribouilloy and Zamorano2010a).
Surgical correction is recommended in cases of severe mitral regurgitation in symptomatic patients with or without left ventricular dysfunction, and for asymptomatic patients with left ventricular dilatation and/or left ventricular ejection fraction <50% (Bonow et al., Reference Bonow, Carabello, Chatterjee, de Leon, Faxon, Freed and Shanewise2008). Because of the asymptomatic status of the twins in our case and the lack of significant regurgitation, regular clinical follow-up and periodical echocardiographic examination were suggested. Early recognition of this rare clinical entity can identify the patients who would benefit from surgical intervention before compensatory left ventricular remodeling and contractile dysfunction develop. Besides this, multiple births have an increased risk of birth defects (Li et al., Reference Li, Ford, Meister and Bodurtha2003). This raises the question of whether when one twin is affected with valvular lesion, the other asymptomatic twin should be examined as well.
This is the first case to our knowledge to present posterior mitral valve cleft in monozygotic twins within a triplet pair, who are discordant regarding the mitral valve lesion with the other dizygotic twin. There is no evidence for the precise genetic or environmental (intrauterine/extrauterine) background of congenital posterior mitral valve cleft. However, previous publications suggest that twins appear to be associated with an increased risk of congenital heart diseases compared with singletons (Bahtiyar et al., Reference Bahtiyar, Dulay, Weeks, Friedman and Copel2007; Campbell et al., Reference Campbell, Copel and Bahtiyar2009). It appears that the interplay of altered placentation in conjunction with a genetic predisposition may play some part in this increased congenital heart disease risk (Bahtiyar et al., Reference Bahtiyar, Dulay, Weeks, Friedman and Copel2007; Bjarnegård et al., Reference Bjarnegård, Enge, Norlin, Gustafsdottir, Fredriksson, Abramsson and Betsholtz2013). Therefore, we assume that beyond genetic effects, the altered local intrauterine environmental factors (altered placental hemodynamics and vascular factors) might also play a role in the development of mitral valve cleft. In order to verify this, further investigations should be performed on larger twin populations.
Consent
Written informed consent was obtained by all three patients for the publication of this case report and any accompanying images.
Glossary
Aorta: The largest artery in the human body originating from the left ventricle via the aortic valve.
Aortic annulus: Fibrous ring surrounding the aortic orifice and serves for the attachment of the cusps of the aortic valve.
Aortosclerosis: Arteriosclerosis of the aorta; a pathological condition that is characterized by thickening and loss of elasticity of aortic wall.
Atrial septal defect: A form of a congenital heart disease that enables blood flow between two compartments of the heart called the left and right atria.
Atrium: A thin walled chamber that allows blood to return to the heart.
Coaptation of mitral valve: Fitting together process of the two surfaces of mitral valve leaflets during valve closure.
Color Doppler: Echocardiographic technique that estimates the average velocity of flow within a vessel by color coding the information.
Commisure: The area where the two valve leaflets come together.
Cordae tendineae: Cord-like tendons that connect the papillary muscles to the tricuspid and mitral valve.
Dyslipidemia: Abnormal level of lipids (e.g., cholesterol and/or fat) in the blood.
Ehlers–Danlos syndrome: Inherited connective tissue disorder caused by a defect in the structure, production, or processing of collagen or proteins that interact with collagen. The manifestations of the disease involve the cardiovascular system (e.g., heart valves), the musculoskeletal system, and skin.
Holosystole: Cardiac systole is the contraction of the cardiac muscle in response to an electrochemical stimulus to the heart's cells. Holosystole refers to the entire phase of cardiac systole.
Infective endocarditis: Inflammation of the inner tissue of the heart (such as valves) caused by infectious agents.
Marfan syndrome: Genetic disorder caused by misfolding of the protein fibrillin-1, which forms fibers in connective tissue. More than 30 different signs and symptoms are variably associated with the Marfan syndrome. The most serious signs involve the cardiovascular system (e.g., dilatation of the aorta, heart valve disease).
Mitral valve annulus: Fibrous ring around the mitral valve.
Papillary muscles: Muscles located in the ventricles that attach to the leaflets of the mitral and tricuspid valves via the cordae tendineae and contract to prevent inversion or prolapse of these valves.
Protosystole: Cardiac systole is the contraction of the cardiac muscle in response to an electrochemical stimulus to the heart's cells. Protosystole refers to the early phase of cardiac systole.
Ventricle: The large chamber that collects and expels blood received from an atrium toward the peripheral beds within the body and lungs.