Depression during pregnancy is a major public health concern. It is highly prevalent and causes considerable suffering and impairment to the mother and has possible adverse consequences for the newborn. Reference Zuckerman, Amaro, Bauchner and Cabral1-Reference Taylor, Paton and Kapur4 Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed antidepressants during pregnancy Reference Taylor, Paton and Kapur4 and until recently were considered safe in this period. Reference Wen, Yand, Garner, Fraser, Olatunbosun and Nimrod5 However, database and case-control studies have reported an association between SSRIs and anencephaly, craniosynostosis, omphalocele and persistent pulmonary hypertension in newborn children, although these associations have not been replicated in other studies. Reference Taylor, Paton and Kapur4,Reference Wogelius, Norgaad, Gislum, Pedersen, Munk and Mortensen6 First-trimester exposure to paroxetine has been associated with cardiovascular malformations in some studies, Reference Kallen and Olausson7,Reference Berard, Ramos, Rey, Blais, St-Andre and Oraichi8 however, other studies have failed to replicate this finding. Reference Taylor, Paton and Kapur4,Reference Alwan, Reefhuis, Rasmussen, Olney and Friedman9
We have conducted a meta-analysis with the aim of examining the suggested association between the use of paroxetine during pregnancy and the risk of cardiovascular defects in newborn children.
Method
We used the search engine Dialog™ (formerly, DataStar®) provided by the National Library of Health that includes the following databases: PubMed, Embase, PsycINFO, Social Sciences Citation Index (SSCI), King's Fund, DH-Data, CINAHL, Allied and Complementary Medicine Database (AMED) and British Nursing Index (BNI). Combinations of the terms ‘SSRI’, ‘selective serotonin reuptake inhibitor(s)’, ‘SRI’, ‘serotonin reuptake inhibitors’, ‘paroxetine’, ‘pregnancy’, ‘congenital malformation(s)’, ‘congenital defect(s)’, ‘cardiovascular malformation(s)’, ‘cardiac defect(s)’, ‘cardiovascular defect(s)’, ‘fetal malformation(s)’ and ‘fetal anomalies’ were used for the search. The search was restricted to articles published in English but there was no exclusion on the basis of country, ethical approval, etc. No grey literature was searched for this review. Each abstract/title and article was scrutinised by two of the authors (N.P. and R.P.) and the differences between them were resolved by consensus. Relevant articles were hand-searched for cross-references. The GlaxoSmithKline website was searched for recent data on paroxetine. To exclude repetitive data-sets, only the study with the most updated data was taken up for analysis. A repeat data search was done in August 2012, after the first review of this article, and results were updated.
Inclusion and exclusion criteria
We included studies that met the following criteria:
-
1 use of SSRIs in the first trimester of pregnancy, with separate data available for paroxetine
-
2 control group of unexposed women available for comparison
-
3 as an outcome, separate data available for congenital cardiovascular defects in newborns, for instance conotruncal heart defects, septal heart defects, ventricular outflow tract obstruction.
Exclusion criteria were:
-
1 papers published on repeat data
-
2 studies with no control group for comparison
-
3 no cardiovascular defect in both study and control group.
Excluded studies are presented in online Table DS1.
The modified QUOROM Flow Chart Reference Mohler, Cook, Eastwood, Olkin, Rennie and Stroup10 (Fig. 1) was used to show the study search process.

Fig 1 Modified QUORON flow chart Reference Mohler, Cook, Eastwood, Olkin, Rennie and Stroup10 describing the search process.
Outcome measure
The outcome measure for this review was cardiovascular malformation in the newborn.
Data collection and analysis
We collected data from the studies that met the selection criteria. The quality of studies was assessed by criteria adapted from Centre for Reviews and Dissemination guidelines. 11 Descriptive data were mainly expressed in actual numbers of exposed mothers and controls. Where exact numbers were not available, frequencies were changed into actual numbers (described odds ratios (ORs) were used to resolve doubts). Results were presented in terms of risk ratio (RR) with 95% confidence intervals. A funnel plot was used to assess publication bias and heterogeneity among studies was analysed by the χ2-test. A random-effect model was applied to combine the data. Subgroup analysis was carried out for cohort and case-control studies separately. Sensitivity analysis was carried out by the sequential removal of studies with maximum weight. Data analysis was performed with Review Manager (RevMan 5.0) for Windows. A checklist recommended by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie12 was used.
Results
The systematic search identified 29 relevant studies. Only 11 studies Reference Wogelius, Norgaad, Gislum, Pedersen, Munk and Mortensen6,Reference Berard, Ramos, Rey, Blais, St-Andre and Oraichi8,Reference Alwan, Reefhuis, Rasmussen, Olney and Friedman9,Reference Bakker, Kerstjens-Frederikse, Buys, de Walle and de Jongvan den Berg13-Reference Malm, Klaukka and Newvonen19,Reference Vial, Bernard, Carlier, Jonville-Bera, Jean-Pastor and Barjhoux21 could be included in the analysis, 7 cohort Reference Wogelius, Norgaad, Gislum, Pedersen, Munk and Mortensen6,Reference Davis, Rubanowice, McPhillips, Raebal, Andrade and Smith14-Reference Reis and Kallen17,Reference Malm, Klaukka and Newvonen19,Reference Vial, Bernard, Carlier, Jonville-Bera, Jean-Pastor and Barjhoux21 and 4 case-control studies Reference Berard, Ramos, Rey, Blais, St-Andre and Oraichi8,Reference Alwan, Reefhuis, Rasmussen, Olney and Friedman9,Reference Bakker, Kerstjens-Frederikse, Buys, de Walle and de Jongvan den Berg13,Reference Louik, Angela, Werler, Hernandez-Diaz and Mitchell18 (Table 1). The total number of individuals included in the meta-analysis was 4514 in the paroxetine group and 1 469 302 in the control group.
Table 1 Characteristics of included studies

| Study | Design and quality a | Description of study | Study group | Control group | Results | Comments |
|---|---|---|---|---|---|---|
| Alwan et al Reference Alwan, Reefhuis, Rasmussen, Olney and Friedman9 | D | Data from National Birth Defects Prevention Study (USA) | 9622 infants with major birth defects | 4092 infants with no major birth defects | No significant association between maternal use of SSRIs and congenital heart defects | Odds ratio adjusted for race/ethnicity, obesity, smoking and income |
| Bakker et al Reference Bakker, Kerstjens-Frederikse, Buys, de Walle and de Jongvan den Berg13 | D | Birth defects registry (The Netherlands) | 678 infants with isolated heart defects | 615 controls with a genetic disorder with no heart defect | Paroxetine associated with increased risk of atrium septum defects | No increased risk for heart defects overall |
| Berard et al Reference Berard, Ramos, Rey, Blais, St-Andre and Oraichi8 | D | Data from Quebec Pregnancy Registry (Canada) Women on antidepressants during first trimester (excluding those on known teratogens) were included | 101 infants with major congenital malformations | 1302 infants without congenital malformations | Exposure to paroxetine above 25 mg/day associated with major congenital and cardiac malformations | Odds ratio adjusted for gestational and maternal age, diabetes, hypertension, depression, medications, number and types of antenatal visits and other sociodemographic variables |
| Davis et al Reference Davis, Rubanowice, McPhillips, Raebal, Andrade and Smith14 | C | Pregnancy outcomes from five managed-care organisations (USA) | 1441 full-term infants exposed to antidepressants | 49 663 full-term infants not exposed to antidepressants | SSRIs and tricyclic antidepressants did not have a consistent link with congenital anomalies 182 infants exposed to paroxetine did not have an increased risk of cardiac septal defects |
Controls could be on other possible teratogenic medicines, no adjustment for confounders |
| Diav-Citrin et al Reference Diav-Citrin, Shechtman, Weinbaum, Wajnberg, Avgil and Di Gianantonio15 | A | Teratology information services from Israel, Italy and Germany | 410 first-trimester paroxetine-exposed pregnancies | 1467 women on non-teratogenic drugs | Twofold increase in overall rate of congenital anomalies in paroxetine group Main risks applied to cardiovascular anomalies |
After adjusting for various confounders significance disappeared |
| Einarson et al Reference Einarson, Pistelli, DeSantis, Malm, Paulus and Panchaud16 | A | From teratology information centres around the world | 1174 infants exposed to paroxetine | Equal number of demographically and clinically matched women on non-teratogenic drugs | The rates of cardiac defects in the paroxetine group and in the unexposed group were both 0.7% (odds ratio 1.1, 95% CI 0.36–2.78) | For meta-analysis actual numbers were derived from frequency and odds ratio |
| Reis & Kallen Reference Reis and Kallen17 | C | Swedish Medical Birth Register | 15 017 infants exposed to antidepressants | General population | Association between paroxetine and congenital heart defects was verified | Adjustments were made for year of delivery, maternal age, parity, smoking and BMI |
| Louik et al Reference Louik, Angela, Werler, Hernandez-Diaz and Mitchell18 | D | Slone Epidemiology Center Birth Defects Study (USA) | 9849 infants with birth defects | 5860 infants without birth defects | Sertraline and paroxetine significantly associated with cardiac defects | Reference group was all the women not exposed to any antidepressants Odds ratio adjusted for maternal age, race/ethnicity, education, year of last menstrual period, study centre, smoking, alcohol, history of birth defect in first-degree relative, BMI, parity, seizure, diabetes, hypertension, infertility and folic acid use |
| Malm et al Reference Malm, Klaukka and Newvonen19 | C | Finnish data | 1782 women with at least one purchase of SSRI Women with chronic illnesses were excluded |
1782 matched controls, as per year of pregnancy, age, geographic area and social status with no drug purchase | Major malformations were not more common in infants of women with SSRI purchase | For meta-analysis, data for paroxetine were extrapolated from Einarson et al Reference Einarson, Pistelli, DeSantis, Malm, Paulus and Panchaud20 |
| Vial et al Reference Vial, Bernard, Carlier, Jonville-Bera, Jean-Pastor and Barjhoux21 | A | French data | 500 women exposed to paroxetine | 500 controls | Incidence of major malformations was 3.6% after paroxetine exposure, compared with 1.8% (RR = 2.03, 95% CI 0.79–5.58) Two major cardiac malformations in each group |
Only abstract is available |
| Wogelius et al Reference Wogelius, Norgaad, Gislum, Pedersen, Munk and Mortensen6 | C | Data from Danish Medical Birth Registry | 1051 women who filled prescription for SSRIs | Reference cohort of 150 780 women with no SSRI prescriptions | Increased risk of congenital malformations after exposure to SSRIs Among offspring of SSRI users, 1.4% had cardiovascular malformation (1% in controls) |
Relative risk adjusted for smoking, birth order, maternal age, birth year, county and prescriptions for anti-epileptics, NSAIDs and antidiabetics Data for paroxetine were extrapolated from Einarson et al Reference Einarson, Pistelli, DeSantis, Malm, Paulus and Panchaud20 |
BMI, body mass index; NSAIDs, non-steroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
a Adapted from Centre for Reviews and Dissemination guidelines: 11 A (highest quality): cohort (prospective study) with concurrent controls, B: cohort (prospective study) with historical controls, C: cohort (retrospective study) with concurrent controls, D: case–control (retrospective) study, E: observational study without control groups or large differences from comparisons between times and/or places.
Quality analysis
As shown in Table 1, the studies that met the selection criteria were from all grades except grade B and the lowest grade E on the Centre for Reviews and Dissemination hierarchy of observational studies. 11
Publication bias
The funnel plot (Fig. 2) shows the relative absence of small-sample sized studies which showed teratogenic effect of paroxetine. In trim-and-fill analysis, three studies on the left side of the plot were trimmed, but the adjusted risk ratio for the main analysis remained significant (RR = 1.23, 95% CI 1.05-1.42).
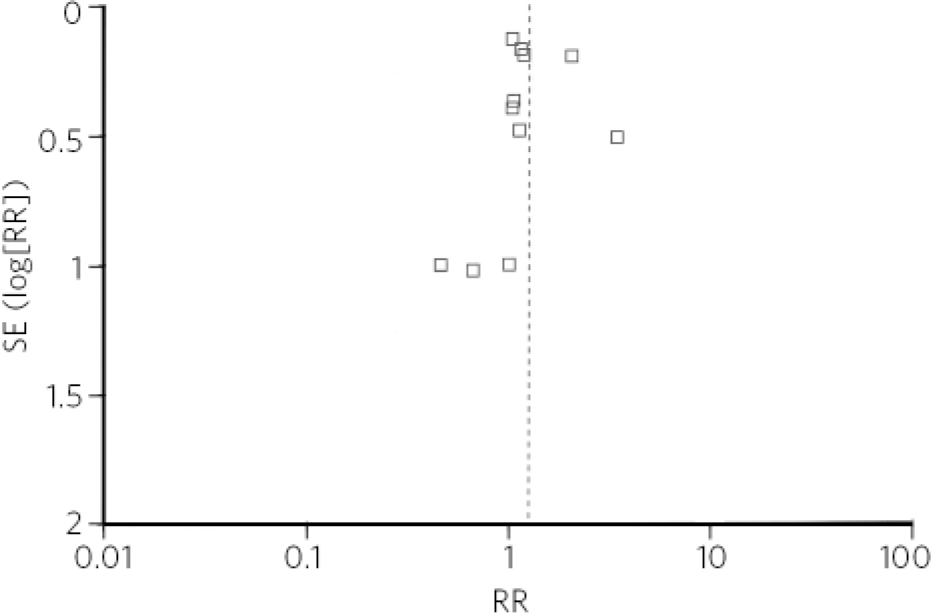
Fig 2 Funnel plot of studies included in the meta-analysis. RR, risk ratio; SE, standard error.
Test of heterogeneity
Examination of the χ2 distribution showed that there was significant heterogeneity between the studies included in the main analysis (Q = 14.34, d.f. = 10, P = 0.1). In the subgroup analysis, there was no significant heterogeneity within case-control (Q = 0.4, d.f. = 3, P = 0.9) and cohort (Q = 8.22, d.f. = 6, P = 0.2) studies.
Pooled results
Paroxetine use in the first trimester of pregnancy was found to be significantly associated with cardiovascular malformations, compared with unexposed controls (RR = 1.25, 95% CI 1.01-1.54) (Fig. 3).
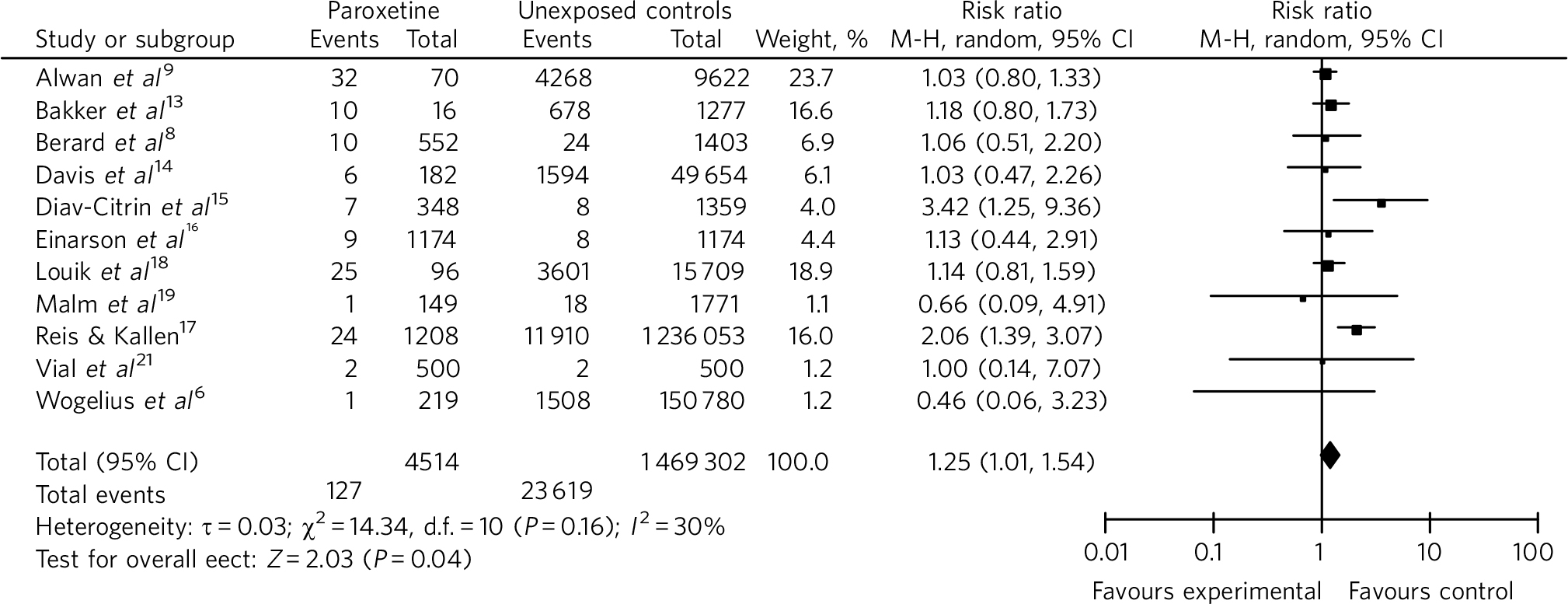
Fig 3 Risk of cardiovascular malformations with first-trimester use of paroxetine in comparison with unexposed controls (forest plot). M-H, Mantel-Haenszel method.
Subgroup analysis
Risk of cardiovascular malformation with paroxetine group became non-significant when data were pooled separately for case-control (RR = 1.09, 95% CI 0.91-1.30) and cohort (RR = 1.52, 95% CI 0.98-2.34) studies.
Sensitivity analysis
In sequential removal of studies with maximum effect sizes, the difference between paroxetine and the unexposed control remained significant after excluding the studies by Alwan et al Reference Alwan, Reefhuis, Rasmussen, Olney and Friedman9 and Louik et al Reference Louik, Angela, Werler, Hernandez-Diaz and Mitchell18 (RR = 1.38, 95% CI 1.02-1.86). Individually, exclusion of studies by Bakker et al Reference Bakker, Kerstjens-Frederikse, Buys, de Walle and de Jongvan den Berg13 (RR = 1.27, 95% CI 0.98-1.64), Louik et al Reference Louik, Angela, Werler, Hernandez-Diaz and Mitchell18 (RR = 1.28, 95% CI 0.98-1.66) or Reis & Kallen Reference Reis and Kallen17 (RR = 1.11, CI 0.94-1.31) made the pooled result non-significant.
Discussion
The validity of meta-analysis of observational studies has always been debated, as observational studies are more prone to biases when compared with the gold-standard randomised controlled trials. Reference Spitzer22 However, a meta-analysis of observational studies seems justified for assessing the teratogenic effect of medications used during pregnancy because experimental studies cannot be conducted and large samples are required to observe rare events such as specific congenital malformations. In recognition of the limitations of meta-analysis of observational studies, we applied a random-effect model (rather than a fixed-effect model) to combine the results, as it can be applied irrespective of the level of heterogeneity of studies. Combining case-control and cohort studies is a well-recognised practice in meta-analysis of epidemiological studies, Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie12,Reference Schlesselman23 although we also carried out a subgroup analysis for case-control and cohort studies separately. Further, we performed a sensitivity analysis to assess the robustness of results. For quality analysis of the studies, the key components of design were considered, as this method has been found to be more appropriate for meta-analysis of observational studies. Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie12 In general, the study met the requirements of the MOOSE guidelines. Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie12
Although more than half of the identified studies were excluded from the analysis, most of them presented repeat data; thus, the combined results can be taken as a fair representation of the identified studies. There may be some doubts as to the reliability of actual numbers, as in some studies numbers were extrapolated from the frequencies and odds ratios; however, this should not affect the results considerably bearing in mind the large size of the collective sample. The apparent discrepancy between sample size and weight for each study (Fig. 1) corroborates the fact that in meta-analysis, weight given to a particular study depends not only on the sample size, but also on the variance of the data.
Underrepresentation of positive studies with small sample size in publication bias analysis could be a reflection of Type II error, a likely outcome in view of the rarity of the occurrence of cardiovascular defects. The trim-and-fill analysis only confirmed the limitation of this method, as it does not take into account the reasons for funnel plot asymmetry other than publication bias.
Our meta-analysis, based on largest collective data sample so far, suggests that offspring of women who are exposed to paroxetine in the first trimester of pregnancy are at a small but significant increased risk of cardiovascular malformations. However, subgroup analysis and sensitivity analysis shows the fragility of this association. It is also possible that the borderline significant results of our meta-analysis could disappear, if the crude numbers used for the combined analysis were adjusted for various confounders such as maternal age, race, smoking, medical comorbidities, concomitant use of possible teratogens, etc.
Results of our meta-analysis fall in line with two other meta-analyses. Reference O'Brien, Einarson, Sarkar, Einarson and Koren24,Reference Wurst, Poole, Ephross and Olshan25 O'Brien et al Reference O'Brien, Einarson, Sarkar, Einarson and Koren24 separately analysed three case-control (n = 30 247) and six cohort (n = 66 409) studies and they did not find any significant association of cardiac malformation with paroxetine exposure. On the other hand, meta-analysis by Wurst et al Reference Wurst, Poole, Ephross and Olshan25 combined ten cohort and four case-control studies (n = 109 958) and found an increased prevalence of cardiac defects with first-trimester paroxetine use (OR = 1.46, 95% CI 1.17-1.82). Whether it is the large sample size which overcomes Type II error and exposes the teratogenic potential of paroxetine or too much heterogeneity (for the sake of large sample size) that brings spurious association remains debatable. In future, an analysis with large but more homogeneous data might provide the answer. In the meantime, our meta-analysis suggests that there is a possibility that exposure to paroxetine could be significantly associated with cardiovascular malformations and in that sense it supports the existing guidelines, Reference Taylor, Paton and Kapur4,26 which advise avoiding paroxetine use in early pregnancy.







eLetters
No eLetters have been published for this article.