Atherosclerosis is a chronic disease of the arteries characterized by inflammation and plaque building in the arterial wall, eventually leading to stenosis of the vessel. Carotid atherosclerosis (CAS), which is the manifestation of atherosclerotic disease in the cervical arteries, is associated with increased risk for cardiovascular diseases (CVDs) (Chambless et al., Reference Chambless, Heiss, Folsom, Rosamond, Szklo, Sharrett and Clegg1997; Ebrahim et al., Reference Ebrahim, Papacosta, Whincup, Wannamethee, Walker, Nicolaides and Lowe1999; Lorenz et al., Reference Lorenz, Markus, Bots, Rosvall and Sitzer2007). CVDs are responsible for 31% of deaths globally, being the leading cause of death worldwide, and of all CVD deaths, 80% are attributable to stroke and myocardial infarction (World Health Organization, 2017). Carotid intima-media-thickness (CIMT), plaque, and stenosis are measurable traits indicating CAS. Several studies have confirmed that increased CIMT is the distance between the lumen-intima and the media-adventitia interfaces as assessed by ultrasound and is a predictor of coronary heart disease and cerebrovascular events, such as stroke (Bots et al., Reference Bots, Hoes, Koudstaal, Hofman and Grobbee1997; Chambless et al., Reference Chambless, Heiss, Folsom, Rosamond, Szklo, Sharrett and Clegg1997, Reference Chambless, Folsom, Clegg, Sharrett, Shahar, Nieto and Evans2000; Howard et al., Reference Howard, Sharrett, Heiss, Evans, Chambless, Riley and Burke1993; Lorenz et al., Reference Lorenz, Markus, Bots, Rosvall and Sitzer2007; Polak et al., Reference Polak, Pencina, Pencina, O'Donnell, Wolf and D'Agostino2011; Salonen & Salonen, Reference Salonen and Salonen1991; van der Meer et al., Reference van der Meer, Bots, Hofman, del Sol, van der Kuip and Witteman2004). Furthermore, CIMT is a marker of subclinical atherosclerosis (Naqvi et al., Reference Naqvi, Mendoza, Rafii, Gransar, Guerra, Lepor and Shah2010), and prospective studies have shown that the subclinical stage of atherosclerosis is associated with clinical coronary artery disease (Kuller et al., Reference Kuller, Shemanski, Psaty, Borhani, Gardin, Haan and Tracy1995).
The tools for non-invasive assessment of CAS include B-mode ultrasound and time-of-flight magnetic resonance angiography, or contrast-enhanced magnetic resonance angiography. Ultrasound is a well-established and widely used method to detect carotid artery pathologies since it is highly repeatable, reproducible, and sensitive (Heiss et al., Reference Heiss, Sharrett, Barnes, Chambless, Szklo and Alzola1991; Stein et al., Reference Stein, Korcarz, Hurst, Lonn, Kendall and Mohler2008). According to a meta-analysis on CIMT reproducibility, intra- and interobserver variability varied between 62% and 97% and between 58% and 100%, respectively (Kanters et al., Reference Kanters, Algra, van Leeuwen and Banga1997). However, the measurement error increased if maximal instead of mean CIMT was registered and if internal CIMT instead of common CIMT was measured (Kanters et al., Reference Kanters, Algra, van Leeuwen and Banga1997). MRI may provide additional information on plaque characteristics (Kerwin et al., Reference Kerwin, Hatsukami, Yuan and Zhao2013). The invasive imaging methods of the CAS include contrast-enhanced computer tomography, magnetic resonance, and catheter (digital subtraction) angiography. Plaque size relative to the resolution of magnetic resonance imaging influences the reliability of magnetic resonance angiography, which ranges between 0.38 and 0.89 for wall and cap thickness and between 0.66 and 0.94 for vessel area (Wasserman et al., Reference Wasserman, Astor, Sharrett, Swingen and Catellier2010). Reliability or measurement error is an important factor to consider since it is a part of unique environmental variance (E), an important driver of the magnitude of heritability estimates.
Twin and family studies along with candidate gene analyses and genome-wide association studies (GWAS) have a crucial role to explore detailed genetic and environmental effects on these important markers of cardiovascular disease. Investigating underlying factors affecting the condition of carotid arteries is essential for future individualized treatment and prevention aspects. The present review aims to overview the studies that investigate the background of phenotypic variances of carotid artery atherosclerosis markers and possible responsible genes, with the focus on recent results.
Methods
To find the most appropriate articles for our topic, searches in PUBMED and the Web of Science database were conducted. First, we searched using the following keywords in the title or abstract: carotid, atherosclerosis, family study, twin study, heritability, heredity, parent offspring, parent child, and atherosclerosis. Second, we performed a separate search in order to review genes associated with carotid artery atherosclerosis with the key words candidate gene, genome-wide association, and carotid. The first search resulted in 152 articles, of which 41 were considered as relevant to our topic, and the second search resulted in 125 articles, of which 22 were included. Articles exclusively in English were considered for inclusion, as well as articles that were the subject of clinical twin research or family studies.
Twin Studies on CAS
The features of carotid arteries have been the focus of many twin studies since the 1980s. The first twin studies aimed to investigate the effect of environmental factors (e.g., smoking) on atherosclerosis in monozygotic (MZ) twins discordant for smoking (Haapanen et al., Reference Haapanen, Koskenvuo, Kaprio, Kesaniemi and Heikkila1989; Lassila et al., Reference Lassila, Seyberth, Haapanen, Schweer, Koskenvuo and Laustiola1988). Recently, CIMT and plaques on several segments of the carotid artery, which are important predictors of future cardiovascular events, have been the focus of investigations in twins (Naqvi & Lee, Reference Naqvi and Lee2014).
Heritability of CIMT and its genetic correlation with other non-vascular traits
CIMT is the distance between the luminal surface of the intima (inner layer of the carotid artery) and the media-adventitia interface measured by ultrasound. Substantial genetic effects on CIMT have been described using the ACE + age model (Medda et al., Reference Medda, Fagnani, Schillaci, Tarnoki, Tarnoki, Baracchini and Stazi2014). Variance in the common CIMT was genetically determined in 31% of participants (Medda et al., Reference Medda, Fagnani, Schillaci, Tarnoki, Tarnoki, Baracchini and Stazi2014). Similar results were observed in the Korean population (Lee et al., Reference Lee, Sung, Lee, Park, Kim, Lee and Song2012a). Using the ACE model, heritability was estimated at 48%, 38%, and 45% regarding common CIMT, bifurcation intima-media thickness (IMT), and internal CIMT, respectively. When taking into account measured covariates (cardiovascular risk factors), the variance was explained by additive genetics in 21% in the common carotid artery (CCA) and 24% in the bifurcation and in 31% in the internal carotid artery (ICA) (Lee et al., Reference Lee, Sung, Lee, Park, Kim, Lee and Song2012a). Cardiovascular risk factors determined the total phenotypic variance in 46%, 37%, and 26% in the CCA, bifurcation IMT, and ICA, respectively (Lee et al., Reference Lee, Sung, Lee, Park, Kim, Lee and Song2012a). Zhao et al. (Reference Zhao, Cheema, Bremner, Goldberg, Su, Snieder and Vaccarino2008) reported higher heritability (69%) of CIMT, assuming equal common environmental factors, and the heritability remained high (59%) after adjusting for age, high-density lipoprotein (HDL), and systolic blood pressure (SBP). However, the study population consisted only of middle-aged male twins. Swan et al. (Reference Swan, Birnie, Inglis, Connell and Hillis2003) found higher MZ than dizygotic correlations regarding far-wall common CIMT, but the significant heritability was not confirmed. Near-wall common CIMT on the right side did not show a higher MZ correlation (Swan et al., Reference Swan, Birnie, Inglis, Connell and Hillis2003); however, far-wall common CIMT is the preferred location to determine CIMT (Wikstrand, Reference Wikstrand2007). The differences observed among segments of the carotid arteries might be attributable to the carotid geometry (Bijari et al., Reference Bijari, Wasserman and Steinman2014; Phan et al., Reference Phan, Beare, Jolley, Das, Ren, Wong and Srikanth2012). Furthermore, Polak et al. (Reference Polak, Person, Wei, Godreau, Jacobs, Harrington and O'Leary2010) suggested that there is a segmental difference regarding effects of cardiovascular risk factors on the carotid arteries. According to their results, fasting glucose and diastolic blood pressure showed a stronger association with common CIMT than with the other segments. Hypertension, diabetes, and current smoking were associated with carotid bulb IMT, and low-density lipoprotein (LDL) cholesterol with ICA IMT (Polak et al., Reference Polak, Person, Wei, Godreau, Jacobs, Harrington and O'Leary2010).
Interestingly, the association of anthropometric parameters showed differences in genetic basis between men and women. In men, the genetic correlation between body mass index (BMI) and IMT in the common and internal carotid arteries remained significant even after adjustment for covariates, whereas in women, these traits did not genetically correlate with CIMT after adjustment (Song et al., Reference Song, Lee, Sung, Kim and Lee2012). Similarly, Juo et al. (Reference Juo, Lin, Rundek, Sabala, Boden-Albala, Park and Sacco2004) found genetic correlation between common CIMT and BMI in a family study conducted on the Hispanic population. In a study conducted on women twin pairs, substantial additive genetic basis (92%) was found regarding the association between carotid-femoral pulse wave velocity and CIMT (Cecelja et al., Reference Cecelja, Jiang, Bevan, Frost, Spector and Chowienczyk2011). However, no independent association was found between these two traits (Cecelja et al., Reference Cecelja, Jiang, Bevan, Frost, Spector and Chowienczyk2011). Kulshreshtha et al. (Reference Kulshreshtha, Goyal, Veledar, McClellan, Judd, Eufinger and Vaccarino2014) implied that cardiovascular health index (which considers blood pressure, fasting glucose, total cholesterol, BMI, physical activity, healthy diet, and smoking) and CIMT are independently associated and this relation has a unique environmental basis.
Heritability of carotid artery plaque features
On a relatively large sample (328 individuals) of Hungarian and Italian twins, additive genetics was responsible for the variance in the presence of carotid plaques in 52% of participants, and this trait was explained by unique environmental factors in 48% (Lucatelli et al., Reference Lucatelli, Fagnani, Tarnoki, Tarnoki, Sacconi, Fejer and Medda2017). Moreover, substantial relation in the co-occurrence of femoral and carotid plaques was found using the Cholesky model (42%) and was highly heritable (77%) (Lucatelli et al., Reference Lucatelli, Fagnani, Tarnoki, Tarnoki, Sacconi, Fejer and Medda2017). Besides, the contribution of unique environmental factors to co-occurrence of these plaques was lower but not negligible (23%) (Lucatelli et al., Reference Lucatelli, Fagnani, Tarnoki, Tarnoki, Sacconi, Fejer and Medda2017). The same study group reported an even higher additive genetic basis of carotid plaque presence (78%), plaque composition (74%), plaque sidedness (74%), and plaque quantity (74%) (Tarnoki et al., Reference Tarnoki, Baracchini, Tarnoki, Lucatelli, Boatta, Zini and Schillaci2012). Although heritability seems to have a strong influence on both CIMT and carotid plaques, there are twin studies emphasizing the role of mainly non-shared environmental factors. Investigating co-twins living in different countries is an ideal model to assess unique environmental effects. Jartti et al. (Reference Jartti, Ronnemaa, Raitakari, Hedlund, Hammar, Lassila and Kaprio2009) described interesting results when comparing CIMT in Finnish twin pairs when one co-twin was resident in Sweden. Being a resident in a lower coronary heart disease risk country had a significant impact on CIMT, but the difference was only significant when moving at an age less than 21 years (Jartti et al., Reference Jartti, Ronnemaa, Raitakari, Hedlund, Hammar, Lassila and Kaprio2009). On the other hand, the genetic susceptibility to have a greater CIMT in certain populations is also confirmed by the results of the same study group, showing that men from Eastern Finland had significantly higher IMT compared to men from Western Finland, and this was independent from their current resident country (Jartti et al., Reference Jartti, Raitakari, Kaprio, Jarvisalo, Toikka, Marniemi and Ronnemaa2002). Table 1 summarizes the twin studies and heritability values on carotid plaque features and CIMT.
TABLE 1 Heritability Values for CAS Markers in Twin Studies
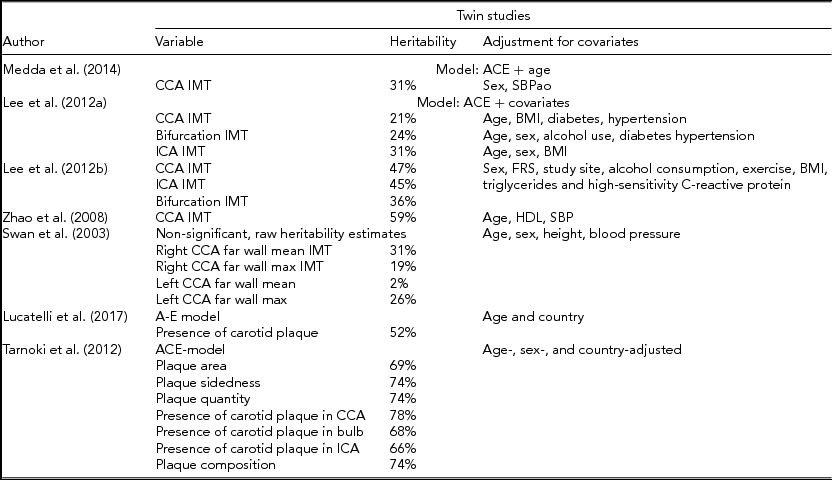
ACE-model: A = additive genetic effects, C = shared environment effects, E = unshared environment effects, BMI = body mass index, CCA = common carotid artery, FRS = Framingham Risk Score, HDL = high-density lipoprotein, ICA = internal carotid artery, IMT = intimamedia thickness, MAP = mean arterial pressure, RSES = Rosenberg Self-Esteem Scale, SBPao = aortic systolic blood pressure, SBP = systolic blood pressure.
Proust et al. (Reference Proust, Empana, Boutouyrie, Alivon, Challande, Danchin and Lacolley2015) conducted an exome-array analysis and reported a heritability of 10.6% attributable to variants on the exome-array regarding common CIMT using genome-wide complex trait analysis (GCTA). However, despite the significant heritability, the large standard errors may indicate inconclusive results. None of the investigated exomes reached the significance thresholds in this study (Proust et al., Reference Proust, Empana, Boutouyrie, Alivon, Challande, Danchin and Lacolley2015). GCTA is a tool to investigate heritability of phenotypes based on the differences in genotype data between cases and controls (Yang et al., Reference Yang, Lee, Goddard and Visscher2011). According to our knowledge, no heritability was estimated using LD score regression for CIMT phenotypes.
Family studies and CAS
Although twin studies are the focus of this article, family studies in regard to CAS also have to be mentioned in brief. In family studies, the heritability of CIMT varied between 16% and 66% (Fox et al., Reference Fox, Polak, Chazaro, Cupples, Wolf, D'Agostino and Framingham Heart2003; Juo et al., Reference Juo, Rundek, Lin, Cheng, Lan, Huang and Sacco2005; Kao et al., Reference Kao, Hsueh, Rainwater, O'Leary, Imumorin, Stern and Mitchell2005; Kuipers et al., Reference Kuipers, Kammerer, Miljkovic, Woodard, Bunker, Patrick and Zmuda2013; Mayosi et al., Reference Mayosi, Avery, Baker, Gaukrodger, Imrie, Green and Keavney2005; Moskau et al., Reference Moskau, Golla, Grothe, Boes, Pohl and Klockgether2005; Ryder et al., Reference Ryder, Pankratz, Dengel, Pankow, Jacobs, Sinaiko and Steinberger2017; Sayed-Tabatabaei et al., Reference Sayed-Tabatabaei, van Rijn, Schut, Aulchenko, Croes, Zillikens and van Duijn2005; Xiang et al., Reference Xiang, Azen, Buchanan, Raffel, Tan, Cheng and Hodis2002). There were two findings for heritability of plaque presence of 23% (Hunt et al., Reference Hunt, Duggirala, Goring, Williams, Almasy, Blangero and Stern2002) and 15% (Dong et al., Reference Dong, Beecham, Slifer, Wang, Blanton, Wright and Sacco2010). Whether heritability is calculated for offspring only or offspring and parents influences the results heavily. The population characteristics – for example, hypertensive or diabetic individuals – also may explain the wide range of heritability results. Although the family study is a good method to assess the difference between generations, it may not differentiate shared environmental and genetic effects, and this can be a cause of differences compared to twin studies (Susser & Susser, Reference Susser and Susser1987). Table 2 summarizes the family and sib studies and heritability values on carotid plaque features and CIMT.
TABLE 2 Heritability Values for CAS and Stiffness Markers in Family- and Sib-Studies
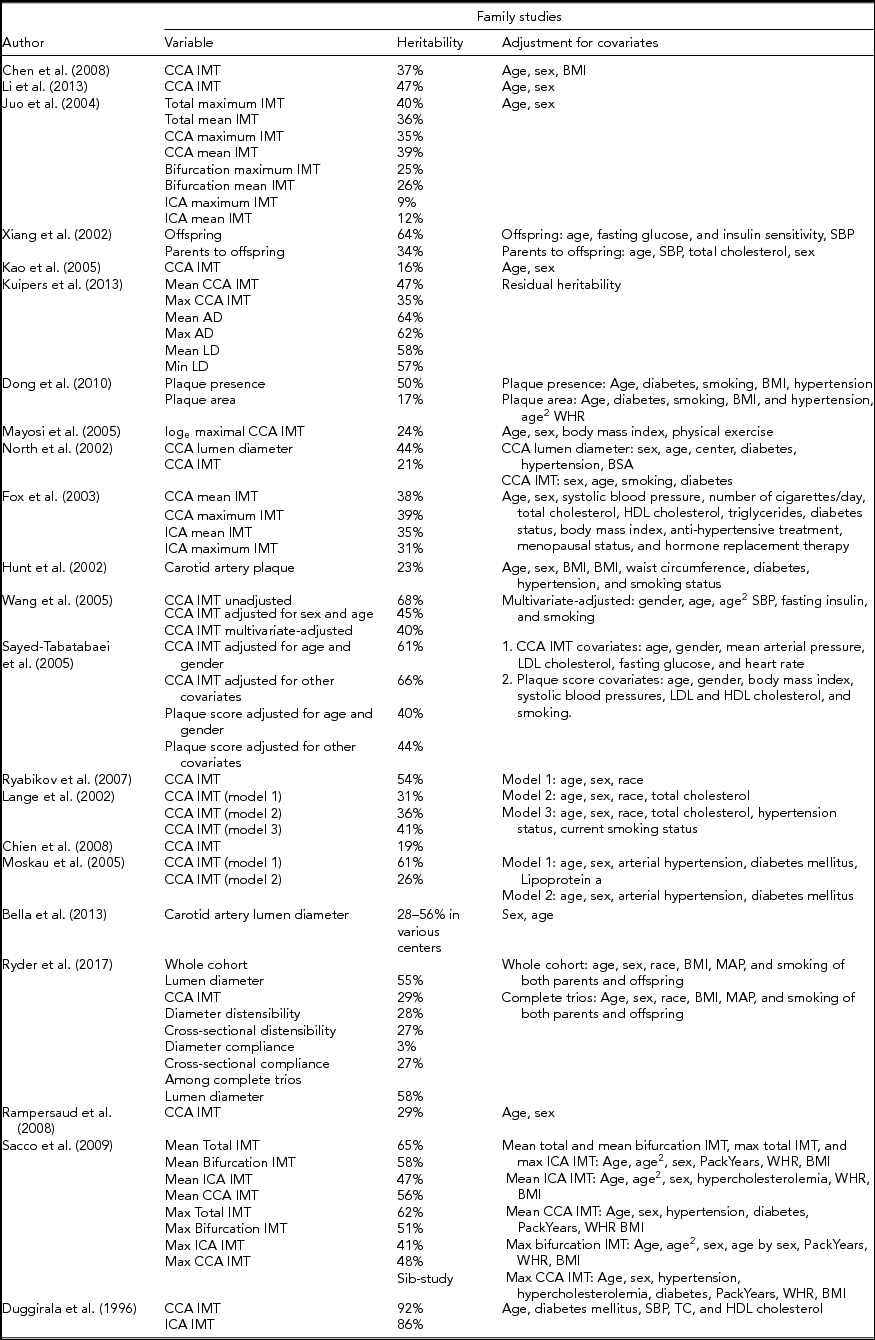
AD = adventitial diameter, BMI = body mass index, BSA = body surface area, CCA = common carotid artery, HDL = high-density lipoprotein, ICA = internal carotid artery, IMT = intima-media thickness, LD = lumen diameter, LDL = low-density lipoprotein, MAP = mean arterial pressure, SBP = systolic blood pressure, TC = total cholesterol, WHR = waist-hip ratio.
The methodology of CIMT measurement may be responsible for the observed differences in heritability values. Far-wall CIMT values are preferred over near-wall CIMT values as layers of the near-wall cannot be accurately visualized and clearly distinguished because the ultrasound beam crosses the arterial wall layers in a different order (from high to low echogenic structures) as compared to far-wall CIMT. Far-wall CIMT, on the other hand, is highly correlated with wall thickness measurements on histological specimens (Pignoli et al., Reference Pignoli, Tremoli, Poli, Oreste and Paoletti1986; Wong et al., Reference Wong, Edelstein, Wollman and Bond1993). Differences also arise in carotid artery segments, as visualization of the CCA is more accurate compared to visualization of the carotid bulb and ICA. This is explained by the CCA being perpendicular to the ultrasound beam. Mean CIMT values have the advantage of better reproducibility; however, they are less sensitive to changes in CIMT (Stein et al., Reference Stein, Korcarz, Hurst, Lonn, Kendall and Mohler2008). Most protocols are made for the registration of CCA far-wall mean IMT. On the contrary, plaques should be screened and registered on the CCA, carotid bulb and ICA segments separately (Stein et al., Reference Stein, Korcarz, Hurst, Lonn, Kendall and Mohler2008).
Other non-modifiable factors, such as gender and ethnic differences, have to be considered when interpreting heritability results of CIMT. Several studies have confirmed that male gender is associated with significantly higher CIMT values both in adults (Mazurek et al., Reference Mazurek, Zmijewski, Czajkowska and Lutoslawska2014) and children (Whincup et al., Reference Whincup, Nightingale, Owen, Rapala, Bhowruth, Prescott and Deanfield2012). Relevant ethnic differences have been observed, with Black African Caribbeans having significantly higher CIMT compared to White Europeans (Markus et al., Reference Markus, Kapozsta, Ditrich, Wolfe, Ali, Powell and Cullinane2001; Whincup et al., Reference Whincup, Nightingale, Owen, Rapala, Bhowruth, Prescott and Deanfield2012). Significantly higher CIMT values were reported among African-American individuals as compared to Whites and Asians (Breton et al., Reference Breton, Wang, Mack, Berhane, Lopez, Islam and Avol2011). Furthermore, the impact of significant covariates on a given phenotype should always be taken into account when interpreting heritability results. Similar heritability estimates with and without adjustment for covariates indicate a more powerful heritability result and the effect of the covariates on the variance. Different models may be created based on the significantly associated covariates. For example, features of carotid plaque were highly heritable when adjusting for age, sex, and country only; when taking into account significant covariates, such as smoking, hyperlipidemia, peripheral arterial disease, and diabetes, the heritability did not change significantly (Tarnoki et al., Reference Tarnoki, Baracchini, Tarnoki, Lucatelli, Boatta, Zini and Schillaci2012).
Genes Associated with Carotid Artery Atherosclerosis
Heritability of carotid artery atherosclerosis traits has been confirmed by the twin and family studies described above in detail, even though the results show great variation in heritability values depending on the methods and the populations investigated. In order to obtain a more detailed understanding of the genes beyond phenotype-based heritability, various studies investigating the exact gene variations have been conducted via linkage analysis, candidate gene association studies, and GWAS. These studies aim to determine the risk or the eventual causative role of specific alleles regarding the CAS phenotype; however, differences between these study designs have to be emphasized. Candidate gene association studies depend heavily on the right choice of genes, which can bias the outcomes. This approach may be successful if there is a presumption or knowledge about the function of the gene of interest. GWAS, on the other hand, can be conducted in order to find an association between a phenotype or disease and genetic variants without any prior knowledge on the function or action of a given gene. GWASs allow the sequencing of the entire genome, and therefore, are less biased by the choice of candidate genes. Furthermore, GWAS is a more powerful method to identify low-penetrance variants. This is also the reason why GWAS studies are well-established methods for the investigation of the genetic background of common genetic variations and complex diseases and phenotypes, such as CIMT and plaque. On the other hand, GWASs are not suitable for the analysis of the genetic background of rare diseases (Wilkening et al., Reference Wilkening, Chen, Bermejo and Canzian2009). Compared to linkage analysis, GWAS has a better resolution. Previous reviews summarized the most relevant genes and single-nucleotide polymorphisms (SNP) (Humphries & Morgan, Reference Humphries and Morgan2004; Juo, Reference Juo2009). Therefore, the current review is restricted to the advances and most relevant studies in this field since the latest reviews (Humphries & Morgan, Reference Humphries and Morgan2004; Juo, Reference Juo2009).
Candidate Gene Analyses and GWAS on CAS
Since atherosclerosis is a complex procedure, prior candidate gene association studies aimed to investigate regulators of the process at several points, such as inflammation and the function of extracellular matrix components.
The matrix-metalloproteinase-3 (MMP-3) and other matrix-metalloproteinases (MMPs)
Members of the MMP family, which are important regulators of the extracellular matrix degradation, were investigated as target genes influencing atherogenesis in both coronary and carotid arteries. The MMP-3 enzyme has broad substrate specificity in extracellular matrix degradation and can regulate other MMPs (Woessner, Reference Woessner1991). Extensive research has been conducted on the 5A/6A polymorphisms found in the promoter region of the MMP-3 gene with regard to both coronary and CAS, as the 5A allele is associated with higher and the 6A allele with lower MMP-3 transcription (Ye et al., Reference Ye, Eriksson, Hamsten, Kurkinen, Humphries and Henney1996), the former leading to decreased plaque stability and the latter leading to (stable) plaque progression. The 5A variant seems to be related to coronary plaque rupture and consequent myocardial infarction and the 6A variant may be related to coronary artery disease (Abilleira et al., Reference Abilleira, Bevan and Markus2006). Increased levels of MMP-3 have been described in patients with vulnerable plaques in a recent study (Hu et al., Reference Hu, Wei, Wang, Lu, Liu and Zhang2018), which is in line with the aforementioned assumption regarding the effect of the 5A variant. The MMP-3 6A allele was significantly associated with greater IMT (Rauramaa et al., Reference Rauramaa, Vaisanen, Luong, Schmidt-Trucksass, Penttila, Bouchard and Humphries2000), even after adjustment for covariates (Djuric et al., Reference Djuric, Zivkovic, Radak, Jekic, Radak, Stojkovic and Alavantic2008; Rundek et al., Reference Rundek, Elkind, Pittman, Boden-Albala, Martin, Humphries and Sacco2002). This was confirmed by a meta-analysis including roughly 180 individuals (Humphries & Morgan, Reference Humphries and Morgan2004). Recent results also indicate that the 5A/6A polymorphism is associated with CIMT progression in patients with type 2 diabetes mellitus (Pleskovic et al., Reference Pleskovic, Letonja, Vujkovac, Starcevic, Caprnda, Curilla and Petrovic2017). A recent GWAS study identified four SNPs on the 11q22.3 region that were independently associated with plasma MMP-12 levels on a genome-wide significant level, but expression quantitative trait loci analysis did not reveal a direct, causative role of these SNPs (Mahdessian et al., Reference Mahdessian, Perisic Matic, Lengquist, Gertow, Sennblad and Baldassarre2017). MMP-8 promoter gene polymorphisms were associated with plaque presence in Caucasian females and elevated MMP-8 mRNA levels in carotid artery plaques were associated with this allele ex vivo; however, the power of this study was limited by the low number of cases (Djuric et al., Reference Djuric, Stankovic, Koncar, Radak, Davidovic, Alavantic and Zivkovic2011). Other MMPs and their gene polymorphisms, such as MMP-14 (Li et al., Reference Li, Jin, Zhu, Chen, Wang, Hu and Zheng2014) and MMP-7 (Hu et al., Reference Hu, Jin, Hu, Zhu, Wang, Lin and Yang2011; Wang et al., Reference Wang, Jin, Zhu, Lin, Hu, Wang and Huang2011), might have a role in plaque vulnerability, but these results have not been confirmed on larger populations.
CDKN2A/B
CDKN2A and 2B are important modulators of cell proliferation. Zhang et al. (Reference Zhang, Wang, Zhang, Zhang, Zhou, Cao and Xu2015) identified the association between the SNP near the CDKN2A/B gene and carotid artery calcification in a population of nearly 900 individuals. The same SNP was significantly associated with plaque presence in a meta-analysis conducted on a much larger sample (den Hoed et al., Reference den Hoed, Strawbridge, Almgren, Gustafsson, Axelsson, Engstrom and Lind2015). The CDKN2A/B gene is located at the 9p21 locus, which is subject of intensive research and the relevance of which in atherosclerosis is undoubted (Holdt & Teupser, Reference Holdt and Teupser2012; Holdt et al., Reference Holdt, Beutner, Scholz, Gielen, Gabel, Bergert and Teupser2010, Reference Holdt, Sass, Gabel, Bergert, Thiery and Teupser2011, Reference Holdt, Hoffmann, Sass, Langenberger, Scholz, Krohn and Teupser2013, Reference Holdt, Stahringer, Sass, Pichler, Kulak, Wilfert and Teupser2016). Results of genome-wide significance of this polymorphism were replicated by large-scale meta-analyses investigating its relation to coronary heart disease (Nikpay et al., Reference Nikpay, Goel, Won, Hall, Willenborg, Kanoni and Farrall2015) and carotid plaque score (Pott et al., Reference Pott, Burkhardt, Beutner, Horn, Teren, Kirsten and Scholz2017). The CDKN2A/B polymorphism was strongly associated with carotid plaque score (Pott et al., Reference Pott, Burkhardt, Beutner, Horn, Teren, Kirsten and Scholz2017), but not with CIMT in a large-scale, multi-ethnic candidate gene association study conducted on more than 8,000 individuals (Vargas et al., Reference Vargas, Manichaikul, Wang, Rich, Rotter, Post and Bluemke2016), which raises the possibility of clinical and subclinical atherosclerosis having a partly different genetic background (Holdt & Teupser, Reference Holdt and Teupser2012).
IL-6 (interleukin-6) and IL-10 (interleukin-10)
Great attention has been dedicated to inflammatory molecules and variations of their genes in the atherogenic process. The role of IL-6 and IL-10 was reviewed previously and conflicting results were found (Humphries & Morgan, Reference Humphries and Morgan2004). Although it is logical that the genetic variants coding these inflammatory molecules affect CAS, results are conflicting regarding IL-6 (Chapman et al., Reference Chapman, Beilby, Humphries, Palmer, Thompson and Hung2003; Mayosi et al., Reference Mayosi, Avery, Baker, Gaukrodger, Imrie, Green and Keavney2005) and IL-10 (Heiskanen et al., Reference Heiskanen, Kahonen, Hurme, Lehtimaki, Mononen, Juonala and Hulkkonen2010; Yu et al., Reference Yu, Jun, Cho, Park, Chung, Shin and Chung2015), and epigenetic regulatory mechanisms are possible (Pessi et al., Reference Pessi, Viiri, Raitoharju, Astola, Seppala, Waldenberger and Monaco2015). In a family study of roughly 800 individuals, the functional polymorphism of IL-6 explained 2.5% of the heritable component of CIMT (Mayosi et al., Reference Mayosi, Avery, Baker, Gaukrodger, Imrie, Green and Keavney2005). Hulkkonen et al. (Reference Hulkkonen, Lehtimaki, Mononen, Juonala, Hutri-Kahonen, Taittonen and Kahonen2009) and Riikola et al. (Reference Riikola, Sipila, Kahonen, Jula, Nieminen, Moilanen and Hulkkonen2009) did not find any association between CIMT and IL-6 gene polymorphisms in a gene-association study of ~2,000 individuals. Cunnington et al. (Reference Cunnington, Mayosi, Hall, Avery, Farrall, Vickers and Keavney2009) could not demonstrate the association between IL-6 gene polymorphisms (which had been associated with coronary artery disease) and CIMT. Since the IL-10 is a molecule of potent anti-inflammatory character, it may have therapeutic implications in the future through gene therapy and is a subject of animal model studies (Dronadula et al., Reference Dronadula, Wacker, Van Der Kwast, Zhang and Dichek2017; Du et al., Reference Du, Dronadula, Tanaka and Dichek2011).
APOE
Although earlier studies emphasize the role of the E4 genotype of the APOE gene in carotid artery atherosclerosis and the atheroprotective role of the E2 genotype (Humphries & Morgan, Reference Humphries and Morgan2004; Paternoster et al., Reference Paternoster, Martinez-Gonzalez, Charleton, Chung, Lewis and Sudlow2010), large-scale meta-analyses did not confirm the relevance of this gene regarding CIMT. Instead of the APOE gene, the APOC1 gene was associated with CIMT (Bis et al., Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011; Geisel et al., Reference Geisel, Coassin, Hessler, Bauer, Eisele, Erbel and Kronenberg2016). The authors speculate whether the significance of APOE gene may be restricted to early atherosclerosis and familial dyslipidemia (Bis et al., Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011).
ACE
The ACE gene codes the angiotensin-converting enzyme which converts angiotensin I to angiotensin II. A well-known association exists between the enzyme and hypertension. The insertion/deletion (I/D) in the non-coding region of this gene affecting the enzyme activity has been associated with increased CIMT. A meta-analysis including more than 9,800 individuals described the significant positive association between the D-allele and CIMT (Sayed-Tabatabaei et al., Reference Sayed-Tabatabaei, Houwing-Duistermaat, van Duijn and Witteman2003). The association was significant only among Whites in low-risk populations, whereas it was significant among both Asians and Whites in high-risk populations (including symptomatic cerebrovascular disease, type I and II diabetes, non-diabetic hemodialysis, and hypertensive patients), indicating relevant ethnic differences possibly in both genetic and environmental effects regarding this trait (Sayed-Tabatabaei et al., Reference Sayed-Tabatabaei, Houwing-Duistermaat, van Duijn and Witteman2003). The D/D genotype had a low frequency amongst Asians (Sayed-Tabatabaei et al., Reference Sayed-Tabatabaei, Houwing-Duistermaat, van Duijn and Witteman2003). Several other studies with smaller hypertensive populations from both Asian (Park et al., Reference Park, Ahn, Lee and Hong2009) and European (Imbalzano et al., Reference Imbalzano, Vatrano, Quartuccio, Di Stefano, Aragona, Mamone and Mandraffino2017) ancestries found association between the D/D phenotype and increased CIMT. Some other studies found no significant association with this trait (Hung et al., Reference Hung, McQuillan, Nidorf, Thompson and Beilby1999), which may depend on smaller sample sizes or other methodological aspects as an association of opposite direction has not been described (Humphries & Morgan, Reference Humphries and Morgan2004).
Paraoxonase-1 (PON-1)
PON-1 is an enzyme that binds to plasma HDL and protects it from oxidation (Litvinov et al., Reference Litvinov, Mahini and Garelnabi2012). PON-1 may affect CAS through its influence on HDL (Kim et al., Reference Kim, Li, Bell, Burt, Vaisar, Hutchins and Jarvik2016) and LDL particles (Mackness et al., Reference Mackness, Durrington and Mackness1998). The genetic variants and expression of the PON-1 gene is highly dependent on environmental influences, such as smoking, and it has been a subject of research regarding epigenetic effects (Aviram & Vaya, Reference Aviram and Vaya2013). Details regarding PON-1 polymorphisms and CAS have recently been reviewed (Lioudaki et al., Reference Lioudaki, Verikokos, Kouraklis, Ioannou, Chatziioannou, Perrea and Klonaris2017), and therefore, they are not further discussed in this article. Briefly, two polymorphisms of the enzyme gene have been described and their effect on CIMT and carotid plaque formation is controversial (Humphries & Morgan, Reference Humphries and Morgan2004; Lioudaki et al., Reference Lioudaki, Verikokos, Kouraklis, Ioannou, Chatziioannou, Perrea and Klonaris2017).
Cholestryl-esther transfer protein (CETP)
CETP is a protein taking part in the cholesterol transport from HDL to very low-density lipoprotein. The inhibition of CETP increases serum HDL levels, and therefore, it is a potential pharmacological target. Millwood et al. (Reference Millwood, Bennett, Holmes, Boxall, Guo and Bian2018) investigated the association between the loss-of-function CETP variant and CIMT. Furthermore, a genetic risk score was created consisting of the loss-of-function variant and four other CETP variants and its relation to CIMT was studied. No significant associations were found in this study involving more than 20,000 Asian individuals (Millwood et al., Reference Millwood, Bennett, Holmes, Boxall, Guo and Bian2018). Two polymorphisms of the enzyme gene, the TaqIB, and the I405V polymorphisms, have been studied but the results are inconclusive regarding their relation to CIMT (Humphries & Morgan, Reference Humphries and Morgan2004). A meta-analysis conducted on circa 2,200 individuals did not find any association between these polymorphisms and CIMT either in the total population of the meta-analysis, nor in Asians and Europeans separately (Li et al., Reference Li, Jin, Zhu, Chen, Wang, Hu and Zheng2014). Neither TaqIB nor other rare variants showed significant associations with CIMT in a study including 855 patients from different ethnicities, despite the fact that serum HDL and CETP levels depended on CETP polymorphisms (Tsai et al., Reference Tsai, Johnson, Kao, Sharrett, Arends, Kronmal and Post2008).
MTHFR
MTHFR is involved in homocysteine metabolism and the C to T substitution at nucleotide 677 in the MTHFR gene has been associated with lower enzyme activity and higher homocysteine levels (Miyaki, Reference Miyaki2010). Increased homocysteine levels are associated with higher cardiovascular risk (Graham et al., Reference Graham, Daly, Refsum, Robinson, Brattstrom, Ueland and Andria1997). Previous reviews have summarized the findings regarding MTHFR gene polymorphisms (Humphries & Morgan, Reference Humphries and Morgan2004; Juo, Reference Juo2009). The association between the MTHFR gene and homocysteine levels is confirmed, but recent candidate gene studies including less than 1,000 individuals did not confirm the gene's direct effect on CIMT (Hernandez-Socorro et al., Reference Hernandez-Socorro, Rodriguez-Esparragon, Celli and Lopez-Fernandez2017; Pramukarso et al., Reference Pramukarso, Faradz, Sari and Hadisaputro2015; Sun et al., Reference Sun, Song, Liu, Fang, Wang, Wang and Hu2017). Whether hyperhomocysteinemia is directly or indirectly related to atherosclerosis (the latter because of renal dysfunction) is to be elucidated (Durga et al., Reference Durga, Verhoef, Bots and Schouten2004).
Other genes
Further novel findings of large-scale meta-analyses include the role of certain SNPs of the EDNRA gene (coding the endothelin receptor type-1) in multiple carotid artery plaque phenotypes (Bis et al., Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011; Hemerich et al., Reference Hemerich, van der Laan, Tragante, den Ruijter, de Borst, Pasterkamp and Asselbergs2015). Regarding the relation of EDNRA gene to CIMT, the results are controversial (Li et al., Reference Li, Chen, Jiang, Simino, Srinivasan, Berenson and Mei2015; Lopez-Mejias et al., Reference Lopez-Mejias, Genre, Garcia-Bermudez, Ubilla, Castaneda, Llorca and Gonzalez-Gay2014); however, gene–environmental interactions regarding this gene have been studied (Li et al., Reference Li, Chen, Jiang, Simino, Srinivasan, Berenson and Mei2015; Reference Li, Chen, Jiang, Simino, Srinivasan, Berenson and Mei2015). Another gene, PINX1, the product of which is a telomerase inhibitor, was associated with common CIMT in the general population (Bis et al., Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011; Geisel et al., Reference Geisel, Coassin, Hessler, Bauer, Eisele, Erbel and Kronenberg2016; Li et al., Reference Li, Chen, Jiang, Simino, Srinivasan, Berenson and Mei2015), although no similar association was found in a population with rheumatoid arthritis (Lopez-Mejias et al., Reference Lopez-Mejias, Genre, Garcia-Bermudez, Ubilla, Castaneda, Llorca and Gonzalez-Gay2014). The SMG6 gene showed significant association with common CIMT with increased heterogeneity across ethnicities, whereas LPA and TRIB1 loci were significantly associated with internal CIMT (Vargas et al., Reference Vargas, Manichaikul, Wang, Rich, Rotter, Post and Bluemke2016).
In the Asian population, the highly significant association between Early B-cell Factor 1 (EBF1) gene SNPs and CIMT progression was emphasized in a large-scale GWAS study (Xie et al., Reference Xie, Myint, Voora, Laskowitz, Shi, Ren and Wu2015), but other results point toward the epigenetic regulation of this gene and its effect on atherosclerosis (Singh et al., Reference Singh, Babyak, Nolan, Brummett, Jiang, Siegler and Hauser2015). In the same study, another SNP near the procadherin 15 (PCDH15) gene was linked to CIMT progression on a genome-wide significant level (Xie et al., Reference Xie, Myint, Voora, Laskowitz, Shi, Ren and Wu2015), and although other similar SNPs in this region have been identified, the relevance of this SNP is not confirmed.
More recently, the ryanodine receptor 3 (RYR3) gene SNPs and their relation to CAS have been investigated. This gene codes a calcium channel regulating intracellular calcium and inflammatory processes. Two variants of this gene showed an association with subclinical and clinical CAS in a subpopulation of men (Shrestha et al., Reference Shrestha, Irvin, Taylor, Wiener, Pajewski, Haritunians and Grunfeld2010), women (Shendre et al., Reference Shendre, Irvin, Aouizerat, Wiener, Vazquez, Anastos and Shrestha2014), and a population of both sexes (Zhi et al., Reference Zhi, Shendre, Scherzer, Irvin, Perry, Levy and Shrestha2015) suffering from human immunodeficiency virus (HIV) as well as clinical manifestation of CAS in a post-mortem study of Japanese elderly (Zhao et al., Reference Zhao, Ikeda, Arai, Naka-Mieno, Sato, Muramatsu and Sawabe2014).
Bis et al. (Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011) conducted a GWAS meta-analysis on ~25,000, ~11,000, and~10,000 individuals regarding carotid artery plaque, internal CIMT, and common CIMT, respectively. Three SNPs reached genome-wide significance regarding common CIMT, including a SNP near the ZHX2 gene on chromosome 8q24, a SNP near the APOC1 gene on chromosome 19q13, and a SNP within the PINX1 gene on 8q23.1. The first two SNPs were associated with decreased common CIMT. More specifically, the rs11781551 near the ZHX2 gene lowered CIMT by 0.8% per copy of the allele, and the G allele of rs445925 near the APOC1 gene lowered common CIMT by 1.6%. The G allele on the third SNP near the PINX1 gene, rs6601530, was associated with increased common CIMT by 0.08% per allele copy. Two SNPs near the PIK3CG and EDNRA genes were associated with plaque presence on a genome-wide significant level. No SNP achieved genome-wide significant association with regard to internal CIMT (Bis et al., Reference Bis, Kavousi, Franceschini, Isaacs, Abecasis, Schminke and Consortium2011).
Melton et al. (Reference Melton, Carless, Curran, Dyer, Goring, Kent and Almasy2013) investigated SNPs associated with CIMT in 772 Mexican American individuals. No genome-wide significant association was detected, but there were some nominally significant SNPs on chromosome 20p11 near the gene PAX1 and on chromosome 2q21 in the gene NCKAP5 (Nck-associated protein 5) in relation to internal CIMT. Another SNP upstream of EXOC3L2 on chromosome 19q13 was nominally significantly associated with common CIMT. The number of cases was small in this study and therefore the study power was relatively low. There was a genetic correlation of 51% between common and internal CIMT, indicating that the genetic basis of these two segments CIMT is partly overlapping (Melton et al., Reference Melton, Carless, Curran, Dyer, Goring, Kent and Almasy2013).
Shendre et al. (Reference Shendre, Wiener, Irvin, Aouizerat, Overton, Lazar and Shrestha2017) performed genome-wide association and admixture analysis on CIMT-related genes in a population of ~1,000 individuals including HIV-positive and negative African-American female individuals (Shendre et al., Reference Shendre, Wiener, Irvin, Aouizerat, Overton, Lazar and Shrestha2017). None of the SNPs reached genome-wide significance, although some SNPs almost reached this level, such as mediator complex subunit 30 and exostosin glycosyltransferase 1 (MED30 and EXT1) genes on chromosome 8 in all women and in the HIV-positive group, catenin delta 2 (CTNND2) gene on chromosome 5, transmembrane and coiled-coil domain family 3 gene, and the NADH: Ubiquinone oxidoreductase subunit A12 (TMCC3 and NDUFA12) in the HIV-positive group and family with sequence similarity 5, member C, and regulator of G-protein signaling 18 genes (FAM5C and RGS18) on chromosome 1 in the HIV-negative group. CTNND2 and TMCC3 | NDUFA12 were significantly associated with local European ancestry. However, the study power was relatively low because of the number of cases (Shendre et al., Reference Shendre, Wiener, Irvin, Aouizerat, Overton, Lazar and Shrestha2017). The relevance of these novel findings remains to be elucidated.
Other GWAS in Relation to CAS (Genetic Risk Score)
Large-scale studies attempted to find associations between certain risk scores and SNPs previously identified by GWAS.
Den Hoed et al. (Reference den Hoed, Strawbridge, Almgren, Gustafsson, Axelsson, Engstrom and Lind2015) investigated the association between genetic risk score consisting of 45 genes and carotid plaque and CIMT in a meta-analysis involving more than 7,000 individuals. The risk alleles of these 45 loci had previously been associated with coronary heart disease. Carotid bulb CIMT and plaque presence showed significant associations with the genetic risk score, but not CCA IMT (den Hoed et al., Reference den Hoed, Strawbridge, Almgren, Gustafsson, Axelsson, Engstrom and Lind2015). Each risk allele of the 45 loci increased the odds of having plaque by 2.8% and that of having increased CIMT by 0.24% (den Hoed et al., Reference den Hoed, Strawbridge, Almgren, Gustafsson, Axelsson, Engstrom and Lind2015). SNPs near CDKN2B/A were significantly associated with plaque presence, but this large-scale meta-analysis failed to demonstrate any such association between plaque presence and SNPs near EDNRA. Furthermore, additional risk-alleles on chromosome 9p21.3, where CDKN2B/A is located, increased the odds of having plaque with an additional 13.9%. With regard to CIMT, no significant association was observed between the individual loci and CIMT of the carotid bulb or CCA; however, the association between risk alleles near the apolipoprotein gene cluster and common CIMT was confirmed (den Hoed et al., Reference den Hoed, Strawbridge, Almgren, Gustafsson, Axelsson, Engstrom and Lind2015). No association was found between SNPs affecting coronary artery disease and common CIMT in a meta-analysis involving roughly 5,000 individuals (Conde et al., Reference Conde, Bevan, Sitzer, Klopp, Illig, Thiery and Markus2011). The different outcomes regarding CIMT of the CCA and bulb might relate to their associations with different conditions as previously described in the literature, as CCA IMT tends to be associated with stroke whereas carotid bulb IMT tends to reflect the risk for ischemic heart disease (Ebrahim et al., Reference Ebrahim, Papacosta, Whincup, Wannamethee, Walker, Nicolaides and Lowe1999).
Conclusion
Although heritability varies widely regarding carotid artery atherosclerosis traits, moderate additive genetic influence seems to determine the variance in these phenotypes. Future international collaborative twin studies (such as on discordant MZ twins) should elucidate how the different environmental interventions and effects influence this genetic susceptibility in various ethnicities. The co-occurrence of CAS with coronary and peripheral atherosclerosis and other diseases have been certified with twin studies. Based on these findings, new therapeutic targets and preventive individualized strategies may be established. Numerous SNPs have been described to increase the risk for development of subclinical or clinical CAS. However, the results are often conflicting, and only a minority of these genes seems to be potential future therapeutic targets. This warrants the need for future research aiming to obtain a deeper knowledge of the exact pathomechanism of these genetic variants and gene-environmental interactions, which would be essential for practical implications of the enormous amount of genes found in GWAS-studies in relation to CAS.
Conflict of Interest
None.




