Asthma is a prevalent disease among children that has been shown to switch from male to female predominance during adolescence by some (Almqvist et al., Reference Almqvist, Worm and Leynaert2008; Asher et al., Reference Asher, Montefort, Björkstén, Lai, Strachan and Weiland2006; Nicolai et al., Reference Nicolai, Pereszlenyiova-Bliznakova, Illi, Reinhardt and von Mutius2003), but not others (Protudjer et al., Reference Protudjer, Lundholm, Bergstrom, Kull and Almqvist2014). This suggests that events associated with puberty may play a role in the gender switch of asthma (Almqvist et al., Reference Almqvist, Worm and Leynaert2008). An association between asthma and height has also been proposed, but is collectively inconclusive (Hauspie et al., Reference Hauspie, Susanne and Alexander1977; Strunk et al., Reference Strunk, Sternberg, Szefler, Zeiger, Bender and Tonascia2009; The Childhood Asthma Management Program Research Group, 2000). Asthma appears to be associated with shorter height (El-Sayed et al., Reference El-Sayed, Hamza, Sayed and Mahmoud2010; Hauspie et al., Reference Hauspie, Susanne and Alexander1976; Reference Hauspie, Gyenis, Alexander, Simon, Susanne and Madách1979; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003; Umlawska et al., Reference Umlawska, Gaszczyk and Sands2013) and delayed growth (Hauspie et al., Reference Hauspie, Susanne and Alexander1977) during adolescence, although the impact of childhood asthma on final adult height remains less understood (Kelly et al., Reference Kelly, Sternberg, Lescher, Fuhlbrigge, Williams and Zeiger2012; Norjavaara et al., Reference Norjavaara, Gerhardsson de Verdier and Lindmark2001). To date, this work has been done exclusively in singletons. For example, Polish children 5–18 years old with asthma of varying severities and duration of disease were shorter than healthy controls, and nearly 5% of those with asthma were very short in stature (Umlawska et al., Reference Umlawska, Gaszczyk and Sands2013). In a retrospective study of Swedish women, childhood asthma was associated with significantly shorter adult height compared to women without a report of childhood asthma (Norjavaara et al., Reference Norjavaara, Gerhardsson de Verdier and Lindmark2001). However, asthma (Fagnani et al., Reference Fagnani, Annesi-Maesano, Brescianini, D’Ippolito, Medda, Nistico and Stazi2008; Lichtenstein & Svartengren, Reference Lichtenstein and Svartengren1997; Thomsen et al., Reference Thomsen, Kyvik and Backer2008; Reference Thomsen, van der Sluis, Kyvik, Skytthe and Backer2010), pubertal staging (Koenis et al., Reference Koenis, Brouwer, van Baal, van Soelen, Peper, van Leeuwen and Hulshoff Pol2013), menarche (van den Berg et al., Reference van den Berg, Setiawan, Bartels, Polderman, van der Vaart and Boomsma2006), and height (Hemani et al., Reference Hemani, Yang, Vinkhuyzen, Powell, Willemsen, Hottenga and Visscher2013) are highly heritable. For example, a dizygous twin has three-fold greater risk of asthma compared to the general population if the co-twin has asthma, compared to the general population (Thomsen et al., Reference Thomsen, van der Sluis, Kyvik, Skytthe and Backer2010). Among monozygous twins, this risk increases to six-fold (Thomsen et al., Reference Thomsen, van der Sluis, Kyvik, Skytthe and Backer2010).
Twin studies provide an ideal way to gain greater understanding of diseases (Mayo, Reference Mayo2009), such as asthma, that are of genetic and environmental etiology. To this end, and in a cohort of Swedish twins followed prospectively from 8–9 years through 19–20 years, we sought to study the association between asthma and pubertal staging in boys and menarche in girls, and height. We first considered these associations in the entire cohort, and subsequently in same-sex twin pairs discordant for asthma. This approach provided the opportunity to take heritability into account.
Methods
Study Design and Subjects
This analysis is based on the overarching Twin Study of Child and Adolescent Development (TCHAD), described elsewhere (Lichtenstein et al., Reference Lichtenstein, Tuvblad, Larsson and Carlström2007). Twin pairs were eligible if they were born in Sweden between May 1985 and December 1986 and their parents could communicate in Swedish. Starting at 8–9 years of age, twins have been prospectively followed at 13–14 years, 16–17 years, and 19–20 years. At 8–9 years, data were collected solely from the parents (n = 1,339), while data were collected from the twins and their parents at 13–14 years (n = 2,263 and n = 1,063, respectively), 16–17 years (n = 2,369 and n = 1,067, respectively), and from twins at 19–20 years (n = 1,569). The population for this study was 2,658 (49.1% boys) twins (1,329 twin pairs).
Variables
Zygosity for same-sex twin pairs was determined using an algorithm with a 95% probability of correctly classifying same-sex twin as either monozygotic or same-sex dizygotic (Lichtenstein et al., Reference Lichtenstein, Tuvblad, Larsson and Carlström2007).
Asthma status (presence vs. absence), based on validated questions from the International Study of Allergy and Asthma in Childhood (Asher et al., Reference Asher, Keil, Anderson, Beasley, Crane, Martinez and Williams1995) and described elsewhere (Mogensen et al., Reference Mogensen, Larsson, Lundholm and Almqvist2011), was ascertained based on parent-report at 8–9 years and 13–14 years, as well as retrospective parent-report from birth to 8–9 years at 8–9 year assessment. Current asthma prevalence was defined as asthma in the 12 months prior to 8–9 years and 13–14 years. Asthma persistence was also considered. Persistent early childhood asthma was defined as a retrospective parent-report of asthma prior to 8–9 years, and parent-report of asthma at 8–9 years. Persistent late childhood asthma from 8–9 years to 13–14 years was defined as asthma presence at both of these time points. Asthma discordance, based on parent-report of current asthma at 8–9 years, as well as at 13–14 years was considered in same-sex twins only.
Self-reported puberty data, including menarche in girls, were collected at 13–14 years. Boys were queried about skin changes, linear growth spurt, pubic hair growth, voice change, and beard growth. Possible responses for each characteristic above included: not yet started; just started; definitely started; complete/fully developed, with 1–4 points allocated respectively. In keeping with the recommendations from the Petersen index (Petersen et al., Reference Petersen, Crockett, Richards and Boxer1988) and described elsewhere (Protudjer et al., Reference Protudjer, Lundholm, Bergstrom, Kull and Almqvist2014), points associated with the latter three characteristics were summed for each boy. Each boy was then classified into mutually exclusive stages: pre-, early, mid-, late or post-puberty. Due to small sample sizes and a narrow age range at 13–14 years, which yielded little variation in pubertal staging, trichotomous categorization of pubertal staging, as previously used by our group (Protudjer et al., Reference Protudjer, Lundholm, Bergstrom, Kull and Almqvist2014), was not possible in the present study. Thus, a binary outcome of pubertal staging for boys was created from the above-described categories: early (pre- or early puberty) versus late (mid-, late-, or post-puberty). Rather than pubertal staging, girls were classified by self-reported menarche status at 13–14 years. As with boys, sample sizes and a narrow age range at 13–14 years mandated the creation of a binary outcome for pubertal staging. Upon further examination of the data, it became clear that the difference in pubertal staging was driven by menarche (no vs. yes). Further, 21 girls had responded to the menarche question but did not respond to all the questions required for ascertainment of pubertal staging. At 16–17 years, two girls (including one with asthma at 8–9 years) reported being unclear if they had reached menarche; these girls were excluded from further analyses. Parent-report of asthma status at 8–9 years was missing for 20 boys and 13 girls.
Data on height were collected via parent-report (8–9 years, 13–14 years) and twin-report (16–17 years, 19–20 years). Measured and reported heights have been shown elsewhere to be well correlated in similar-aged study populations (Brettschneider et al., Reference Brettschneider, Ellert and Schaffrath Rosario2012; Legleye et al., Reference Legleye, Beck, Spilka and Chau2014; Yoshitake et al., Reference Yoshitake, Okusa, Sasaki, Kunitsugu and Hobara2012). Height data from all waves were reviewed for implausible values. This led to the exclusion of height data at 8–9 years for two twins who had parent-reported heights of <50 cm (range 26–34 cm), and at 16–17 years for 22 twins, who self-reported heights <100 cm (range 0.5–84 cm).
Confounding Variables
Using directed acyclic graphs (Moodie & Stephens, Reference Moodie and Stephens2010), which permit visual description of epidemiological relationships, we identified potential confounders. Based on their associations to asthma, and pubertal staging, menarche or height, age (Nicolai et al., Reference Nicolai, Pereszlenyiova-Bliznakova, Illi, Reinhardt and von Mutius2003) and birth weight (Örtqvist et al., Reference Örtqvist, Lundholm, Carlström, Lichtenstein, Cnattingius and Almqvist2009; Wang et al., Reference Wang, Dinse and Rogan2012) were included as confounders in our regression models for the entire cohort. Age was calculated as the difference between date of questionnaire completion and date of birth. Birth weight for all twins was obtained from the Swedish Medical Birth Register, held by the National Board of Health and Welfare.
Statistical Analyses
Descriptive statistics included n, percentage, and mean ± standard deviation, and tests for normal distribution of height. For the entire cohort, logistic regression was used to analyze associations between exposure variables (current asthma, persistent early- or late-childhood asthma) and dichotomous outcomes. Also, for the entire cohort, linear regression was used for the continuous outcome of height. Standard errors were in both cases estimated using the sandwich estimator for clustered observations, to account for within-pair correlations. For within-pair analyses, that is, analyses with adjustment for everything twins share within pairs, conditional linear regression was used. For within-pair analyses, only birth weight was considered as a confounder, as each twin pair had the same age. Undeterminable zygosity was classified as unknown (n = 17); these participants were subsequently excluded from within-pair analyses.
If the effect of the exposure variable does not differ between the within-pair analyses and those of the entire cohort, then genetic and shared environmental factors contribute little to the association. As the within-pair analysis was not stratified on zygosity, this analysis cannot completely (100%) account for the heritability of asthma, puberty, and height.
For logistic regression analyses, odds ratios (OR) with corresponding 95% confidence intervals (95% CI) are presented. For linear regression and conditional linear regression analyses, means and standard errors, estimated from the regression models at the mean of the covariates, are presented graphically, with estimated coefficients, 95%CI and p-values presented in text. All analyses were stratified by sex. Statistical significance was set at p < .05. Analyses were performed with STATA 11.0 (StatCorp LP, College Station, TX, USA).
Ethical permission was received from Regional Ethical Review Board of the Karolinska Institutet, Stockholm, Sweden.
Results
Characteristics of the 2,658 (1,306 [49.1%] boys) twins included in this study are presented in Table 1. Participants responded to questionnaires at mean ages of 8.7 ± 0.5 years, 13.7 ± 0.5 years, 16.7 ± 0.5 years, and 19.7 ± 0.5 years. At 8–9 years and 13–14 years, asthma prevalence amongst boys was 8.2% and 10.2%, respectively. The corresponding numbers for girls were 4.2% and 4.9%. At 19–20 years, 11.1% of boys and 10.6% of girls had self-reports of asthma. At age 13–14, an approximately equal number of boys were in early versus late puberty and most girls (705/1,046 [66.6%]) had reached menarche.
TABLE 1 Study Population of a Cohort of Twins
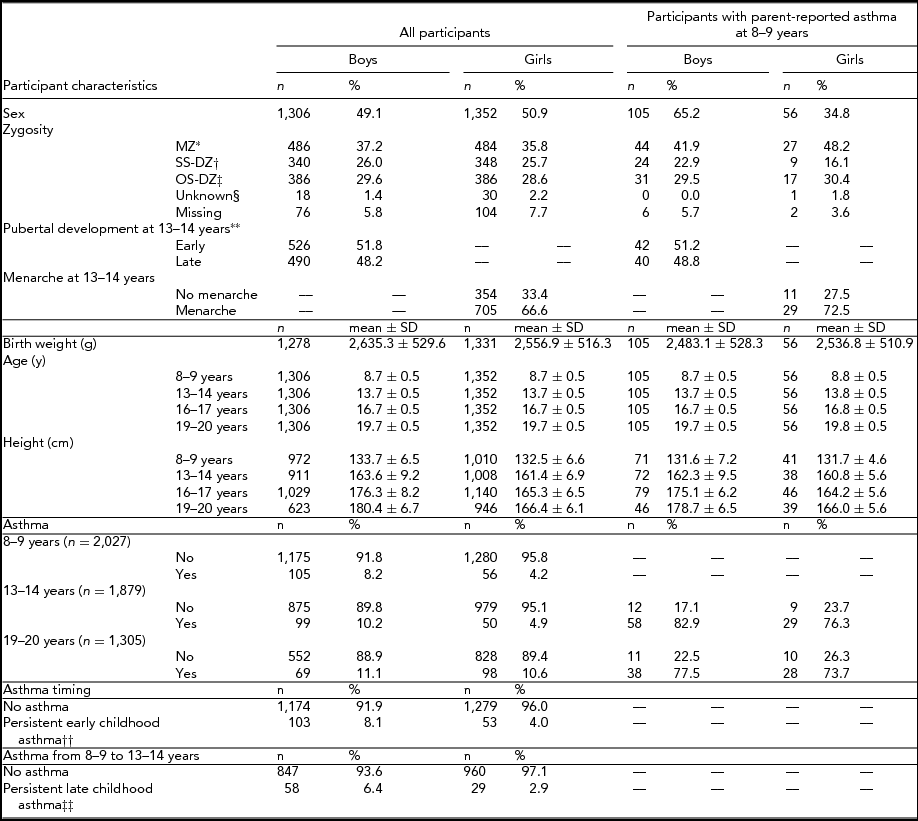
*Monozygotic twins; †Same sex dizygotic twins; ‡Opposite sex dizygotic twins; §Unknown zygosity; **Pre/early versus mid/late/post as per Peterson et al. (Reference Petersen, Crockett, Richards and Boxer1988); ††Retrospective parental report of asthma prior to 8–9 years and parental report of asthma at 8–9 years; ‡‡Parental report of asthma at 8–9 years. and 13–14 years.
In the entire cohort, no statistically significant associations were found between current asthma at either 8–9 years, or 13–14 years, or persistent early- or late-childhood asthma, and self-reported pubertal staging at 13–14 years for boys or menarche in girls (Table 2).
TABLE 2 Unadjusted and Adjusted Odds Ratios for Asthma in Association with Pubertal Staging/Menarche in a Cohort of Twins
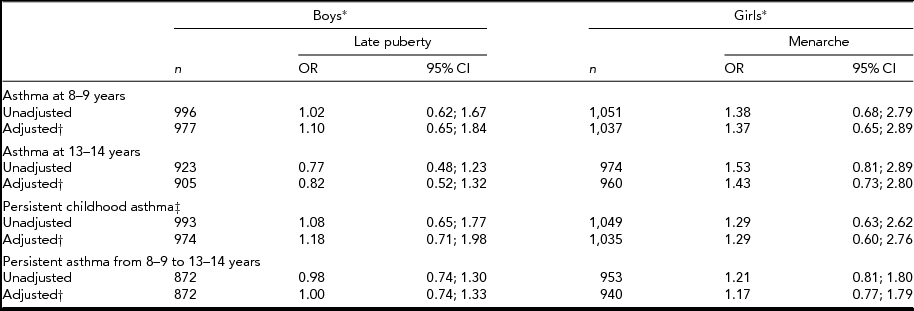
*Reference groups are early puberty (boys) and no menarche (girls); †Adjusted for age at respective visit(s) and birth weight; ‡Based on retrospective and current parent-report of asthma at 8–9 years.
Figure 1 is a presentation of the adjusted estimates of mean height from linear regression for the entire cohort by asthma presence versus absence. Cross-sectionally, boys with asthma were shorter than boys without asthma at 8–9 years (on average, 1.86 [0.17–3.56] cm, p = .03) and at 13–14 years (on average, 2.94 [0.98–4.91] cm, p = .003) but did not reach statistical significance at 19–20 years (on average, 1.74 (-0.08–3.56) cm, p = .06). The corresponding cross-sectional findings for girls did not reach statistical significance at any of these time points (e.g., 8–9 years (0.95 (-0.55–2.46) cm, p = .215). In longitudinal analyses, asthma at 8–9 years was not statistically significantly associated with height differences through 19–20 years. Asthma at 13–14 years was associated with shorter height in boys at 16–17 years (on average 2.12 (0.62–3.62) cm, p = .006), but not with height at 19–20 years. Corresponding numbers for girls did not reach statistical significance (for example: the result for girls with asthma at 13–14 years and height at 16–17 years was 1.15 (-0.82–3.11) cm, p = .254).
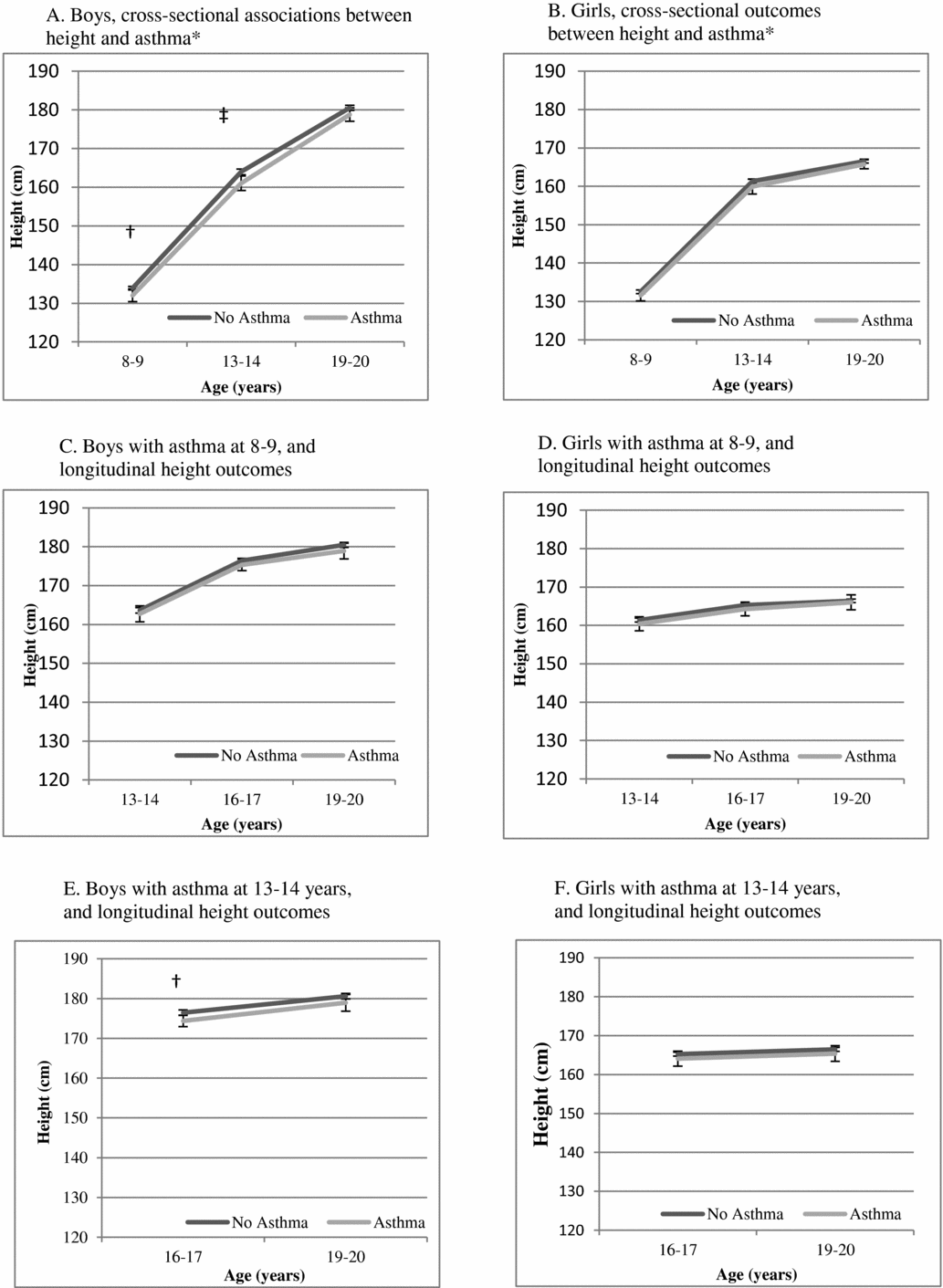
FIGURE 1 Mean height with 95% confidence intervals, estimated from linear regression models with asthma as a predictor of height (cm) from 8–9 years through 19–20 years, adjusted for birth weight and age in a cohort of Swedish twins
Table 3 is a summary of the descriptive characteristics for same-sex twin pairs by asthma discordance. Among same-sex twins, there were 38 and 36 twin pairs of boys who were discordant for asthma at 8–9 years and 13–14 years, respectively. The corresponding numbers for girls were 24 and 20. The numbers for within-pair analyses on pubertal stage were too small for analyses as there were no more 4–6 female pairs and 13–15 male pairs who were discordant for both asthma and puberty stage (Table 3).
TABLE 3 Zygosity, Puberty, and Height in Asthma Discordant Same-Sex Twin Pairs
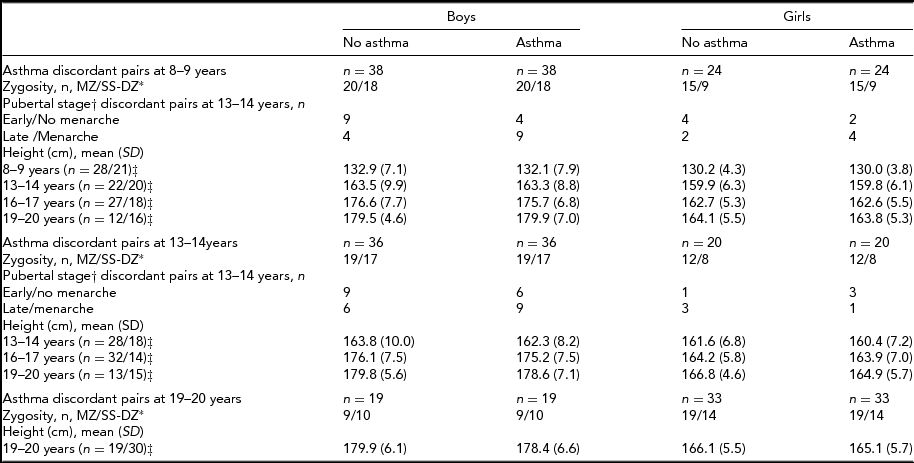
*Monozygotic twins (MZ); Same sex dizygotic twins (SS-DZ); †Pre/early versus mid/late/post as per Peterson et al. (Reference Petersen, Crockett, Richards and Boxer1988) for boys and menarche for girls; ‡ (number of boy pairs/number of girl pairs, with information available for both twins).
Figure 2 is a presentation of the estimated mean heights for same-sex asthma discordant twin pairs, when adjusted for everything the twins have in common within the pair (conditional analyses). In both cross-sectional and longitudinal analyses within pairs, associations between asthma and height were attenuated and not statistically significant for either sex.
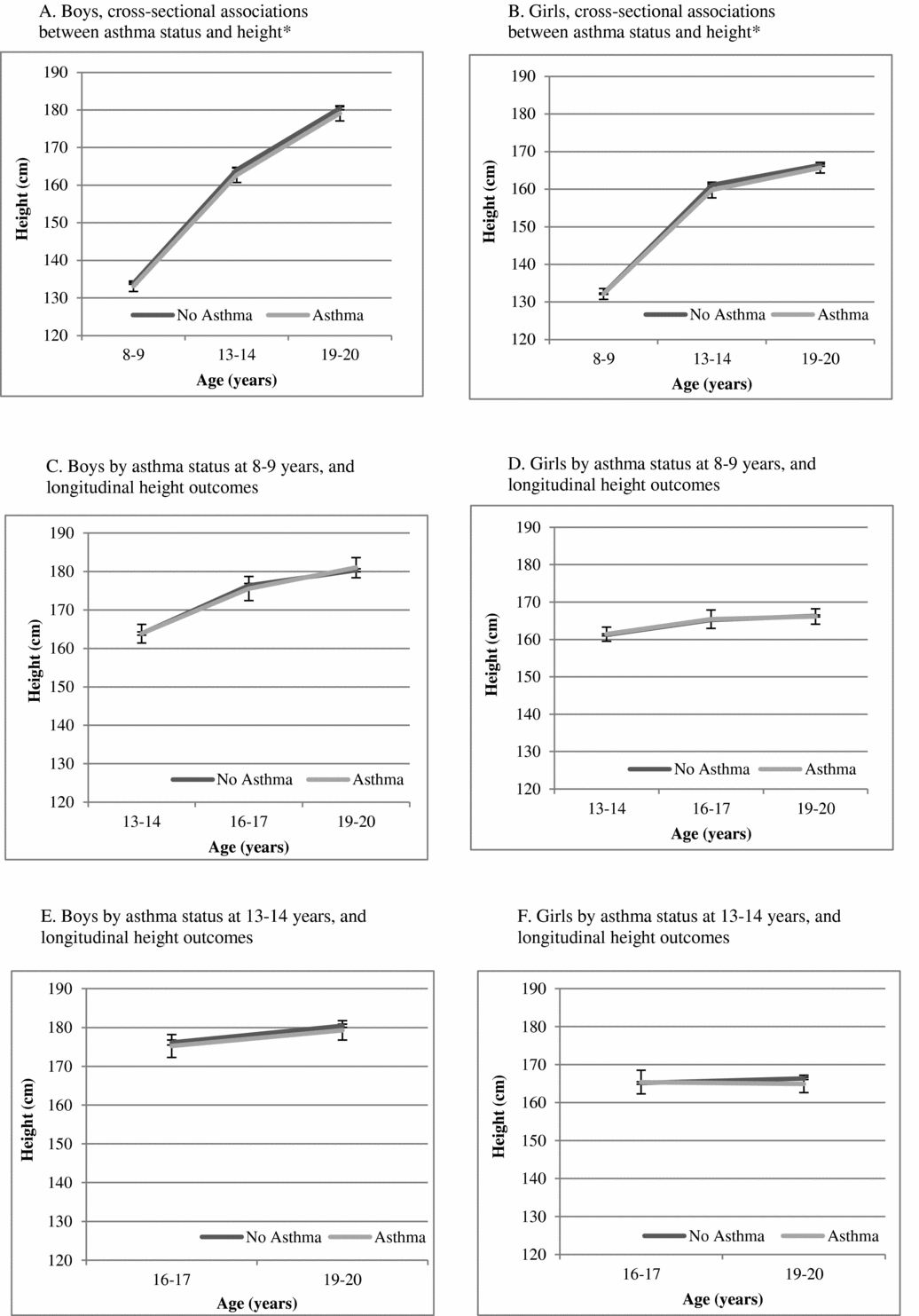
FIGURE 2 Mean height with 95% confidence intervals, estimated from conditional linear regression models with asthma as a predictor of height (cm) from 8–9 years through 19–20 years, adjusted for birth weight, using twin pairs that are discordant for asthma in a sample of same-sex twins.
Discussion
In a cohort of twins, we identified some statistically significant cross-sectional and longitudinal associations between asthma and shorter height among boys but not girls. These differences were attenuated and no longer statistically significant in within-pair analyses in twin pairs discordant for asthma status. Our observations of shorter height among those with asthma compared to those without asthma are consistent with some (El-Sayed et al., Reference El-Sayed, Hamza, Sayed and Mahmoud2010; Hauspie et al., Reference Hauspie, Susanne and Alexander1976; Reference Hauspie, Gyenis, Alexander, Simon, Susanne and Madách1979; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003; Umlawska et al., Reference Umlawska, Gaszczyk and Sands2013), but not all (Kaplan et al., Reference Kaplan, Brush and Mascie-Taylor1987; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003) reports from singletons. But, the use of twin data permits greater insight into the potential causality of associations between asthma and height than was possible in reports from singletons. The estimates from within-pair analyses were attenuated compared to those of the entire cohort, suggesting partial confounding by genes or shared familial environment. In other words, our findings provide modest evidence that differences in height among boys by asthma status may be partly attributable to genes or shared familial environment, rather than asthma. This is interesting, as there has been a concern about the impact of asthma on height for many years (El-Sayed et al., Reference El-Sayed, Hamza, Sayed and Mahmoud2010; Hauspie et al., Reference Hauspie, Susanne and Alexander1976; Reference Hauspie, Susanne and Alexander1977; 1979; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003; The Childhood Asthma Management Program Research Group, 2000; Umlawska et al., Reference Umlawska, Gaszczyk and Sands2013). However, caution is warranted when interpreting the collective results in relation to familial confounding as the confidence intervals from the within-pair analyses are wide enough to cover the estimates from the cohort analysis.
Our null findings between asthma and self-reported pubertal staging in the entire cohort are consistent with some (Neville et al., Reference Neville, McCowan, Thomas and Crombie1996; Skrzypuklec et al., Reference Skrzypuklec, Doniec, Drosdzol, Nowosielski and Pawlinska-Chmara2007), but not all studies (Drosdzol et al., Reference Drosdzol, Wilk and Nowosielski2007; Hauspie et al., Reference Hauspie, Susanne and Alexander1977; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003). An inconclusive pattern in the collective results may be attributable to different methods, such as categorization by age (Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003; Neville et al., Reference Neville, McCowan, Thomas and Crombie1996) or validated scales (Drosdzol et al., Reference Drosdzol, Wilk and Nowosielski2007; Hauspie et al., Reference Hauspie, Susanne and Alexander1977), of establishing pubertal staging. In contrast to newer ways of determining pubertal staging (Gasser et al., Reference Gasser, Molinari and Largo2013), the validated Peterson index, which we applied to our data on boys, does not include information on height to establish pubertal staging (Petersen et al., Reference Petersen, Crockett, Richards and Boxer1988).
The adolescent growth spurt is a marker of sexual maturation (WHO Expert Committee on Physical Status, 1995). Boys reach their pubertal debut at approximately 11.5 years, approximately a year later than girls. Boys’ progression through puberty lasts for ~3.5 years, and is shorter than that for girls (~4.2 years). For both boys and girls, the growth spurt begins in puberty and is completed by late puberty. On average, girls reach their growth spurt by ~12 years and boys reach their growth spurt 2 years later (WHO Expert Committee on Physical Status, 1995). In our study, self-reported pubertal staging was during the 13–14 year assessment, the age at which boys begin to reach their growth spurt, but 1–2 years after girls would have reached their growth spurt on average. In cross-sectional analyses of asthma and height in the entire cohort, boys with asthma were significantly shorter than boys without asthma at 8–9 years and at 13–14 years, but did not differ significantly by 19–20 years. Similar to some previous reports on 13- to 14-year-old boys (Hauspie et al., Reference Hauspie, Susanne and Alexander1976; Reference Hauspie, Susanne and Alexander1977; 1979), and in keeping with those of girls (Drosdzol et al., Reference Drosdzol, Wilk and Nowosielski2007), 13-to 14-year-old boys, but not girls with asthma in the entire cohort were statistically significantly shorter than their same-sex peers without asthma. No statistically significant differences between asthma and height were found for girls in either cross-sectional or longitudinal analyses in the entire cohort or within-pair analyses. One may speculate that the null findings at 8–9 years and 13–14 years may, in part, be attributable to a low prevalence of asthma among girls at these ages, thereby potentially limiting our ability to identify statistically significant observations. By 19–20 years, more than twice as many girls reported asthma than at the previous time points. As such, we would have anticipated that differences in height by asthma status at 19–20 years would have been identified if such differences existed. From adolescence onward, girls typically (Almqvist et al., Reference Almqvist, Worm and Leynaert2008; Nicolai et al., Reference Nicolai, Pereszlenyiova-Bliznakova, Illi, Reinhardt and von Mutius2003) but not always (Protudjer et al., Reference Protudjer, Lundholm, Bergstrom, Kull and Almqvist2014) have a higher prevalence of asthma than in childhood. Girls with asthma in adolescence often have a more severe disease phenotype than boys, as evidenced by higher emergency room visits and hospitalization rates (Akinbami et al., Reference Akinbami, Moorman and Liu2011). Although our data precluded consideration to disease severity, disease severity is associated with a greater odds of asthma persistence (Tai et al., Reference Tai, Tran, Roberts, Clark, Gibson, Vidmar and Robertson2014) and oral steroid use (Schofield, Reference Schofield2014), both of which have been associated with delayed attainment of adult height (Martin et al., Reference Martin, Landau and Phelan1981).
Within-pair analyses of asthma and height yielded a pattern of results that was attenuated compared to that of the entire cohort. Taken collectively, the differences in the results of analyses of the entire cohort and within-pair analyses suggests that the association between asthma and height is confounded by genetic and/or shared environmental factors.
We acknowledge the weaknesses of our study. First, height was ascertained by parent-reports at 8–9 years and 13–14 years, and by twin-report at 16–17 years and 19–20 years. Although measured height is the gold standard, this was impractical in a large cohort such as TCHAD. Importantly, others have shown a strong correlation between reported and measured heights (Brettschneider et al., Reference Brettschneider, Ellert and Schaffrath Rosario2012; Legleye et al., Reference Legleye, Beck, Spilka and Chau2014; Yoshitake et al., Reference Yoshitake, Okusa, Sasaki, Kunitsugu and Hobara2012) in studies of children of similar ages to the twins in the present study. Second, small sample sizes precluded consideration of asthma medication or stratification by zygosity. Nonetheless, our approach permitted adjustment for at least 50% of segregating genes, which all dizygotic twins, and indeed all siblings share. Moreover, twins share a common environment from conception and through childhood. Third, although pubertal development exists along a continuum, we were only able to establish a dichotomous variable for pubertal staging, thereby limiting the sensitivity of our pubertal staging classification.
To our knowledge, this is one of the first reports on asthma and height using a twin design. Our findings in the entire cohort correspond well with the associations of asthma and height in singletons (El-Sayed et al., Reference El-Sayed, Hamza, Sayed and Mahmoud2010; Hauspie et al., Reference Hauspie, Susanne and Alexander1976; Reference Hauspie, Gyenis, Alexander, Simon, Susanne and Madách1979; Moudiou et al., Reference Moudiou, Theophilatou, Prifits and Papadimitriou2003; Umlawska et al., Reference Umlawska, Gaszczyk and Sands2013). Thus, our results both are generalizable to singletons and extend the understanding of asthma and height.
Conclusion
In a cohort of Swedish twins, we identified that twin boys, but not girls with asthma were of shorter height than those without asthma. Estimates from within-pair analyses were attenuated and non-significant, suggesting the association is partly confounded by genetic or familial environmental factors.
Acknowledgments
We acknowledge financial support from the Swedish Research Council (Grant no. 2011-3060) and through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework Grant no. 340-2013-5867, the Strategic Research Program in Epidemiology at Karolinska Institutet, the Swedish Heart Lung Foundation (Contract no. 20120480), the Swedish Asthma and Allergy Association (Grants no. 2010048-K, 2011051-K, 2012056-K, and 2013049-K) and the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (ALF; Contract no. 20130422 and 20140322). The Swedish Twin Registry was financially supported by the Swedish Ministry of Education and Research. Jennifer Protudjer was first supported by European Respiratory Society Long Term Research Fellowship no. 117-2011, then a Canadian Institutes of Health Research Post-Doctoral Fellowship during this project.







