Mania or a manic episode is a severe phase in the course of bipolar disorders, the condition termed ‘manic—depressive illness’ by Kraepelin in the 19th century. A contemporary textbook described the difficulty of managing a patient with mania — using drugs of limited efficacy, mainly sedative or anaesthetic, such as chloral and urethane, or potentially toxic drugs, such as potassium bromide. The sedative anticonvulsant paraldehyde was also used. Without sedation the patient would need three or four attendants, skilled in ‘a certain kind of tact’, and spacious, safe accommodation (Reference CloustonClouston, 1896). Thomas Clouston stated the need for a drug in mania as follows: “what we want and have not yet got is a medicine that will cause really natural, restful, refreshing sleep, and one that will stay or slacken the morbid energising of the brain cells in the convolutions, without affecting the appetite or the nutrition.”
Fifty years later the use of bromides remained extensive, but barbiturates such as amylobarbitone (amobarbital) were in use, and paraldehyde remained popular (Reference Sargant and SlaterSargant & Slater, 1946). Electroconvulsive therapy (ECT) was also being used in mania, following its introduction in 1935.
The situation changed dramatically, first in 1949 when John Cade in Australia discovered the effects of lithium in mania (Reference CadeCade, 1949), and in 1952 with the development of the first antipsychotic phenothiazine, chlorpromazine. It remained to Schou, working in Aarhus, Denmark, to establish the use of lithium in mania on the basis of a controlled study (Reference Schou, Juel-Nielson and StromgrenSchou et al, 1954), and by 1968 the prophylactic efficacy of lithium in bipolar disorder was established. Cade's discovery can be regarded as the beginning of the modern era of effective drug treatment in psychiatry (Reference ShorterShorter, 1997).
By 1963, the use of lithium in mania was recommended for difficult cases, but haloperidol was considered particularly useful (Reference Sargant and SlaterSargant & Slater, 1963). However, experience over the following 30 years indicated that lithium is far short of an ideal treatment, even when administered expertly to ‘concordant’ patients. There is a striking difference between the results of early controlled trials and the outcome on lithium in open clinical practice, where a smaller proportion of patients appear to be ‘lithium responders’; some even doubt the validity of the clinical trials (Reference MoncrieffMoncrieff, 1997).
The benefits of lithium therapy have to be balanced against the difficulties in its management — with poor compliance in patients with bipolar disorder and the occurrence of withdrawal mania — and the risks of side-effects and toxicity (Reference CooksonCookson, 1997). The latter includes the possibility of permanent neurological sequelae with cerebellar damage (Reference SchouSchou, 1984).
Since the 1970s anticonvulsant drugs, first carbamazepine and more recently valproate and, in open studies, lamotrigine and topiramate, have displayed antimanic effects in at least a proportion of patients (Reference Dunn, Frye and KimbrellDunn et al, 1998).
MODE OF ACTION OF LITHIUM AND THE MOOD STABILISERS
Lithium has numerous effects on biological systems, especially at high concentrations (see Reference Wood and GoodwinWood & Goodwin, 1987). As the smallest alkaline cation, lithium can substitute for sodium, potassium, calcium and magnesium in biological systems. It penetrates cells via sodium and other channels but is extruded less efficiently than sodium by the sodium—potassium active transport system. Thus, the plasma—cell ratio for lithium is much lower than that for sodium. Within the cell, lithium interacts with systems that normally involve other cations, including transmitter release and second-messenger systems, such as adenylate cyclase, the phosphoinositol cycle and protein kinase C; these actions effectively block particular transmitters and hormones. Receptor upregulation is also reduced, perhaps accounting for the value of lithium as an adjunct to other drugs. One of the major sites of action of anticonvulsant drugs is the membrane ionic channels for conductance of cations — sodium, calcium and potassium; these control the electrical excitability of neurons and the release of transmitters (Reference UptonUpton, 1994). Because they show selectivity for particular channels, anticonvulsant drugs may alter cation function in a more specific way than lithium. High doses of antipsychotic drugs can also interfere with these channels, and their actions could be quantified in the same way as receptor-blocking affinities are (Task Force, 1991).
RECENT PRACTICE
The recognition of the shortcomings of lithium has required a reconsideration of the role of antipsychotic agents and of the so-called ‘mood stabilisers’ (lithium and the anticonvulsants) in bipolar disorder.
It was noted that while doses of antipsychotic drugs given to psychiatric inpatients in a private university centre did not increase from 1989 to 1993, there was a doubling (to 84%) in the proportion of cases prescribed adjunctive treatment with anticonvulsants and a rise to 70% in the number receiving adjunctive lithium (Reference Baldessarini, Kando and CentorrinoBaldessarini et al, 1995).
The preference of American (New York) psychiatrists for high-potency antipsychotics in mania was illustrated in a study by Chou et al (Reference Chou, Zito and Vitral1996) of 528 patients with mania in New York state hospitals in 1990. Ninety-two per cent received antipsychotic medication, 61% lithium, 54% both and 22% benzodiazepines — mainly lorazepam — during the first 3 weeks of hospitalisation for mania. Eleven per cent received an anticonvulsant. Haloperidol (38% of prescriptions) was the most widely used antipsychotic drug, followed by fluphenazine (20%) and chlorpromazine (18%).
SELECTION OF PATIENTS
Other papers in this supplement indicate the shortcomings of mood stabilisers as monotherapy for bipolar disorder (see Reference Calabrese, Rapport and SheltonCalabrese et al, 2001, this supplement). The seminal paper by Bowden et al (Reference Bowden, Brugger and Swann1994) comparing valproate with lithium or placebo in a 3-week parallel-group, double-blind study provides the strongest evidence for the use of valproate in mania, but also demonstrates the problem. The study enrolled 179 patients, half of whom had been unresponsive to lithium previously. The proportion of patients showing 50% improvement on the mania rating scale of the Schedule for Affective Disorders and Schizophrenia (SADS-M) (Reference Endicott and SpitzerEndicott & Spitzer, 1978) was greater for valproate (48%) and lithium (49%) than for placebo (25%), an ‘effect size’ or ‘absolute risk reduction’ of 24%, corresponding to a ‘number needed to treat’ (NNT) (Reference Laupacis, Sackett and RobertsLaupacis et al, 1988) of about 4 (95% CI 3-22). Few patients had a return to normal functioning within 3 weeks. Thus, in routine practice for acute mania, both lithium and valproate are useful as adjuncts to antipsychotic drugs, rather than as monotherapy. Lithium has a delayed effect, taking a few days to begin, and 2-8 weeks to approach its full effect on mania — sometimes even longer. This limits lithium as a treatment for acute mania and makes monotherapy risky for all but the mildest cases.
The study also validated the concept of lithium non-responders, as patients identified as such before entry to the study showed less improvement on lithium than did other patients. Carbamazepine is licensed in the UK for use in patients with bipolar disorder for whom lithium is not suitable; valproate was licensed for use in bipolar illness in the UK in 2001, although widely prescribed before that.
Patients who respond to lithium tend to be those with classical mania rather than the mixed or schizoaffective form (Reference Himmelhoch, Mulla and NeilHimmelhoch et al, 1976; Reference Goodnick and MeltzerGoodnick & Meltzer, 1984; Reference Swann, Secunda and KatzSwann et al, 1986). Patients with elated-grandiose mania showed a better response than those with the destructive-paranoid form in one study (Reference Murphy and BeigelMurphy & Beigel, 1974), but not in another (Reference Swann, Secunda and KatzSwann et al, 1986). Dysphoric mania was less likely to improve (Reference Post, Rubinow and UhdePost et al, 1989). Overall, 36% of patients with mixed mania and 75% of patients with pure episodes responded well to lithium (Reference McElroy, Keck and PopeMcElroy et al, 1992). Patients with a rapid-cycling phase of illness are often less responsive to lithium (Reference Dunner and FieveDunner & Fieve, 1974; Reference Dilsaver, Swann and ShoaibDilsaver et al, 1993). Neurological signs predict a poor response to lithium (Reference Himmelhoch, Neil and MayHimmelhoch et al, 1980).
For long-term prophylaxis it has been suggested that patients who have already experienced more than two mood episodes are less likely than others to benefit from lithium (Reference Gelenberg, Kane and KellerGelenberg et al, 1989). A history of more than 10 episodes has also been linked to a low probability of responding to lithium (Reference Swann, Bowden and CalabreseSwann et al, 1999). However, some patients improve after multiple mood episodes. For instance, Robert Lowell's bipolar illness improved on lithium although he had experienced more than 20 episodes before commencing therapy (Reference JamisonJamison, 1994).
OFFICIAL GUIDELINES
In 1980, an American Psychiatric Association (APA) task force recommended antipsychotic pharmacotherapy for the reduction of manic excitement and for extremely unstable bipolar patients. In contrast, the 1994 guidelines of the APA advise the use of antipsychotic agents in mania only as ‘adjuncts’ to mood stabilisers (particularly lithium or valproate) or to ECT, when mania is associated with ‘agitation’, dangerous behaviour or psychosis (American Psychiatric Association, 1994). These guidelines recognise also that antipsychotic agents or benzodiazepines may be useful to ‘enhance compliance’ with mood stabilisers, or while the latter drugs are developing their effect. The guidelines do not define what is meant by ‘agitation’ in the context of mania, and this is ambiguous.
The guidelines appear to have been based upon four important principles:
-
(a) Mood stabilisers are effective in manic states generally, although having a delayed action.
-
(b) Antipsychotics are beneficial by virtue of reducing hyperactivity or by sedation, rather than by a more general antimanic effect.
-
(c) Antipsychotics, but not mood stabilisers, reduce psychotic symptoms in mania.
-
(d) Antipsychotics should be avoided because they produce unacceptable side-effects, particularly tardive dyskinesia; it is stated that they do so to a greater extent than in schizophrenia. The alternative medications (mood stabilisers) are therefore safer for patients with bipolar disorder.
The consensus panel of 61 experts, reporting from North Carolina in 1996, appeared to confirm that at least among expert opinion, lithium or valproate was regarded as the first-line treatment for mania (Frances et al, 1996). However, it seems that the questionnaire on which the consensus was based did not offer the option of antipsychotic drug therapy alone as a treatment for acute mania in any of its forms. Antipsychotic therapy was offered as an opinion only in addition to mood stabilisers, and was preferred by the panel over benzodiazepines to induce sleep and sedation in patients exhibiting mania with psychotic features. When asked to rate different types of antipsychotic drugs for use in mania (as adjuncts), 75% chose conventional high-potency drugs such as haloperidol as either the antipsychotic of choice, or as ‘first-line’ treatment. This is surprising considering that the high-potency drugs are less sedative, having less affinity to block histamine receptors than the low-potency ones (such as chlorpromazine). Few (13%) favoured adding an antipsychotic to induce sleep; benzodiazepines were preferred for this by 82%.
More recent practice guidelines from the Department of Veterans Affairs recommend first-line treatment of mania with lithium for 3 weeks, followed by a change to ‘a different mood stabiliser’, if there is no response, or a combination with an anti-convulsant if there is a partial response (Reference Bauer, Callahan and JampalaBauer et al, 1999). If the patient also has psychotic features (delusions or hallucinations), antipsychotic drugs should be given if they are judged to be needed. It is acknowledged that mood stabilisers may take several weeks for a maximal response, and that during this time the patient with mania may require ‘adjunctive’ antipsychotic medication for ‘severe agitation’. It is stated that antipsychotics may exacerbate post-manic depressive episodes or induce rapid cycling; but this is a matter requiring further study, as the evidence for it is slight.
CURRENT PRACTICE
The use of antipsychotic therapy in mania was reviewed by Chou (Reference Chou1991), McElroy et al (Reference McElroy and Strakowski1996), Gelenberg & Hopkins (Reference Gelenberg and Hopkins1996), Soares et al (Reference Soares, Mallinger and Gerson1997), Licht (Reference Licht1998) and by Tohen & Zarate (Reference Tohen and Zarate1998).
In Europe, psychiatrists view the above guidelines with scepticism. There, antipsychotic drugs are widely regarded as first-line treatment for acute mania (Reference LichtLicht, 1998); lithium is added for patients who are known to have responded to it in the past or as an adjunct for those who still have mania after about 2 weeks of adequate doses of an antipsychotic agent.
In view of the APA guidelines and the consensus panel report, it might be expected that American psychiatrists would use antipsychotic drugs sparingly, and less often than their counterparts in other countries. However, the difference in approach may be more theoretical than real (Reference Cookson, Sachs, Buckley and WaddingtonCookson & Sachs, 2000). In centres in both the USA and Europe, surveys show that the majority of patients with mania are discharged from hospital still taking antipsychotic medication and remain on it after 6 months (Reference Licht, Gouliaev and VestergaardLicht et al, 1994; Reference Keck, McElroy and StrakowskiKeck et al, 1996; Reference McElroy and StrakowskiMcElroy et al, 1996).
Table 1 shows the proportions of patients receiving treatment with antipsychotic drugs, during the acute phase, at the time of discharge from hospital and at 3-6 months follow-up in centres in the USA and in Europe. Figures for manic episodes treated in East London are closely similar to those of Licht et al (Reference Licht, Gouliaev and Vestergaard1994) in Aarhus, where the use of lithium has been extensively investigated (further details available from the author upon request). Both in the acute phase and at 3-6 months, the proportion of patients in the USA on antipsychotic medication is at least as high as in European centres. This might mean that nearly all American in-patients with mania have psychosis, and therefore require treatment for agitation or dangerous behaviour. Alternatively, it may signify that the guidelines are more conservative about the use of antipsychotic medication than the clinical situation is judged to warrant.
Table 1 Antipsychotic treatment of mania

| Place | n | Percentage on antipsychotic medication | ||||
|---|---|---|---|---|---|---|
| In-patient | On discharge | 3-6 months | 1-2 years | |||
| Harrow et al (Reference Harrow, Goldberg and Grossman1990) | Chicago | 73 | 34 | |||
| Verdoux & Bourgeois (Reference Verdoux and Bourgeois1993) | France | 44 | 72 | 52 | ||
| Licht et al (Reference Licht, Gouliaev and Vestergaard1994) | Aarhus | 125 | 90 | 74 | ||
| Sernyak et al (Reference Sernyak, Griffin and Johnson1994) | Yale | 40 | 95 | |||
| Keck et al (Reference Keck, McElroy and Strakowski1996) | Ohio | 77 | 68 | |||
| Chou et al (Reference Chou, Zito and Vitral1996) | New York | 528 | 92 | |||
| Read et al 1 | London | 100 | 88 | 74 | 49 | |
As further evidence of current practice, in a large cohort of in-patients with bipolar disorder in over 100 psychiatric units in the USA about half were not prescribed mood stabilisers (Reference Edell, Tunis and GreenwoodEdell et al, 1999). On the other hand, a survey of 102 patients with bipolar disorder (of whom 72 were in remission) in a private practice setting in Texas, found typical antipsychotic drugs in use in only seven patients, but clozapine in 19 (Reference Tyler, Stopfel and MyersTyler et al, 1999).
An important objection to the APA guidelines' advocacy of mood stabilisers as first-line treatment is that in practice therapy is commenced with two types of agent at the same time. This vitiates the principle of making one change in treatment at a time in order to be able to determine whether a particular intervention is effective — a useful principle when only a proportion of patients are thought to be responders to lithium, carbamazepine or valproate. This might not matter if antipsychotic agents were effective only by virtue of their sedative effects; it should be possible to withdraw the drug once the manic state has settled. However, there is evidence that they may have a much broader effect in mania, independent of sedation.
It is possible that reluctance to use antipsychotic medication in bipolar disorder may restrict prescribers in a broader way. For example, the clinician may hesitate to use an antidepressant drug in case this leads to a manic phase that cannot be rapidly corrected without using an antipsychotic. Also, patients may be exposed to the adverse effects of clozapine in order to avoid extrapyramidal side-effects, even if these would be only short-term with typical antipsychotic agents.
How effective is chlorpromazine in acute mania?
The first antipsychotic drug, chlorpromazine, was effective in mania in a placebo-controlled trial (Reference KleinKlein, 1967). It was also the first such agent to be widely compared with lithium in randomised, double-blind controlled trials. A summary by Davis et al (Reference Davis, Wang and Janicak1993), of trials in which categorical outcomes were given, showed a response rate in four trials (145 patients) of 23% with chlorpromazine, compared with 63% for lithium, an ‘effect size’ or absolute difference in risk of 39%, which would correspond to an NNT of 2.6. This difference appears impressive, but the original studies had many shortcomings. They did not use rating scales specific for mania, and lacked a clear definition of response (‘remission or marked improvement’). There was also potential for unblinding because of the faster effects of chlorpromazine and its sedative properties. The number of patients included was usually small by current standards.
A much larger study by Prien et al (Reference Prien, Caffey and Klett1972) enrolled 255 patients on either lithium or chlorpromazine. This study drew the influential conclusion that in the more highly active or psychotic cases, chlorpromazine was superior to lithium, both in reducing hyperactivity and in overall improvement. However, for the other group of ‘mildly active’ patients, lithium was slightly superior to chlorpromazine in eventual effect, although equal with regard to improvement in mood and ideation. These findings may have been particularly influential in forming the opinion that antipsychotic drugs should be used for mania with psychosis, and be used to sedate (reduce hyperactivity) and to control dangerous behaviour. On the other hand, lithium did not appear to have more specific antimanic effects than chlorpromazine in the mildly active patients.
Is sedation required for antipsychotics to improve mania?
Chlorpromazine was the most widely investigated antipsychotic agent in early comparative trials, but evidence has emerged that drugs with more specific dopamine-receptor blocking actions have antimanic properties, although these drugs — such as pimozide and sulpride — are less sedative, being without blocking actions at histamine or noradrenalin α 1 receptors. An open study in which pimozide was compared with thioridazine or chlorpromazine, described antimanic actions (Reference Post, Jimerson and BunneyPost et al, 1980). In a comparative trial with pimozide, chlorpromazine produced a greater initial improvement in ratings, with sedation reducing hyperactivity, but pimozide brought about a similar degree of improvement to chlorpromazine at 2 weeks (Reference Cookson, Silverstone and WellsCookson et al, 1981). Patients on chlorpromazine tended to resist increases in dosage, because they felt sedated.
Another group of non-sedative antipsychotic drugs, the benzamides, is selective for the D2 class of dopamine receptors as opposed to D1. The first of these, sulpiride, was reported to be effective in mania in an open study, in combination with lithium (Reference Christie, Whalley and HunterChristie et al, 1989). In our own experience the benzamide drug, remoxipride, did not bring about sustained improvement in a series of 10 patients with mania in doses shown to be effective in schizophrenia (J. Cookson & M. Mitchell, presented at the Fourth Congress of Biological Psychiatry, Philadelphia, 1985; further details available from the author upon request). This is interesting in relation to the effects on prolactin; whereas sulpiride causes a greater rise in prolactin levels than does haloperidol, remoxipride causes a lesser rise than haloperidol. This suggests that the three drugs act to different extents on different subtypes of the D2 receptors in the pituitary and in the limbic system. However, others reported that remoxipride does have antimanic properties when used in combination with haloperidol (Reference Chouinard and SteinerChouinard & Steiner, 1986) and in AIDS-related secondary mania (Reference Scurlock, Singh and CatalanScurlock et al, 1995). Another benzamide drug reportedly effective in mania is sultopride (Reference Rappard and ParrRappard & Parr, 1981).
The efficacy of these non-sedative drugs highlights the importance of dopamine receptor blockade in the antimanic effects of antipsychotic drugs.
Haloperidol in mania
Since haloperidol is reportedly the most commonly prescribed antipsychotic drug in mania (Reference Chou, Zito and VitralChou et al, 1996), the study by Shopsin et al (Reference Shopsin, Gershon and Thompson1975) is important. This compared haloperidol (up to 36 mg per day) with chlorpromazine (up to 1800 mg per day) and lithium in a 3-week study with only 10 patients in each treatment group. Lithium produced a broader improvement in manic symptoms than the antipsychotic therapy; seven patients were well enough to be discharged after treatment with lithium compared with only one patient on chlorpromazine and two patients on haloperidol. However, the clinical ratings (Clinical Global Impression) showed almost identical levels of improvement at 3 weeks for lithium and haloperidol, both of which were superior to chlorpromazine. The authors described haloperidol as being more effective than lithium in reducing hyperactivity, whereas lithium produced greater effects on mood and ideation; however, the Brief Psychiatric Rating Scale (Reference Overall and GorhamOverall & Gorham, 1962) did not show significant differences in individual items for the three treatments. Haloperidol had a more pronounced antimanic effect than chlorpromazine but this was not accompanied by sedation. The authors argued that the control of symptoms by haloperidol was ‘a suppressive lid’, as opposed to the ‘more total normalisation of affect, ideation and behaviour’ on lithium.
The high rate of remission within 3 weeks of lithium therapy in this study is in contrast to the study by Bowden et al (Reference Bowden, Brugger and Swann1994), described above. Conversely, the very low rate of remission on haloperidol contrasts with the dose-finding study of 47 severely ill patients with mania by Rifkin et al (Reference Rifkin, Doddi and Karajgi1994), in which 45% of patients, treated with haloperidol, achieved remission within 6 weeks. The latter study was important in failing to show a significantly greater improvement with doses of haloperidol above 10 mg per day and up to 80 mg per day. However, the numbers involved were small and the confidence limits overlapped in all three dosage ranges. There was a distinct trend for patients on 30 mg per day or more to do better than those on only 10 mg per day. Further studies are needed to identify the optimal doses for haloperidol in mania, as opposed to schizophrenia.
In patients who remain disturbed on standard doses of haloperidol, additional sedation may be achieved by giving benzodiazepines, especially lorazepam, a practice that carries less risk of toxicity than using higher doses of antipsychotic drug (Reference Busch, Miller and WeidenBusch et al, 1989; Reference Lenox, Newhouse and CreelmanLenox et al, 1992).
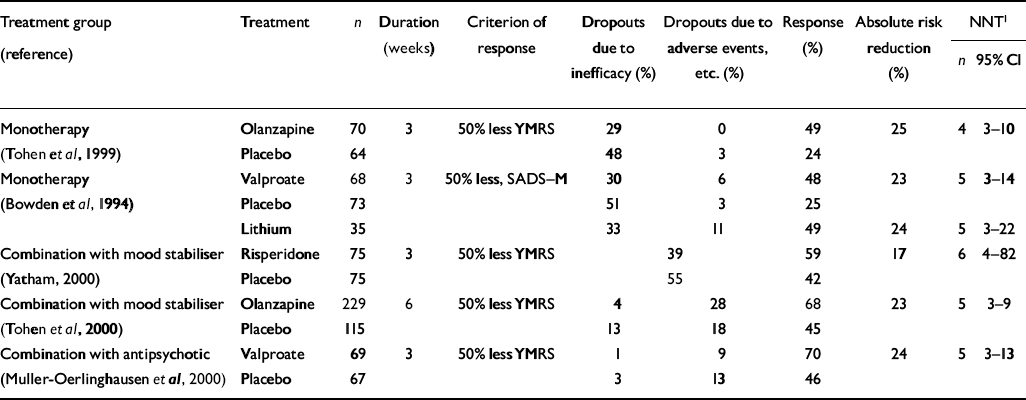
If, as Shopsin et al (Reference Shopsin, Gershon and Thompson1975) found, lithium is superior to haloperidol, and acts through different mechanisms to exert its antimanic effect, one would expect that patients treated with a combination of lithium and haloperidol would respond significantly better than patients treated with either drug alone. One small study (with seven patients in each group and therefore lacking sufficient power to explore the question conclusively) has addressed this comparison in mania (Reference Garfinkel, Stancer and PersaudGarfinkel et al, 1980). Haloperidol appeared superior to lithium alone, and the combination gave no additional advantage during 3 weeks of treatment. Conversely, in a 3-week study with 136 patients, the addition of valproate to conventional antipsychotic therapy (mainly haloperidol) produced greater improvement than the addition of placebo (Reference Muller-Oerlinghausen, Retzow and HennMuller-Oerlinghausen et al, 2000); 70% improved — at least 50% reduction in Young Mania Rating Scale (YMRS) scores — compared with 46% on antipsychotic drug plus placebo (NNT=5; 95% CI 3-13) (see Table 2).
Table 2 Numbers needed to treat in placebo-controlled trials in mania
| Treatment group (reference) | Treatment | n | Duration (weeks) | Criterion of response | Dropouts due to inefficacy (%) | Dropouts due to adverse events, etc. (%) | Response (%) | Absolute risk reduction (%) | NNT1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 95% CI | |||||||||
| Monotherapy | Olanzapine | 70 | 3 | 50% less YMRS | 29 | 0 | 49 | 25 | 4 | 3-10 |
| (Reference Tohen, Sanger and McElroyTohen et al, 1999) | Placebo | 64 | 48 | 3 | 24 | |||||
| Monotherapy | Valproate | 68 | 3 | 50% less, SADS-M | 30 | 6 | 48 | 23 | 5 | 3-14 |
| (Reference Bowden, Brugger and SwannBowden et al, 1994) | Placebo | 73 | 51 | 3 | 25 | |||||
| Lithium | 35 | 33 | 11 | 49 | 24 | 5 | 3-22 | |||
| Combination with mood stabiliser | Risperidone | 75 | 3 | 50% less YMRS | 39 | 59 | 17 | 6 | 4-82 | |
| (Reference YathamYatham, 2000) | Placebo | 75 | 55 | 42 | ||||||
| Combination with mood stabiliser | Olanzapine | 229 | 6 | 50% less YMRS | 4 | 28 | 68 | 23 | 5 | 3-9 |
| (Reference Tohen, Jacobs and MeyersTohen et al, 2000) | Placebo | 115 | 13 | 18 | 45 | |||||
| Combination with antipsychotic | Valproate | 69 | 3 | 50% less YMRS | 1 | 9 | 70 | 24 | 5 | 3-13 |
| (Reference Muller-Oerlinghausen, Retzow and HennMuller-Oerlinghausen et al, 2000) | Placebo | 67 | 3 | 13 | 46 | |||||
ENDOCRINE CORRELATES OF ANTIMANIC EFFECTS OF ANTIPSYCHOTIC AGENTS
Cortisol
Elevated serum cortisol levels are found in mania. During treatment with pimozide, cortisol levels return gradually towards normal, with a time course similar to that of clinical improvement (Reference CooksonCookson, 1985). In contrast, during treatment with haloperidol, there appears to be a dissociation between an early normalisation of cortisol levels within 3 days, and a more gradual clinical improvement during 2 weeks of treatment (Reference Cookson, Silverstone and WilliamsCookson et al, 1985). This apparent difference between haloperidol and pimozide may be related to the different pharmacology of the two drugs. Both drugs block dopamine receptors, but haloperidol in addition blocks noradrenalin α 1 receptors in humans (Reference Szabadi, Gaszner and BradshawSzabadi et al, 1981). Alpha-1-adrenoreceptors are known to be involved in the control of cortisol secretion (Reference Rees, Butler and GoslingRees et al, 1970), and blockade of these receptors is thought to contribute to the sedative effects of antipsychotic drugs (Reference Peroutka and SnyderPeroutka & Snyder, 1980). It may account for the early transient sedative effects seen in mania with haloperidol (Reference Cookson, Moult and WilesCookson et al, 1983). Levels of noradrenalin in the cerebrospinal fluid are raised in mania (Reference Post, Lake and JimersonPost et al, 1978), and blockade of noradrenalin receptors by haloperidol may be part of the mechanism of the drug's antimanic effect.
Prolactin
Dopamine (D2) receptors in the prolactin-secreting cells of the anterior pituitary gland are blocked by classical antipsychotic agents. The resulting elevation of serum prolactin levels provides a biological marker of this dopamine receptor blockade. During treatment with haloperidol, prolactin levels increase in the plasma over the course of 14 days, with a pattern of rise similar to that of clinical improvement. However, more detailed analysis using intravenous ‘test doses’ of haloperidol shows an initial rise in serum prolactin levels, which is partly transient after the first intravenous dose and cannot be exceeded by giving larger doses of haloperidol (Reference Cookson, Moult and WilesCookson et al, 1983). This is followed by a gradual rise during the following 2-4 weeks. Within 2 weeks, prolactin secretion by the pituitary gland becomes sensitive over a wider range of doses of haloperidol than in the drugnaïve patient. These compensatory changes, in the control of prolactin secretion, are thought to include changes in the level of dopamine in the portal pituitary blood supply during prolonged treatment with haloperidol. If similar compensatory changes occur in the dopamine pathways of the limbic system, doses of haloperidol that are effective in the drug-naïve patient with mania may not be sufficient to control patients in later stages of mania who have received prolonged treatment with antipsychotic drugs.
ANTIPSYCHOTICS AS MOOD STABILISERS
Antipsychotic drugs are used extensively in the maintenance treatment of patients with bipolar disorder (Reference SachsSachs, 1990; Reference Sernyak, Godleski and GiffinSernyak et al, 1997). For patients who are unresponsive to lithium (and anticonvulsants), and particularly for those whose compliance with oral medication is poor, depot formulations of antipsychotic drugs have the advantage of providing a sustained and reliable delivery of a drug for periods of weeks. For rapid-cycling bipolar disorder, antipsychotics such as haloperidol decanoate stabilise mood swings (Reference Lowe and BatchelorLowe & Batchelor, 1990).
In the large clinics run by community psychiatric nurses for patients on depot medication, most patients have a diagnosis of schizophrenia or paranoid state; however, there is usually a proportion of patients with a diagnosis of bipolar disorder. Two retrospective studies using a ‘mirror image’ design have examined the effects of depot treatment in those patients in community clinics. One study reported an improvement in the frequency only of manic episodes (Reference White, Cheung and SilverstoneWhite et al, 1993). The other study found an improvement in manic, depressive and mixed episodes (Reference Littlejohn, Leslie and CooksonLittlejohn et al, 1994). These naturalistic studies suggest that long-term treatment with antipsychotic drugs enables a proportion of patients with bipolar disorder to experience periods of greater stability. The mechanisms involved may be primarily antimanic, but result in less subsequent depression, as is argued for lithium (Reference Kukopoulos, Reginaldi and LaddomaKukopoulos et al, 1980). Most of the older antipsychotic drugs do not have antidepressant properties, although they may be useful in combination with monoamine reuptake inhibitors for agitated or psychotic depression. One hope for the new atypical antipsychotic agents is that by virtue of their action to block noradrenalin α2 receptors, they may exert antidepressant effects; these drugs might lessen the post-manic phase of depression and be more helpful than the older antipsychotic agents in patients with mixed affective states.
ANTIPSYCHOTICS, LITHIUM AND NEUROLOGICAL SIDE-EFFECTS
Lithium can increase extrapyramidal (Parkinsonian) side-effects in patients on antipsychotic drugs (Reference Tyrer, Alexander and ReganTyrer et al, 1980) and can itself produce cogwheel rigidity in a small minority of patients (Reference Asnis, Asnis and DunnerAsnis et al, 1979). In contrast to antipsychotic-induced parkinsonism, this does not improve with anticholinergic drugs. Cerebellar tremor and incoordination are signs of toxicity.
Combinations of high levels of lithium with high does of antipsychotic drugs, including haloperidol, have been associated with severe neurological symptoms, hyperthermia, impaired consciousness and irreversible brain damage (Reference Cohen and CohenCohen & Cohen, 1974; Reference Loudon and WaringLoudon & Waring, 1976). The conditions resemble both lithium toxicity (Reference SchouSchou, 1984) and neuroleptic malignant syndrome. However, the severity of lithium neurotoxicity bears little relationship to serum lithium levels (Reference Hansen and AmdisenHanson & Amdisen, 1978), and it can occur with serum levels in the usual therapeutic range (Reference West and MeltzerWest & Meltzer, 1979). Antipsychotic drugs can increase intracellular lithium levels, suggesting a possible mechanism for this interaction (Reference Von Knorringvon Knorring, 1990).
Subsequent studies have demonstrated the safety of combining haloperidol (up to 30 mg per day) with lithium at levels of up to 1 mmol/l (see Reference Batchelor and LoweBatchelor & Lowe, 1990). When combining lithium with antipsychotic medication, blood levels should generally be maintained below 1 mmol/l. Staff should be advised to observe and report the development of neurological symptoms. Lithium toxicity should be assumed in patients taking lithium who have vomiting, severe nausea, cerebellar signs or disorientation. The diagnosis of lithium toxicity should be based upon clinical judgement and not upon the blood level. Lithium should be temporarily discontinued if such symptoms develop and should only be restarted (at an adjusted dose) when the patient's condition has improved or an alternative cause of the symptoms has been found.
TARDIVE DYSKINESIA
Tardive dyskinesia (TD) is a side-effect of long-term treatment with antipsychotic drugs. In schizophrenia, patients receiving intermittent treatment may be at greater risk of developing TD than those receiving antipsychotic drugs consistently for many years (Reference Van Harten, Hoek and Matroosvan Harten et al, 1998). Affective disorder is a risk factor for the development of TD, as is chronic schizophrenia (Reference Kane and SmithKane & Smith, 1982; Reference Hamra, Nasrallah and ClancyHamra et al, 1983; Reference Mukherjee, Rosen and CaracciMukherjee et al, 1986). However, the rate of TD in patients with bipolar disorder was found to be no higher than in a matched group of patients with schizophrenia (Reference Hunt and SilverstoneHunt & Silverstone, 1991). The availability of treatments such as lithium and anticonvulsants, which are effective in bipolar disorder and not in schizophrenia, encourages the view that TD is more avoidable in bipolar disorder. However, the recent doubt cast upon the effectiveness of lithium in clinical practice highlights the continuing need for the development of antipsychotic drugs that are less prone to produce TD, such as clozapine, and that are also effective in bipolar disorder.
ATYPICAL ANTIPSYCHOTIC DRUGS IN MANIA
Clozapine has been reported to be effective in open studies in mania, including treatment-resistant patients (Reference McElroy, Dessain and PopeMcElroy et al, 1991; Reference Tohen and ZarateTohen & Zarate, 1998), and in a randomised trial (Reference Suppes, Webb and PaulSuppes et al, 1999). Some patients had rapid cycling disorders so that spontaneous improvement would have contributed to the findings. In a comparative trial, clozapine appeared as effective as chlorpromazine in mania over 3 weeks (Reference Barbini, Scherillo and BenedettiBarbini et al, 1997).
Many of the new atypical antipsychotic drugs developed in the wake of clozapine have potent activity for blocking 5-HT2 receptors. This is thought to protect against Parkinsonian side-effects by an interaction on dopamine neurons to enhance dopamine release, while the drugs also block subtypes of D2 receptors post-synaptically. Since dopamine agonists are able to trigger mania in predisposed individuals (Reference Gerner, Post and BunneyGerner et al, 1976; Reference Turner, Cookson and WassTurner et al, 1984), these atypical antipsychotic drugs carry a theoretical risk of triggering mania. Therefore, controlled trials are essential to explore their value in bipolar disorder.
For olanzapine there is evidence of antimanic efficacy from a placebo-controlled trial with 134 patients (Reference Tohen, Sanger and McElroyTohen et al, 1999). It produced a 50% reduction in the YMRS score in 3 weeks in 49% compared with 24% on placebo, an absolute risk reduction of 25% and an NNT of 4 (95% CI 3-10) (Table 2). The high placebo response rates of 25% in this study and that of Bowden et al (Reference Bowden, Brugger and Swann1994) are in the context of clinical trials, in which placebos are used to take account of non-specific factors including nursing interventions, the use of night sedation and ‘rescue medication’ with lorazepam in the first 10 days, and the discontinuation of previous medication. Also, a proportion of patients in these studies had rapid-cycling conditions and some improvement in mania could be expected as the disorder progressed.
Olanzapine has also been found to be useful in mixed episodes of bipolar disorder (Reference Zullino and BaumannZullino & Baumann, 1999). In contrast to haloperidol and risperidone, it does not produce a sustained elevation of plasma prolactin levels. Further studies are needed to explore the efficacy of this drug in comparison to haloperidol in mania.
Ziprasidone (80-160 mg daily) has also been found to be effective in mania in comparison with placebo in a 3-week study of 195 patients (Keck & Ice, 2000).
Further data are also needed to define the efficacy of risperidone in mania. In a comparative trial, in which additional lorazepam was permitted, risperidone was of similar efficacy to haloperidol or lithium (Reference Segal, Berk and BrookSegal et al, 1998). Risperidone improves mania in patients with AIDS, who are particularly prone to developing extrapyramidal side-effects with typical antipsychotics (Reference Singh, Golledge and CatalanSingh et al, 1997).
Two more recent studies of risperidone (1-6 mg daily) added for 3 weeks to treatment with lithium or valporate (or carbamazepine in one study) have demonstrated an advantage of risperidone over placebo (Reference Sachs and GhaemiSachs & Ghaemi, 2000; Reference YathamYatham, 2000). These large studies included 158 and 150 patients, respectively, and the former study included a group on haloperidol. In the latter study improvement rates (50% reduction in YMRS) were 59% for risperidone compared with 42% for placebo added to a mood stabiliser (Table 2). These figures indicate an absolute risk reduction of 17%, and an NNT of approximately 6 (95% CI 4-82). Risperidone was effective in patients with or without features of psychosis (Reference Petty, Sachs and BowdenPetty et al, 2000). A 6-week study with even greater power compared olanzapine (5-20 mg daily) with placebo, in combination with a mood stabiliser (lithium or valproate) in 344 patients with mania or mixed bipolar episodes (Reference Tohen, Jacobs and MeyersTohen et al, 2000). The rates of improvement (YMRS score reduction of 50% or more) were 68% on olanzapine and 45% on placebo. This absolute risk reduction of 23% corresponds to an NNT of 5 (95% CI 3-9). The effect was more significant in patients on valproate than in those on lithium. Interestingly, patients with mixed states showed markedly greater rates of improvement on olanzapine than placebo in combination with mood stabiliser, 43% compared with 9.5%, with an NNT of approximately 3 (95% CI 2-6).
Both risperidone, possibly in a dose-related manner (Reference Lane, Lin and ChangLane et al, 1998), and olanzapine (Reference Lindenmayer and KlebanovLindenmayer & Klebanov, 1998) — but not clozapine — have been described as worsening or precipitating mania in predisposed patients, mainly those with schizophrenia, schizoaffective disorder or organic brain disease. No evidence of such exacerbation of mania was found in the placebo-controlled trials of either risperidone or olanzapine described above, but patients were already receiving mood stabilisers in some studies.
FUTURE PROSPECTS
Antipsychotic drugs remain an important option for the first-line treatment of mania in all but the mildest cases. Their use in high doses can be reduced by concomitant benzodiazepine treatment. Lithium or another mood stabiliser will often be required in addition, and for prophylaxis. Table 2 summarises the results of influential clinical trials including those using combinations of antipsychotic drugs with mood stabilisers. It can be seen that response rates are generally higher with combination therapy than with monotherapy.
Important topics for further investigation are the efficacy and safety of new atypical antipsychotic drugs in comparison with the conventional antipsychotics in longer-term treatment, and the place of newer anticonvulsants in combination with antipsychotics in bipolar disorder.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Antipsychotic drugs are widely used and are often effective in mania.
-
▪ Antipsychotic therapy is often continued for 6 months after an episode of mania.
-
▪ Lithium remains a first-line treatment for mania and the prophylaxis of bipolar disorder.
LIMITATIONS
-
▪ Early clinical trials in mania involved small numbers.
-
▪ Placebo-controlled trials in mania have until recently been few, but large-scale placebo-controlled trials of atypical antipsychotics in mania are being reported.
-
▪ Guidelines and clinical practice are changing as new treatments become available.





eLetters
No eLetters have been published for this article.