Dementia with Lewy bodies (DLB) is the second most common cause of neurodegenerative dementia after Alzheimer's disease. Reference McKeith, Galasko, Kosaka, Perry, Dickson and Hansen1,Reference Geser, Wenning, Poewe and McKeith2 The overlapping clinical symptoms of DLB and Alzheimer's disease make differentiation between the disorders difficult. This has led to the use of neuroimaging methods, for example single photon emission computed tomography (SPECT), positron emission tomography (PET), and structural and functional magnetic resonance imaging (MRI), to enhance diagnostic accuracy. Neuroimaging has provided important information on differences in specific structures between individuals with Alzheimer's disease and DLB that attempt to explain the varying symptoms. Reference Small3 In general, neuroimaging changes have been less well investigated in DLB compared with Alzheimer's disease and the neurobiological changes underpinning the core features in DLB remain unclear.
Using resting-state functional MRI (fMRI), regional correlations in spontaneous low-frequency fluctuations (at <0.1 Hz) in the blood oxygen level-dependent (BOLD) signal have been reported, Reference Biswal, Yetkin, Haughton and Hyde4 and interpreted as depicting functional connectivity. Reference Fox and Raichle5 Resting-state studies in Alzheimer's disease show functional connectivity abnormalities with the hippocampus and posterior cingulate cortex, but results are conflicting, with some reporting decreases, Reference Gili, Cercignani, Serra, Perri, Giove and Maraviglia6,Reference Greicius, Srivastava, Reiss and Menon7 and others increases. Reference Wang, Zang, He, Liang, Zhang and Tian8,Reference Zhang, Wang, Xing, Liu, Ma and Yang9 Previously, we investigated cortical connectivity in DLB and Alzheimer's disease and using a seed-region approach showed abnormal functional connectivity with the posterior cingulate in DLB and with the hippocampus in Alzheimer's disease. Abnormalities in participants with DLB and Alzheimer's disease were characterised by increased connectivity. Reference Kenny, Blamire, Firbank and O'Brien10 Only one other group has investigated resting-state connectivity in DLB. Reference Galvin, Price, Yan, Morris and Sheline11 Galvin et al focused on precuneus connectivity only and using the whole structure as the seed region showed both increased connectivity with the putamen and inferior parietal cortex and decreased connectivity with the medial prefrontal cortex, frontoparietal operculum and primary visual cortex. Reference Galvin, Price, Yan, Morris and Sheline11
This study used resting-state fMRI to compare participants with DLB with those with Alzheimer's disease and with controls, focusing on subcortical connectivity from the caudate nucleus, putamen and thalamus. Connectivity was hypothesised to be abnormal in individuals with DLB compared with those with Alzheimer's disease and the controls in the following regions: the caudate, because of its role in emotional regulation and the greater depression severity in DLB; the putamen, because abnormalities in structural pathology and neurotransmitter function here are associated with Parkinsonian symptoms in DLB; Reference O'Brien, Colloby, Fenwick, Williams, Firbank and Burn12,Reference Walker, Costa, Walker, Shaw, Gacinovic and Stevens13 and the thalamus (mediodorsal nucleus), because it is involved in the maintenance of consciousness Reference Perry and Perry14 and fluctuating cognition is a core feature of DLB. Reference McKeith, Galasko, Kosaka, Perry, Dickson and Hansen1
Method
Participants
The study involved 47 participants aged over 60 years: 15 with DLB, 16 with Alzheimer's disease and 16 controls. Recruitment of the participants with DLB and Alzheimer's disease was from clinical old age psychiatry, geriatric medicine and neurology out-patient services and the controls by local advertisement or from partners of participants. The participants were the same as those in a previous paper. Reference Firbank, Blamire, Teodorczuk, Teper, Burton and Mitra15 The study was approved by the local ethics committee and all participants gave signed informed consent for participation, following an explanation of the full procedure by an experienced clinician. The participants with DLB (DLB group) met consensus criteria for probable DLB: presence of two or more core features (fluctuating cognition, visual hallucinations and/or Parkinsonism) Reference McKeith, Galasko, Kosaka, Perry, Dickson and Hansen1,Reference McKeith, Dickson, Lowe, Emre, O'Brien and Feldman16 and the participants with Alzheimer's disease (Alzheimer's disease group) fulfilled National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria for probable Alzheimer's disease. Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan17 Diagnoses were made by consensus between two experienced clinicians, a method previously validated against autopsy diagnosis. Reference McKeith, Ballard, Perry, Ince, O'Brien and Neill18 Of the 15 individuals in the DLB group, 9 had undergone a 123I-labelled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (123I-FP-CIT) SPECT scan as part of their clinical diagnostic assessment and all demonstrated reduced dopamine transporter uptake in the basal ganglia consistent with their diagnosis.
Detailed physical, neurological and neuropsychiatric examinations were carried out. Cognitive and neuropsychiatric examinations involved: Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh19 and Cambridge Cognitive (CAMCOG) examination to assess cognitive status, Reference Roth, Tym, Mountjoy, Huppert, Hendrie and Verma20 Geriatric Depression Scale (GDS) to assess depressive symptoms, Reference Sheikh and Yesavage21 Neuropsychiatric Inventory (NPI) to assess neuropsychiatric symptoms, Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein22 Clinical Assessment of Fluctuation Scale (CAFS) to assess fluctuating cognition Reference Walker, Ballard, Ayre, Wesnes, Cummings and McKeith23 and the motor subsection of the Unified Parkinson's Disease Rating Scale (UPDRS III) for motor features of Parkinsonism. Reference Fahn, Elton, Fahn, Marsden, Calne and Goldstein24 Exclusion criteria were severe concurrent illness (apart from dementia in the DLB and Alzheimer's disease groups), the presence of space occupying lesions on MRI, stroke history and any contraindications to MRI. Controls had no history of psychiatric illnesses as self-reported and as assessed by an experienced psychiatrist.
Imaging
Participants were scanned using a 3 Tesla MRI system (Intera Achieva scanner, Philips Medical System, Eindhoven, The Netherlands). An 8-channel head coil was used to collect resting-state fMRI scans using a gradient-echo echo-planar imaging sequence. The scan timings and parameters were: 25 axial slices, 128 volumes, anterior-posterior acquisition, in-plane resolution: 2 × 2 mm, slice thickness: 6 mm, repetition time (TR) = 3000 ms, echo time (TE) = 40 ms, field of view: 260 × 150 × 260 mm, acquisition time: 6.65 min. Conventional structural three-dimensional T 1-weighted scans were also collected.
Image analysis
Analysis used the methods described by Fox et al: Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle25 removal of non-brain structures, head motion correction, spatial smoothing (6 mm full-width at half maximum) and temporal band-pass filtering between 0.009 and 0.08 Hz to remove low-frequency drift and high-frequency noise respectively. A study-specific functional brain template was created as the participants in this study were elderly, therefore they would be expected to have more brain atrophy than that in a general standard space template, which is based on younger individuals. To create the study-specific brain template, one participant was registered to the standard space echo planar imaging template from Statistical Parametric Mapping (SPM5, www.fil.ion.ucl.ac.uk/spm/), all other participants were registered to this individuals and then averaged using fslmaths. Seeds that had been placed in patient space were transformed to standard space for analysis. All analysis was performed using standard tools from the FSL software package (version 5, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg26
Seed regions of 2 × 2 voxels (446 mm) were placed manually for each participant on the functional image in the left and right head of caudate nucleus, putamen and thalamus (mediodorsal nucleus). The mean BOLD signal time-series was extracted from each seed and cross-correlated with all other brain voxels to determine functional connectivity. Reference Woolrich, Ripley, Brady and Smith27 To ensure non-neuronal fluctuations did not confound analysis, time series from seeds placed in white matter and cerebrospinal fluid, and a whole brain mask to remove the global signal, were included in the linear regression analysis as covariates of no interest. Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle25
A three-group comparison was carried out to investigate connectivity differences between groups for each seed region, by comparing their data on a voxel by voxel basis. Reference Beckmann, Jenkinson and Smith28,Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith29 Z (Gaussianised T/F) statistic images were thresholded using pixel clusters determined by Z>2.3/P<0.05 (corrected for multiple comparisons). Reference Worsley, Jezzard, Matthews and Smith30 The peak connectivity cluster coordinates were converted from Montreal Neurological Index (MNI) space to Talairach space Reference Talairach and Tournoux31 using GingerALE (version 2.1, www.brainmap.org) on Windows Reference Lancaster, Tordesillas-Gutierrez, Martinez, Salinas, Evans and Zilles32 and entered into Talairach Client to assign Talairach labels. Reference Lancaster, Woldorff, Parsons, Liotti, Freitas and Rainey33 One-way analysis of variance was used to compare demographic factors across groups and the independent-sample t-test for comparisons between groups (P≤0.05) using SPSS (version 15.0.1) on Windows.
Results
Demographics
Table 1 shows the clinical characteristics of the study participants. Groups were comparable for age (P = 0.29, d.f. = 2, F = 1.26) and gender (P = 0.47, χ2 = 0.532, d.f. = 1). As expected, the control group had significantly higher scores on cognitive tests (MMSE and CAMCOG) and lower scores on measures of motor features (UPDRS) and depression (GDS), compared with individuals in the DLB and Alzheimer's disease groups. There were no significant differences between the DLB and Alzheimer's disease groups in age at onset of dementia, duration of dementia, MMSE or total CAMCOG scores. Consistent with the known preservation of memory in DLB, participants in this group had significantly higher scores than those in the Alzheimer's disease group on the CAMCOG memory subscore (P = 0.022), although still significantly lower than that of the control group. As expected, UPDRS, NPI, CAFS and GDS scores were significantly higher in the DLB group compared with the Alzheimer's disease group, indicating greater severity in the DLB group of the motor features of Parkinsonism (P<0.001), neuropsychiatric disturbances (P = 0.002), fluctuating cognition (P = 0.006) and depressive symptoms (P = 0.001).
At the time of study, 24 participants were taking acetylcholinesterase inhibitors: 14 participants with Alzheimer's disease (donepezil: n = 9 and galantamine: n = 5) and 10 with DLB (donepezil: n = 5, galantamine: n = 4 and rivastigmine n = 1). No participants were on memantine. Eight participants (six with DLB and two with Alzheimer's disease) were taking antidepressants (citalopram, mirtazapine, trazodone, venlafaxine or paroxetine) and one person with DLB was taking a non-benzodiazepine (zopiclone) as a hypnotic.
Caudate functional connectivity
Online Fig. DS1 shows the connectivity maps for the left and right caudate in each group (the coordinates are provided in online Table DS1). Statistical comparison of group results showed significant differences in connectivity between the DLB and control groups bilaterally with the head of caudate nucleus (Fig. DS1) and between the Alzheimer's disease and control groups for the right caudate (Fig. DS1(b)). In the DLB compared with the control group the left caudate showed abnormal connectivity with the parahippocampal gyrus (left), posterior cingulate (right) and precuneus (bilateral). The right caudate showed abnormal connectivity in the DLB compared with the control group with the posterior cingulate (bilateral), precuneus (left) and culmen (right), and in the Alzheimer's disease compared with the control group with the posterior cingulate (bilateral) and precuneus/cuneus (bilateral) (Fig. DS1 and Table DS1). In the DLB group,
Table 1 Demographic and neuropsychological data of participants
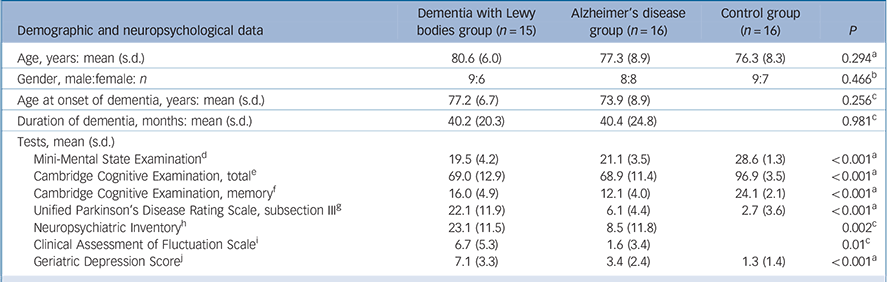
| Demographic and neuropsychological data | Dementia with Lewy bodies group (n = 15) |
Alzheimer's disease group (n = 16) |
Control group (n = 16) |
P |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 80.6 (6.0) | 77.3 (8.9) | 76.3 (8.3) | 0.294Footnote a |
| Gender, male:female: n | 9:6 | 8:8 | 9:7 | 0.466Footnote b |
| Age at onset of dementia, years: mean (s.d.) | 77.2 (6.7) | 73.9 (8.9) | 0.256Footnote c | |
| Duration of dementia, months: mean (s.d.) | 40.2 (20.3) | 40.4 (24.8) | 0.981Footnote c | |
| Tests, mean (s.d.) | ||||
| Mini-Mental State ExaminationFootnote d | 19.5 (4.2) | 21.1 (3.5) | 28.6 (1.3) | <0.001Footnote a |
| Cambridge Cognitive Examination, totalFootnote e | 69.0 (12.9) | 68.9 (11.4) | 96.9 (3.5) | <0.001Footnote a |
| Cambridge Cognitive Examination, memoryFootnote f | 16.0 (4.9) | 12.1 (4.0) | 24.1 (2.1) | <0.001Footnote a |
| Unified Parkinson's Disease Rating Scale, subsection IIIFootnote g | 22.1 (11.9) | 6.1 (4.4) | 2.7 (3.6) | <0.001Footnote a |
| Neuropsychiatric InventoryFootnote h | 23.1 (11.5) | 8.5 (11.8) | 0.002Footnote c | |
| Clinical Assessment of Fluctuation ScaleFootnote i | 6.7 (5.3) | 1.6 (3.4) | 0.01Footnote c | |
| Geriatric Depression ScoreFootnote j | 7.1 (3.3) | 3.4 (2.4) | 1.3 (1.4) | <0.001Footnote a |
a. The P-value was calculated using the χ2 test.
b. The P-values were calculated using the one-way ANOVA.
c. The P -values were calculated using the independent-samples t-test.
d. Control group>Alzheimer's disease and dementia with Lewy bodies (DLB) groups (P<0.001, d.f. = 2, F = 36.61).Footnote a
e. Control group>Alzheimer's disease, DLB groups (P<0.001, d.f. = 2, F = 40.46).Footnote a
f. Control group>Alzheimer's disease, DLB groups (P<0.001, d.f. = 2, F = 41.09);Footnote a DLB group>Alzheimer's disease group (P = 0.022, d.f. = 29, t = −2.42).Footnote c
g. Control, Alzheimer's disease groups<DLB group (P<0.001, d.f. = 2, F = 29.24)Footnote a (i.e. participants with DLB had greater motor features). Control group<Alzheimer's disease group (P = 0.023, d.f. = 30, t = −2.40)Footnote c (i.e. participants with Alzheimer's disease had greater motor features).
h. DLB group v. Alzheimer's disease group (P = 0.002, d.f. = 27, t = −3.38)Footnote c (i.e. participants with DLB had greater neuropsychiatric symptoms).
i. DLB group v. Alzheimer's disease group (P = 0.01, d.f. = 24, t = −2.8)Footnote c (i.e. participants with DLB had greater fluctuation).
j. Control group v. Alzheimer's disease group (P = 0.006, d.f. = 30, t = −2.95);Footnote c Alzheimer's disease group v. DLB group (P = 0.001, d.f. = 29, t = −3.60);Footnote c control group v. DLB group (P<0.001, d.f. = 29, t = −6.39).Footnote c
there were no regions of significantly less connectivity compared with the Alzheimer's disease or control groups, or in the Alzheimer's disease group compared with the control group.
Putamen functional connectivity
Selected putamen connectivity maps for each group are shown in online Fig. DS2 (the coordinates are provided in online Table DS2). Altered putamen connectivity was specific to the DLB group. Group comparisons showed abnormal connectivity in the DLB compared with the Alzheimer's disease group between the putamen (bilateral) and pre- and postcentral gyrus, inferior parietal and transverse temporal regions (all left hemisphere), and between the DLB and control groups with the left putamen and pre- and postcentral gyrus and inferior parietal regions (all left hemisphere) (Fig. DS2 and Table DS2).
Thalamus functional connectivity
Thalamic connectivity for each group is shown in online Fig. DS3 (the coordinates are provided in online Table DS3). Aberrant connectivity was found in the DLB and Alzheimer's disease groups compared with controls with the left thalamus (Fig. DS3(a)), and in the DLB group compared with the control group with the right thalamus (Fig. DS3(b)). Abnormal left thalamus connectivity was shown in the DLB group compared with the controls with the cingulate (bilateral), insula (right) and frontal regions (bilateral) and in the Alzheimer's disease group compared with controls with the pre- and postcentral gyrus and inferior parietal regions (all right hemisphere). For the right thalamus, the DLB group showed altered connectivity compared with the control group in frontal and limbic regions (all right hemisphere) (Fig. DS3 and Table DS3). The DLB and Alzheimer's disease groups did not show less connectivity than controls between the thalamus (bilateral) and any other brain regions.
Discussion
Resting-state fMRI was used to investigate functional connectivity in participants with DLB compared with participants with Alzheimer's disease and controls. The main findings were abnormal connectivity between subcortical and cortical regions in the DLB group, and to a lesser extent the Alzheimer's disease group, compared with controls. In the DLB group, compared with controls, greater connectivity was seen with the head of caudate nucleus (bilateral), putamen (left) and mediodorsal nucleus of the thalamus (bilateral). In the Alzheimer's disease group compared with controls connectivity was greater with the caudate nucleus (right) and thalamus (left), and in the DLB group compared with the Alzheimer's disease group with the putamen (bilateral). The DLB and Alzheimer's disease groups did not show significantly less connectivity with any seeds compared with controls, the DLB group did not show less connectivity compared with the Alzheimer's disease group.
Compared with controls, the DLB and Alzheimer's disease groups showed altered connectivity in some common brain regions; between the caudate and limbic (posterior cingulate cortex) and parietal (precuneus) regions and between the thalamus and frontal (precentral gyrus) regions. However, there were also important differences between the DLB and Alzheimer's disease groups even though the groups were matched for age and dementia severity. The DLB group showed altered connectivity between the putamen and frontal (left precentral gyrus), temporal (left transverse temporal gyrus) and parietal (left inferior parietal and postcentral gyrus) regions compared with the Alzheimer's disease group.
Caudate
Previous studies using SPECT and diffusion tensor imaging (DTI) have shown abnormalities in the caudate nucleus in individuals with DLB Reference O'Brien, Colloby, Fenwick, Williams, Firbank and Burn12,Reference Bozzali, Falini, Cercignani, Baglio, Farina and Alberoni34 and using resting-state fMRI we showed greater caudate functional connectivity in people with late-life depression compared with controls. Reference Kenny, O'Brien, Cousins, Richardson, Thomas and Firbank35 In this study, participants with DLB showed greater connectivity compared with controls between the caudate (bilaterally) and default mode network (a resting-state network involved in attending to environmental stimuli) Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman36 regions of the posterior cingulate cortex and precuneus (bilaterally). In the Alzheimer's disease group, connectivity was greater compared with controls between the caudate (right) and the posterior cingulate cortex and precuneus (bilaterally). The findings here of greater caudate connectivity (bilaterally) in those with DLB could be related to the role the caudate plays in emotional regulation. Depression is known to be more common in people with DLB Reference McKeith, Dickson, Lowe, Emre, O'Brien and Feldman16 and consistent with this the DLB group in this study showed significantly greater depression severity than those with Alzheimer's disease and the controls.
Putamen
Neuroimaging studies investigating the putamen in people with DLB have previously shown greater atrophy on structural MRI, Reference Cousins, Burton, Burn, Gholkar, McKeith and O'Brien37 tissue organisation abnormalities on DTI Reference Bozzali, Falini, Cercignani, Baglio, Farina and Alberoni34 and dopamine transporter loss on SPECT. Reference O'Brien, Colloby, Fenwick, Williams, Firbank and Burn12 Importantly, Cousins et al Reference Cousins, Burton, Burn, Gholkar, McKeith and O'Brien37 and O'Brien et al Reference O'Brien, Colloby, Fenwick, Williams, Firbank and Burn12 showed that these abnormalities are not present in Alzheimer's disease and so are specific to DLB. In this study we also showed that the putamen is affected in DLB, with the DLB group showing greater connectivity with the putamen compared with the Alzheimer's disease and control groups. Connectivity was greater in the DLB group compared with the Alzheimer's disease (bilateral) and control (left) groups in similar brain regions: frontal (precentral), parietal (inferior and postcentral) and temporal (transverse) (all left hemisphere). Altered putamen connectivity was specific to DLB, with no other significant differences between groups found. The putamen is involved in the control of motor functions, therefore abnormalities in connectivity with this structure in DLB could be associated with Parkinsonian symptoms that are characteristic of DLB.
Thalamus
The thalamus is thought to be involved in maintenance of consciousness Reference Perry and Perry14 and at post-mortem increased nicotinic receptor binding is shown in individuals with DLB with disturbances of consciousness compared with individuals with DLB but without such disturbances and controls. Reference Pimlott, Piggott, Ballard, McKeith, Perry and Kometa38 The DLB group showed altered connectivity compared with controls between the thalamus (bilaterally) and frontal (precentral gyrus) and limbic (cingulate) (mainly right hemisphere) regions. The Alzheimer's disease group showed altered connectivity compared with controls between the left thalamus and frontal (precentral gyrus) and parietal (inferior parietal and postcentral gyrus) regions (all right hemisphere). The thalamus had not been predicted to be affected in Alzheimer's disease, but previously structural measures have shown greater thalamic grey matter loss in Alzheimer's disease than DLB. Reference Burton, Karas, Paling, Barber, Williams and Ballard39 Similar to the findings for the caudate, connectivity was affected bilaterally in the DLB group and unilaterally in the Alzheimer's disease group. Changes in connectivity between the thalamus and cingulate, both key areas implicated in attention, were specific to the DLB group.
Findings in relation to other studies
Resting-state functional connectivity in Alzheimer's disease has previously been investigated by a number of groups, but studies have generally focused on using posterior cingulate cortex or hippocampus seeds only and findings have differed with some reporting increased, Reference Wang, Zang, He, Liang, Zhang and Tian8,Reference Zhang, Wang, Xing, Liu, Ma and Yang9 others decreased Reference Gili, Cercignani, Serra, Perri, Giove and Maraviglia6,Reference Greicius, Srivastava, Reiss and Menon7 and within the same study both increased and decreased Reference Supekar, Menon, Rubin, Musen and Greicius40,Reference Wang, Liang, Wang, Tian, Zhang and Li41 connectivity in Alzheimer's disease. Similar to our findings in this present study, we previously showed, using cortical seed regions, greater/increased connectivity in individuals with DLB and Alzheimer's disease compared with controls. Connectivity with a seed placed in the posterior cingulate cortex (right) was increased in participants with DLB compared with controls, whereas hippocampal (left) connectivity was increased in participants with Alzheimer's disease compared with controls. Reference Kenny, Blamire, Firbank and O'Brien10
There has only been one study outside our group investigating resting-state connectivity in DLB and this study used a different approach investigating bilateral precuneus connectivity only and reported both increased (with the putamen and inferior parietal cortex) and decreased (medial prefrontal cortex, frontoparietal operculum and primary visual cortex) connectivity. Reference Galvin, Price, Yan, Morris and Sheline11 However, that study Reference Galvin, Price, Yan, Morris and Sheline11 was in younger participants with DLB (mean age 72 years) with a higher MMSE score (mean 25) compared with our group (mean age 81 years, mean MMSE 20). As the participants in the Galvin et al study Reference Galvin, Price, Yan, Morris and Sheline11 are at an earlier stage of dementia, this could mean that they are better able to compensate for brain damage by increasing connectivity with some brain regions and decreasing connectivity with others.
A task-state study in participants with DLB showed significantly decreased deactivations (for example posterior cingulate cortex) in individuals with DLB and Alzheimer's disease compared with controls. Reference Sauer, Ffytche, Ballard, Brown and Howard42 This could be linked with our finding of increased connectivity between the caudate and posterior cingulate in the DLB and Alzheimer's disease groups compared with controls. This proposes the theory that mechanisms to suppress a region when it is not required in the task state may be inhibited in dementia and therefore regions not required for normal brain functioning at rest remain abnormally active. It could also be that there is a dysfunction in the competitive/opposing relationship between brain regions known to deactivate when a task is performed (default mode network regions) and brain regions known to activate when a task is performed (task positive/attentional networks). Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle25 The findings from this study support the compensatory recruitment hypothesis. Reference Zhang, Wang, Xing, Liu, Ma and Yang9 Regions not affected in dementia could be recruited to compensate for the poorer functioning of affected regions, therefore greater connectivity is observed as circuits compensate for damage elsewhere.
Strengths and limitations
Strengths of this study were the good match of groups for participant numbers, gender and age. The model-based technique used requires prior hypotheses for seed selection, which were formed based on previous neuroimaging study findings showing these regions to be abnormal, and enabled the investigation of spontaneous low-frequency fluctuation correlations between a seed and all other voxels.
This resting-state fMRI study investigated connectivity in DLB with subcortical brain regions. Previous studies in Alzheimer's disease have used different seeds and have either investigated connectivity between two specific seeds or within a seed only, thus potentially missing important changes elsewhere in the brain. A previous study in DLB placed a different seed (precuneus) bilaterally using coordinates. Reference Galvin, Price, Yan, Morris and Sheline11 In contrast, we placed seeds manually in each participant, meaning the seed was less likely to be affected by atrophy differences between participants, and connectivity was investigated separately for each hemisphere. Resting-state studies benefit from simplicity of experimental design, no task has to be practised or performed, meaning they are advantageous in individuals who are cognitively impaired for whom it may be more difficult to adhere to a task.
This study also has some limitations. The results may be biased by the seed selected and connectivities of interest may be missed if they do not show connectivity with the seed. In contrast, model-free methods do not require predefined seeds or a temporal model, although their lack of specificity means results can be hard to interpret. The number of participants in each group was relatively small; with larger group sizes more significant differences may have been observed. However, other resting-state fMRI studies have had comparable numbers, Reference Zhang, Wang, Xing, Liu, Ma and Yang9 and others have had less. Reference Gili, Cercignani, Serra, Perri, Giove and Maraviglia6 Additionally, participants can have overlapping DLB and Alzheimer's disease pathology and therefore subsequent autopsy correlation with pathological burden, as has been carried out in structural MRI studies, Reference Burton, Barber, Mukaetova-Ladinska, Robson, Perry and Jaros43 would be informative.
The participants in the DLB and Alzheimer's disease groups in this study were not medication free and as certain medications have been shown to affect functional connectivity this must be taken into account. The majority of the participants in the DLB and Alzheimer's disease groups (24 out of 31) were taking acetylcholinesterase inhibitors at the time of scanning, with all participants (apart from one) taking either donepezil or galantamine. Previously, medications have been shown to decrease connectivity, for example an electroencephalogram (EEG)/fMRI study showed donepezil decreased default mode network connectivity in healthy older participants Reference Balsters, O'Connell, Martin, Galli, Cassidy and Kilcullen44 and a study investigating antidepressant treatment (citalopram) showed decreased functional connectivity in control participants between the amygdala and ventral medial prefrontal cortex. Reference McCabe and Mishor45 These findings provide support for our study findings not being related to medication effects as we showed increased connectivity in the DLB and Alzheimer's disease groups compared with controls. Additionally, we identified significantly different putamen connectivity patterns in the DLB compared with the Alzheimer's disease group, despite the participants taking similar medications and even though more participants with Alzheimer's disease (88%) were taking acetylcholinesterase inhibitors than those with DLB (67%) we showed greater connectivity in the DLB group.
Memantine, an N-methyl-d-aspartate (NMDA) receptor antagonist, has been shown to increase precuneus resting-state functional connectivity in Alzheimer's disease, Reference Lorenzi, Beltramello, Mercuri, Canu, Zoccatelli and Pizzini46 however none of the people with dementia in this study were taking memantine and in a previous study we showed no significant differences in precuneus connectivity in the same participants studied here. Reference Kenny, Blamire, Firbank and O'Brien10 In late-life depression, we reported no significant difference in resting-state caudate functional connectivity between participants taking medication compared with those not, but as group sizes were small this would need to be investigated further. Reference Kenny, O'Brien, Cousins, Richardson, Thomas and Firbank35 Increased resting-state functional connectivity has been reported in a number of studies in depression, Reference Perrin, Merz, Bennett, Currie, Steele and Reid47,Reference Sheline, Price, Yan and Mintun48 Sheline et al showed increased connectivity in the affective network (involving the caudate) in depression. Reference Sheline, Price, Yan and Mintun48 The DLB group in this study had significantly greater depression severity (as assessed by GDS score) compared with the Alzheimer's disease and control groups, and similarly to Sheline et al we show increased connectivity with the caudate in the DLB group, with our connectivity map (Fig. DS1) showing strong similarities with the affective network described by Sheline et al Reference Sheline, Price, Yan and Mintun48 even though some of our participants were taking antidepressants. It is important that medication effects on connectivity are investigated further, for example before and after treatment and how treatment might affect cognitive measures, however this would comprise a separate study itself.
In conclusion, the main findings of this study were in individuals with DLB greater subcortical functional connectivity with the head of caudate nucleus, which may be related to depressive symptoms; the putamen, which may be as a result of motor symptoms of Parkinson's disease; and the thalamus, which may be linked to fluctuations in cognition. These results are in agreement with our previous study that also showed greater connectivity in DLB and Alzheimer's disease with cortical seed regions. Reference Kenny, Blamire, Firbank and O'Brien10 In combination, these studies provide support for the theory that in DLB and Alzheimer's disease connectivity may increase in the earlier stages of disease with new brain areas recruited to compensate for loss of others, before activity is decreased later in the course of the disease. Further studies investigating longitudinal changes and treatment effects are needed to explore these hypotheses further.
Funding
This work was supported by the Medical Research Council, Alzheimer's Research UK and the UK NIHR Biomedical Research Centre for Ageing and Age-related Disease award to the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Acknowledgements
The authors thank the study participants for their invaluable contribution and the North East Dementia and Neurodegenerative Diseases Research Network (DeNDRoN) for help with participant recruitment.




eLetters
No eLetters have been published for this article.