Studies of those predisposed to schizophrenia for genetic reasons have consistently found lower IQ scores compared with controls prior to the onset of formal illness (Reference Niemi, Suvisaari and Tuulio-HenrikssonNiemi et al, 2003). After illness onset, scores are lower in populations with schizophrenia than in the general population (Reference Dunkley, Rogers and DavidDunkley & Rogers, 1994; Reference Barber, Pantelis, Bodger, Pantelis, Nelson and BarnesBarber et al, 1996) with some individuals showing striking deficits (Reference Cunningham Owens and JohnstoneCunningham Owens & Johnstone, 1980; Reference Buhrich, Crow and JohnstoneBuhrich et al, 1988). A series of more specific cognitive problems, largely relating to memory and executive function, have now been well replicated as associated with schizophrenia (Reference FrithFrith, 1992). In addition, the onset of formal illness may be heralded by cognitive decline (Johnstone et al,Reference Johnstone, Cosway and Lawrie2002a ,Reference Johnstone, Lawrie and Cosway b ). Thus the relationship between schizophrenia and impairments in cognitive ability at all stages – pre-illness, during acute illness and post-illness – is now acknowledged.
At 3%, the point prevalence of schizophrenia in populations considered to have mild idiopathic intellectual disability (DSM–IV mild mental retardation; American Psychiatric Association, 1994) is some three times that in the general population (Reference TurnerTurner, 1989). Our previous studies have compared people where schizophrenia is comorbid with learning disability and individuals with learning disability alone and schizophrenia alone (Reference Doody, Johnstone and SandersonDoody et al, 1998; Reference Sanderson, Best and DoodySanderson et al, 1999). Structural brain changes in the sample with comorbidity resembled those of the sample with schizophrenia alone. In addition, they had high rates of positive family histories of schizophrenia as well as high rates of chromosomal variants and abnormalities (Reference Muir, Davidson and DoodyMuir et al, 1998). These results suggest that within the population with intellectual disability there may be individuals whose cognitive difficulties are part of a schizophrenic illness yet to become clinically manifest.
Where cognitive difficulties precede the onset of psychotic symptomatology and illness, perhaps by some years, early onset would be likely to come to attention through intellectual disability in the school years and these young people would thus enter special needs education. If the intellectual disability were significant then any subsequent psychiatric disorder would most probably be managed in the setting of specialist learning disability services and affected individuals would not come to the attention of general psychiatric services. Indeed the learning disability components might be regarded as justifying exclusion from the more general schizophrenia phenotype. Thus, for administrative reasons, such individuals might be difficult to detect. Furthermore, in the wider population of individuals with learning disabilities, they are likely to represent only a small minority.
In the Edinburgh High-Risk Study of Schizophrenia (EHRS), scores on the Child Behavior Checklist (CBCL; Reference Achenbach, Howell and QuayAchenbach et al, 1991) and the Structured Inventory for Schizotypy (SIS; Reference Kendler, Lieberman and WalshKendler et al, 1989) were significant predictors of the development of psychotic symptoms and among the most important predictors of the later development of a formal schizophrenic illness (Miller et al, Reference Miller, Byrne and Hodges2002a ,Reference Miller, Byrne and Hodges b ; Reference Johnstone, Ebmeier and MillerJohnstone et al, 2005). Wide-ranging neuropsychological impairments, principally in memory and executive function, were demonstrable in many more individuals than were ever likely to develop schizophrenia, but these were worse in those who became ill (Reference Byrne, Clafferty and CoswayByrne et al, 2003; Reference Johnstone, Ebmeier and MillerJohnstone et al, 2005). In addition those at high risk had demonstrable differences in structural brain parameters with thalami and amygdala–hippocampal complexes significantly smaller than in controls (Lawrie et al, Reference Lawrie, Whalley and Kestelman1999, Reference Lawrie, Whalley and Abukmeil2001), and those who developed psychotic symptoms and subsequent schizophrenia showed reductions in grey matter not evident in those who remained well (Reference Lawrie, Whalley and AbukmeilLawrie et al, 2002; Reference Job, Whalley and JohnstoneJob et al, 2005). Further dynamic reductions in temporal lobe size appeared to precede the onset of illness (Reference Job, Whalley and JohnstoneJob et al, 2005).
This longitudinal study follows the general design of the EHRS and investigates the clinical and mental state of a cohort of over 240 young people aged between 13 and 22 years receiving special educational support because of low attainment presumed to be due to intellectual disability. Together with their siblings and unrelated controls, they were recruited to examine whether within the young population with educational difficulties there are those whose cognitive impairments are, at least in part, due to psychotic illness which is yet to become manifest.
METHOD
Study design
The 19 education authorities in Scotland covering districts within reasonable travelling distance of Edinburgh were approached and 18 agreed to participate. Two hundred and seventy-three schools and colleges providing education for students with mild learning disability were then contacted and 99 responded positively. Inclusion policies for students with special educational needs are not universally operational in Scotland and education is provided in special schools as well as in integrated classes in mainstream schools. There are schools catering for particular groups such as children with autism, those with emotional, behavioural, but not intellectual difficulties, those with multiple handicaps and those with sensory impairments, but most special education is directed to those with cognitive difficulties. Measured IQ is nowadays rarely used in educational settings but it is generally considered that most requiring special education would be functioning at an IQ of 80 or below, placing them in the range with intellectual disability or borderline intellectual disability. We omitted the schools/colleges for specialist groups and directed our enquiries to the generality of special needs education. Head teachers were asked to concentrate on those with a presumed IQ in the range of 50–80 (i.e. ‘mild’ and ‘borderline’ intellectual disability). Within this group, those with Down syndrome or other syndromal disorders, major sensory impairments, absence of speech or major cerebral palsy were excluded, as were individuals with clear severe or profound intellectual disability. Schools sent explanatory letters to relevant families on our behalf, with the families expressing their willingness to participate on an ‘opt in’ basis by identifying themselves directly to the research team (Fig. 1).
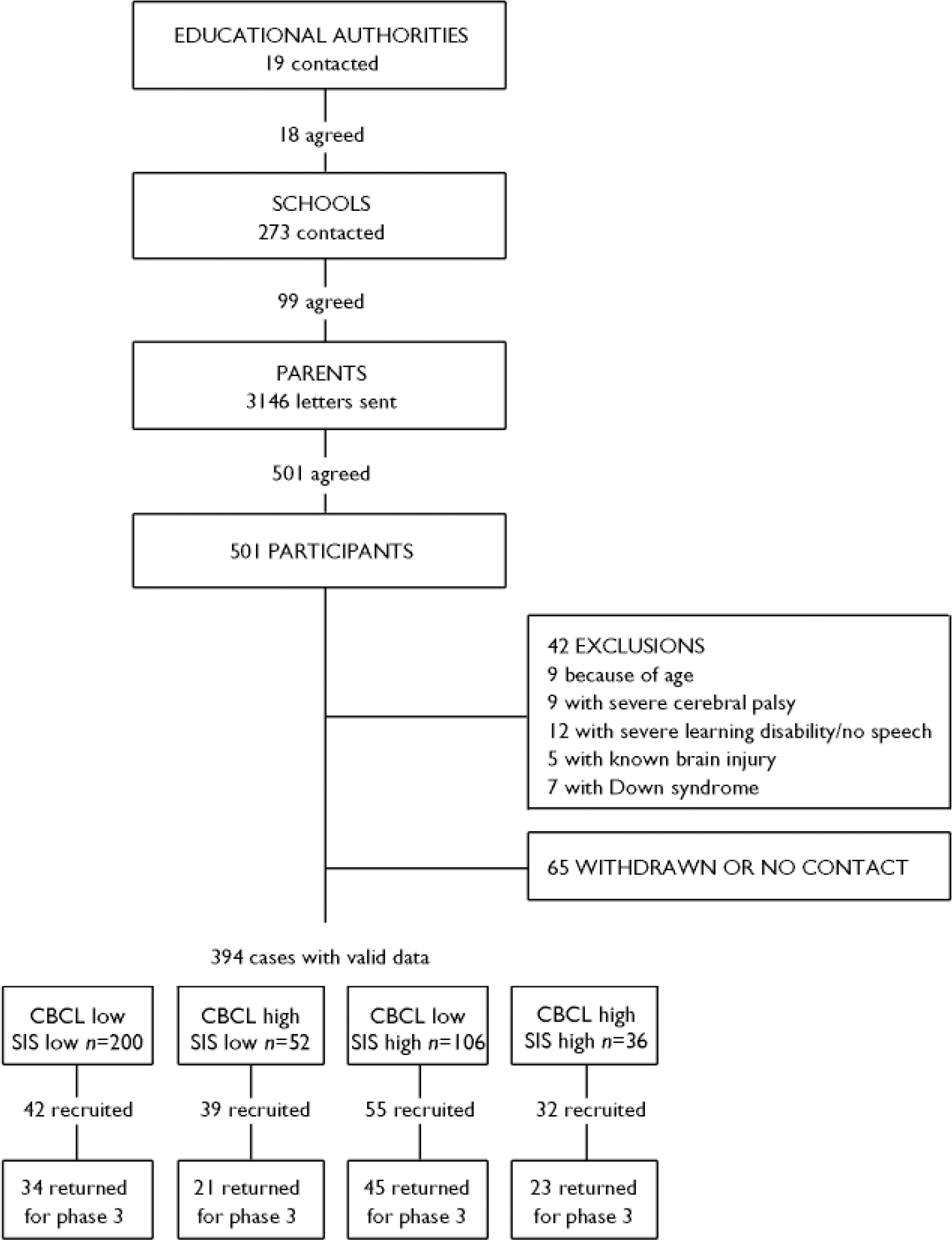
Fig. 1 Flow chart illustrating the recruitment process. CBCL, Child Behaviour Checklist; SIS, Structured Inventory for Schizotypy.
In addition, two comparison groups were recruited comprising: (a) the participants' siblings (also aged 13–22 years) and (b) age-matched controls with no history of psychiatric disorder or special educational requirements recruited through youth and voluntary organisations in the areas from which sample participants came.
Clinical assessments
Phase 1
The families of 501 individuals initially agreed to participate, but in 36 cases, the family subsequently withdrew. The CBCL was therefore completed for 465 participants by one of the research team, who visited the participant's home and interviewed the parents (usually the mother). The CBCL has been validated for use in those with learning disabilities (Reference Epstein and CullinanEpstein & Cullinan, 1984; Reference Schachter, Pless and BruckSchachter et al, 1991; Reference Crijnen, Achenbach and VerhulstCrijnen et al, 1999; Reference Dekker and KootDekker & Koot, 2003). Of these 465 participants, 42 were excluded and an additional 28 did not participate further (Fig. 1).
The SIS was conducted on all but one of the remaining participants, leaving 394 (Fig. 1). This instrument has not been widely used in people with intellectual disability and a pilot investigation (by E.C.J. and D.G.C.O.) was undertaken at a local school for young people with intellectual disability to determine its feasibility in young people with this level of intellectual functioning. Sixteen young people were selected on the basis that they were considered by the teachers and the visiting consultant paediatrician to have an IQ of between 50 and 70. There were no participants in whom the interview could not be conducted. Satisfactory interrater reliabilities were obtained, similar satisfactory results being obtained in subsequent training sessions with other relevant staff (reliability of individual scores, Pearson's rho=0.962; reliability of global scores, Pearson's rho=0.760).
It is known that CBCL scores are higher in individuals with intellectual disability and it was anticipated that a similar situation would arise with the SIS. In fact, the CBCL scores were more than double those in the EHRS whereas SIS scores were elevated by some 16% in general. Cut-offs were therefore based on percentages of individuals in the EHRS who scored above and below the cut-offs, rather than transposing the absolute cut-off points themselves.
Phase 2 (time 1)
Generally 4–6 months after the baseline assessment, participants attended the Division of Psychiatry for the day. The plan for neuropsychology testing was modified from the EHRS to accommodate issues related to age and ability, preserving an emphasis on memory and executive function. The Wechsler Intelligence Scale for Children III (WISC–III; Reference WechslerWechsler, 1992) and the Wechsler Adult Intelligence Scale III (WAIS–III; Reference WechslerWechsler, 1999) (as appropriate to the individual's age) were used to determine IQ in all participants at baseline. Aspects of memory were assessed using the Rivermead Behavioural Memory Test (RBMT; Reference Wilson, Cockburn and BaddeleyWilson et al, 1985) and executive function with the Behavioural Assessment of Dysexecutive Syndrome (BADS; Reference Wilson, Evans and EmslieWilson et al, 1998). The Present State Examination (Reference Wing, Cooper and SartoriusWing et al, 1974), which was utilised in the EHRS, was considered too long and complex for assessment of mental state in this population. The Clinical Interview Schedule (CIS; Reference Goldberg, Cooper and EastwoodGoldberg et al, 1970) was chosen as an alternative as it is relatively brief, covers key areas for establishing ‘caseness’ in major psychiatric disorders with reliability, and is acceptable to young people with intellectual disability (Reference Davidson, Humphreys and JohnstoneDavidson et al, 1995). Psychotic phenomena were classified according to Krawiecka et al (Reference Krawiecka, Goldberg and Vaughan1977). All clinical ratings were performed with the rater masked to CBCL/CIS status.
After clinical assessments, participants had a structural magnetic resonance imaging scan but the scan results are not included in the present report.
Phase 3 (time 2)
All 247 participants who completed the second phase of clinical assessments were invited to return for a third phase, and 185 (75%) of them reattended (184 with useful data: 33 siblings, 28 unrelated controls, 34 CBCL low/SIS low, 21 CBCL high/SIS low, 45 CBCL low/SIS high and 23 CBCL high/SIS high). The third phase of clinical assessments consisted of reassessment with the CIS and Positive and Negative Syndrome Scale (Reference Kay, Fiszbein and OplerKay et al, 1987), together with repeat neuropsychological assessments for all participants.
RESULTS
Recruitment to the study groups was successfully completed, with a total of 247 participants: 79 controls (47 siblings and 32 unrelated controls) and 168 young people with mild intellectual disability. Slight oversampling (planned sample 240) was conducted to even out initial gender imbalances. Almost all subjects were White and born in the UK, with English as their first language. The demographic details at baseline are shown in Table 1.
Table 1 Baseline demographic characteristics of participants

| Siblings (n=47) | Unrelated controls (n=32) | CBCL low/SIS low (n=42) | CBCL high/SIS low (n=39) | CBCL low/SIS high (n=55) | CBCL high/SIS high (n=32) | |
|---|---|---|---|---|---|---|
| Gender, n | ||||||
| Male | 25 | 12 | 24 | 26 | 35 | 24 |
| Female | 22 | 20 | 18 | 13 | 20 | 8 |
| Age, years: mean (s.d.) | 16.8 (2.2) | 16.4 (1.7) | 16.4 (1.5) | 15.3 (1.4) | 15.6 (1.7) | 15.9 (1.9) |
| Full-scale IQ (range) | 97.5 (70–135) | 105.1 (65–143) | 73.6 (42–125) | 72.1 (47–107) | 74.9 (46–107) | 74.3 (40–131) |
| IQ > 100, n (%) | 19 (40.4) | 21 (65.6) | 3 (7.1) | 2 (5.1) | 4 (7.3) | 3 (9.4) |
| CBCL score, mean (s.d.) | 27.7 (24.5) | 14.9 (13.2) | 51.9 (22.8) | 109.8 (20.8) | 57.2 (19.7) | 111.7 (18.3) |
| SIS score, mean (s.d.) | 21.6 (0.84) | 18.5 (6.3) | 21.0 (6.2) | 23.1 (5.1) | 38.1 (7.5) | 38.3 (7.7) |
CBCL, Child Behavior Checklist; SIS, Structured Inventory for Schizotypy
Mental state assessments and neuropsychological tests were successfully completed in all cases.
CIS assessments
The CIS assessments show wide-ranging psychopathology. The usual scheme of scoring is that items are assessed using a 5-point scale with a score of 2 or more indicating results within the morbid range. Scores of 3 or 4 did occur in at least one of the 25 items of the CIS in 53 individuals (48 young people with mild intellectual disability and 5 controls). We therefore decided to divide the scores into three categories: 0, absent; 1, not clearly morbid; and 2 or more, morbid. Not all items showed differences between the six groups, but significant differences were found (Table 2).
Table 2 Symptoms and signs on the Clinical Interview Schedule for participants with mild intellectual disabilities and in controls 1

| Symptoms and signs | CIS score >2, % within each group | P-value 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siblings | Unrelated controls | SIS low/CBCL low | SIS low/CBCL high | SIS high/CBCL low | SIS high/CBCL high | |||||||
| Symptoms | ||||||||||||
| Excessive concern with bodily functions | ||||||||||||
| First follow-up | 4.3 | 6.3 | 4.8 | 5.1 | 7.3 | 9.4 | NS | |||||
| Second follow-up | 6.1 | 3.6 | 2.9 | 9.5 | 8.9 | 26.1 | 0.041 | |||||
| Fatigue | ||||||||||||
| First follow-up | 14.9 | 3.1 | 7.1 | 7.7 | 12.7 | 34.4 | 0.003 | |||||
| Second follow-up | 6.1 | 7.1 | 20.0 | 14.3 | 26.7 | 52.2 | <0.001 | |||||
| Sleep disturbance | ||||||||||||
| First follow-up | 8.5 | 3.1 | 7.1 | 10.3 | 5.5 | 21.9 | NS | |||||
| Second follow-up | 3.0 | 10.7 | 17.1 | 28.6 | 8.9 | 39.1 | 0.003 | |||||
| Irritability | ||||||||||||
| First follow-up | 4.3 | 6.3 | 16.7 | 5.1 | 18.2 | 40.6 | <0.001 | |||||
| Second follow-up | 6.1 | 3.6 | 11.4 | 38.1 | 22.2 | 47.8 | <0.001 | |||||
| Lack of concentration | ||||||||||||
| First follow-up | 4.3 | 0 | 16.7 | 5.1 | 18.2 | 40.6 | <0.001 | |||||
| Second follow-up | 0 | 0 | 14.3 | 14.3 | 20.0 | 26.1 | 0.010 | |||||
| Depression | ||||||||||||
| First follow-up | 6.4 | 3.1 | 11.9 | 12.8 | 21.8 | 31.3 | 0.008 | |||||
| Second follow-up | 6.1 | 0 | 11.4 | 19.0 | 11.1 | 21.7 | NS | |||||
| Depressive thoughts | ||||||||||||
| First follow-up | 4.3 | 0 | 4.8 | 10.3 | 10.9 | 31.3 | <0.001 | |||||
| Second follow-up | 9.1 | 0 | 0 | 9.5 | 4.4 | 8.7 | NS | |||||
| Anxiety | ||||||||||||
| First follow-up | 10.5 | 15.6 | 19.0 | 10.3 | 10.9 | 31.3 | <0.001 | |||||
| Second follow-up | 9.1 | 0 | 17.1 | 4.8 | 24.4 | 34.8 | 0.004 | |||||
| Obsessions/compulsions | ||||||||||||
| First follow-up | 4.3 | 9.4 | 16.7 | 12.8 | 20.0 | 37.5 | 0.003 | |||||
| Second follow-up | 12.1 | 0 | 14.3 | 14.3 | 17.8 | 26.1 | NS | |||||
| Signs | ||||||||||||
| Slowness | ||||||||||||
| First follow-up | 2.1 | 0 | 4.8 | 5.1 | 14.5 | 21.9 | 0.004 | |||||
| Second follow-up | 6.1 | 0 | 5.7 | 9.5 | 24.4 | 17.4 | 0.015 | |||||
| Depression | ||||||||||||
| First follow-up | 0 | 0 | 0 | 5.1 | 7.3 | 9.4 | NS | |||||
| Second follow-up | 0 | 0 | 2.9 | 19.0 | 2.2 | 8.7 | 0.008 | |||||
| Flattening of affect | ||||||||||||
| First follow-up | 0 | 0 | 4.8 | 7.7 | 3.6 | 6.3 | NS | |||||
| Second follow-up | 0 | 0 | 2.9 | 19.0 | 20.0 | 13.0 | 0.004 | |||||
| Delusions | ||||||||||||
| First follow-up | 2.1 | 3.1 | 2.4 | 0 | 9.1 | 25.0 | <0.001 | |||||
| Second follow-up | 0 | 0 | 11.4 | 0 | 11.1 | 30.4 | <0.001 | |||||
| Hallucinations | ||||||||||||
| First follow-up | 4.3 | 0 | 9.5 | 5.1 | 18.2 | 40.6 | <0.001 | |||||
| Second follow-up | 0 | 0 | 14.3 | 0 | 17.8 | 30.4 | 0.001 | |||||
| Incoherence | ||||||||||||
| First follow-up | 0 | 0 | 2.4 | 0 | 1.8 | 12.5 | 0.005 | |||||
| Second follow-up | 0 | 0 | 0 | 0 | 2.2 | 0 | NS | |||||
| Poverty of speech | ||||||||||||
| First follow-up | 2.1 | 0 | 9.5 | 17.9 | 14.5 | 25.0 | 0.006 | |||||
| Second follow-up | 9.1 | 0 | 14.3 | 19.0 | 35.6 | 21.7 | 0.003 | |||||
1. Items showing no significant difference are excluded
2. Chi squared test used (d.f.=5) in analysis of frequencies of absent and morbid symptoms across all six groups
Clearly, no group is without psychopathology but morbid scores tend to be higher in the groups with mild intellectual disability. The most dramatic differences relate to delusions and hallucinations, which are present at a score of 2 or more in 8 (25.0%) and 13 (40.6%) of those in the SIS high/CBCL high group, slightly fewer (5 (9.1%) and 10 (18.2%) respectively) in the SIS high/CBCL low group, but were otherwise uncommon in the other groups (Table 2).
As noted in Table 1, there was a wide range of IQ scores in the groups with intellectual disability (even though the mean score was within the 50–80 range), and there were 12 individuals with an IQ of 100 or more. When these 12 were compared with the remainder, there were no significant differences in the presence of psychopathology items scoring 2 or more on the CIS (i.e. in the clearly morbid range). Interestingly, however, the proportion with delusions, hallucinations and obsessions were higher in this group (delusions 25 v. 7.1%, hallucinations 25 v. 16.9% and obsessions 35 v. 19.9%). This high level of psychopathology might explain why those of average IQ were in special needs education. Nevertheless, even if this group with higher IQ is removed from the analysis, significant between-group differences still remain for all psychotic items of symptomatology and for obsessions.
We went on to consider the CIS results in a hierarchical system of six categories similar to that used in the EHRS (Reference Johnstone, Abukemeil and ByrneJohnstone et al, 2000), where 6=any positive symptoms (delusions, hallucinations, incoherence or incongruity) scoring at least 3 (marked) or 4 (severe); 5=any positive symptom scoring 2 (moderate); 4=any positive symptom scoring 1 (mild – not necessarily morbid); 3=any negative symptoms (flattening of affect, poverty of speech, retardation) scoring at least 2; 2=any non-specific symptom (i.e. those not listed above) scoring at least 2; 1=none of the above. Table 3 provides CIS results for groups with mild intellectual disability and controls at first follow-up and second follow-up, and overall results based on the highest ratings using the above system across both assessments. Nine young people with mild intellectual disability (5.4%) and no controls had a highest CIS rating in the most severe category. Of these 9, 7 (78%) were assessed at baseline as high-scoring on both the SIS and CBCL, and 2 (22%) were high-scoring on the SIS and low-scoring on the CBCL. Severity ratings on the CIS demonstrated significant differences across the four groups with mild intellectual disability for ratings at first and second follow-up phases, and the highest ratings across both assessments (Kruskal–Wallis test, first follow-up, n=168, χ2=24.9, d.f.=3, P<0.001; second follow-up, n=124, χ2=12.9, d.f.=3, P=0.005; highest-ever rating, n=168, χ2=25.9, d.f.=3, P<0.001). Comparison of severity ratings for all groups with mild intellectual disability according to SIS classification (high or low) also demonstrated significant differences for ratings at first and second follow-up and the highest ratings across both assessments (Mann–Whitney test, first follow-up, n=168, U=2394, Z=–3.67, P<0.001; second follow-up, n=124, Z=–3.36, P=0.001; highest-ever rating: n=168, Z=–4.40, P<0.001).
Table 3 Categorisation of participants according to Clinical Interview Schedule at first and second follow-up and highest ratings 1

| Categorisation according to Clinical Interval Schecule, n (%) 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||||
| First follow-up (n=247) | |||||||||||
| Siblings | 21 (44.7) | 14 (29.8) | 1 (2.1) | 8 (17.0) | 3 (6.4) | 0 | |||||
| Unrelated controls | 19 (59.4) | 7 (21.9) | 0 | 5 (15.6) | 1 (3.1) | 0 | |||||
| CBCL low/SIS low | 12 (28.6) | 15 (35.7) | 3 (7.1) | 6 (14.3) | 6 (14.3) | 0 | |||||
| CBCL high/SIS low | 14 (35.9) | 6 (15.4) | 6 (15.4) | 10 (25.6) | 3 (7.7) | 0 | |||||
| CBCL low/SIS high | 17 (30.9) | 6 (10.9) | 4 (7.3) | 16 (29.1) | 10 (18.2) | 2 (3.6) | |||||
| CBCL high/SIS high | 2 (6.3) | 5 (15.6) | 0 | 7 (21.9) | 13 (40.6) | 5 (15.6) | |||||
| Second follow-up (n=185) | |||||||||||
| Siblings | 19 (57.6) | 7 (21.2) | 2 (6.1) | 5 (15.2) | 0 | 0 | |||||
| Unrelated controls | 19 (67.9) | 8 (28.6) | 0 | 1 (3.6) | 0 | 0 | |||||
| CBCL low/SIS low | 14 (40.0) | 5 (14.3) | 2 (5.7) | 6 (17.1) | 8 (22.9) | 0 | |||||
| CBCL high/SIS low | 2 (9.5) | 8 (38.1) | 5 (23.8) | 6 (28.6) | 0 | 0 | |||||
| CBCL low/SIS high | 5 (11.1) | 4 (8.9) | 10 (22.2) | 17 (37.8) | 8 (17.8) | 1 (2.2) | |||||
| CBCL high/SIS high | 3 (13.0) | 3 (13.0) | 0 | 7 (30.4) | 7 (30.4) | 3 (13.0) | |||||
| Highest-ever ratings (n=247) | |||||||||||
| Siblings | 16 (34.0) | 14 (29.8) | 2 (4.3) | 12 (25.5) | 3 (6.4) | 0 | |||||
| Unrelated controls | 15 (46.9) | 11 (34.4) | 0 | 5 (15.6) | 1 (3.1) | 0 | |||||
| CBCL low/SIS low | 7 (16.7) | 11 (26.2) | 3 (7.1) | 9 (21.4) | 12 (28.6) | 0 | |||||
| CBCL high/SIS low | 9 (23.1) | 6 (15.4) | 7 (17.9) | 14 (35.9) | 3 (7.7) | 0 | |||||
| CBCL low/SIS high | 5 (9.1) | 5 (9.1) | 5 (9.1) | 22 (40.0) | 16 (29.1) | 2 (3.6) | |||||
| CBCL high/SIS high | 2 (6.3) | 4 (12.5) | 0 | 5 (15.6) | 14 (43.8) | 7 (21.9) | |||||
CBCL, Child Behavior Checklist; SIS, Structured Inventory for Schizotypy
1. Differences between groups were assessed with Mann–Whitney U-test at first follow-up, U=4268.0, Z=–4.67, P<0.001; at second follow-up, U=1545.0, Z=–6.74, P<0.001; and the highest-ever ratings across both assessments, U=3428.5, Z=–6.28, P<0.001
2. 1=no symptoms; 2=any non-specific symptom; 3=any negative symptom; 4=any mild positive symptom; 5=any moderate positive symptom; 6=any severe positive symptom
Three of the nine young people with scores in category 6 have clearly developed schizophrenia, with sustained fully held delusions and hallucinations. They are now receiving treatment. A fourth has hallucinations only but these are persistent and have worsened, now scoring severe on the CIS having scored moderate at time 1. Schizophrenia is the most likely diagnosis and treatment is being considered. The remaining five have clinically significant delusional or hallucinatory symptoms which are not yet considered sufficiently sustained to justify a diagnosis of schizophrenia although it is clearly a possibility.
Neuropsychology
The full range of RBMT and BADS scores on the six groups at first and second follow-up is shown in Table 4.
Table 4 Neuropsychological performance at first (n=245) and second (n=183) follow-up

| Neuropsychological test | Score: mean (s.d.) | P-value 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siblings | Unrelated controls | SIS low/CBCL low | SIS low/CBCL high | SIS high/CBCL low | SIS high/CBCL high | |||||||
| RBMT story immediate recall | ||||||||||||
| First follow-up | 7.16 (3.08) | 7.49 (3.54) | 4.80 (2.98) | 4.47 (3.77) | 4.57 (2.99) | 3.94 (2.78) | <0.001 | |||||
| Second follow-up | 7.03 (2.34) | 8.39 (3.16) | 4.64 (3.11) | 4.29 (2.40) | 3.94 (2.48) | 4.98 (3.49) | <0.001 | |||||
| RBMT pictures | ||||||||||||
| First follow-up | 9.89 (0.38) | 9.94 (0.25) | 9.81 (0.94) | 9.84 (0.55) | 9.87 (0.39) | 9.84 (0.74) | NS | |||||
| Second follow-up | 10.00 (0) | 9.96 (0.19) | 9.97 (0.17) | 9.86 (0.36) | 9.89 (0.32) | 9.45 (1.18) | 0.005 2 | |||||
| RBMT route immediate recall | ||||||||||||
| First follow-up | 10.91 (0.58) | 10.84 (0.88) | 10.50 (1.33) | 10.32 (1.58) | 10.87 (0.72) | 10.81 (0.75) | 0.026 | |||||
| Second follow-up | 11.00 (0) | 11.00 (0) | 11.00 (0) | 11.00 (0) | 10.67 (1.17) | 10.41 (1.68) | 0.019 | |||||
| RBMT face recognition | ||||||||||||
| First follow-up | 5.00 (0) | 4.94 (0.25) | 4.95 (0.22) | 4.92 (0.27) | 4.84 (0.42) | 5.00 (0) | 0.032 | |||||
| Second follow-up | 5.00 (0) | 4.96 (0.19) | 4.94 (0.24) | 4.81 (0.51) | 4.91 (0.29) | 4.91 (0.29) | NS | |||||
| RBMT orientation | ||||||||||||
| First follow-up | 8.66 (0.70) | 8.84 (0.45) | 7.93 (1.57) | 7.39 (1.90) | 7.85 (1.35) | 7.74 (1.71) | <0.001 | |||||
| Second follow-up | 9.00 (0) | 8.93 (0.38) | 7.97 (1.51) | 7.95 (1.56) | 8.09 (1.29) | 8.14 (1.64) | <0.001 | |||||
| RBMT appointment | ||||||||||||
| First follow-up | 1.77 (0.48) | 1.88 (0.34) | 1.60 (0.59) | 1.71 (0.52) | 1.58 (0.57) | 1.29 (0.64) | <0.001 3 | |||||
| Second follow-up | 1.97 (0.18) | 1.93 (0.26) | 1.63 (0.60) | 1.81 (0.40) | 1.51 (0.55) | 1.55 (0.51) | <0.001 | |||||
| RBMT story delayed recall | ||||||||||||
| First follow-up | 6.41 (3.29) | 5.80 (2.71) | 3.85 (2.91) | 3.93 (3.33) | 3.73 (2.69) | 3.66 (2.59) | <0.001 | |||||
| Second follow-up | 6.45 (2.46) | 7.32 (2.90) | 4.06 (2.86) | 3.93 (2.24) | 3.32 (2.31) | 4.14 (3.44) | <0.001 | |||||
| RBMT route delayed recall | ||||||||||||
| First follow-up | 10.94 (0.44) | 10.84 (0.88) | 10.26 (1.58) | 10.26 (1.74) | 10.78 (0.94) | 10.84 (0.64) | 0.006 | |||||
| Second follow-up | 11.00 (0) | 11.00 (0) | 10.86 (0.85) | 10.86 (0.66) | 10.73 (0.89) | 10.41 (1.68) | NS | |||||
| RBMT message delayed | ||||||||||||
| First follow-up | 2.91 (0.28) | 2.94 (0.25) | 2.86 (0.42) | 2.68 (0.53) | 2.64 (0.62) | 2.84 (0.37) | 0.006 | |||||
| Second follow-up | 2.94 (0.25) | 2.96 (0.19) | 2.89 (0.40) | 2.81 (0.40) | 2.89 (0.32) | 2.82 (0.40) | NS | |||||
| RBMT first name | ||||||||||||
| First follow-up | 1.72 (0.65) | 1.88 (0.42) | 1.36 (0.91) | 1.05 (0.96) | 1.33 (0.86) | 1.19 (0.91) | <0.001 | |||||
| Second follow-up | 1.91 (0.39) | 1.89 (0.32) | 1.57 (0.74) | 1.48 (0.87) | 1.33 (0.85) | 1.55 (0.86) | 0.004 | |||||
| RBMT second name | ||||||||||||
| First follow-up | 1.83 (0.52) | 1.81 (0.59) | 1.05 (0.96) | 1.16 (0.95) | 1.31 (0.92) | 1.10 (0.94) | <0.001 | |||||
| Second follow-up | 1.97 (0.18) | 1.86 (0.53) | 1.51 (0.78) | 1.24 (1.00) | 1.56 (0.79) | 1.27 (0.94) | 0.001 | |||||
| RBMT belonging | ||||||||||||
| First follow-up | 3.47 (0.95) | 3.87 (0.34) | 3.43 (0.83) | 3.24 (0.88) | 3.36 (0.89) | 3.10 (0.94) | <0.001 | |||||
| Second follow-up | 3.94 (0.25) | 3.96 (0.19) | 3.54 (0.82) | 3.62 (0.59) | 3.60 (0.81) | 3.68 (0.48) | 0.009 | |||||
| RBMT profile | ||||||||||||
| First follow-up | 21.38 (2.62) | 22.16 (2.13) | 18.33 (3.56) | 17.24 (5.02) | 18.02 (3.70) | 17.29 (2.83) | <0.001 | |||||
| Second follow-up | 22.85 (1.28) | 22.86 (1.63) | 19.11 (3.63) | 19.00 (3.33) | 18.13 (4.21) | 18.36 (4.02) | <0.001 | |||||
| RBMT screen | ||||||||||||
| First follow-up | 10.21 (1.57) | 10.75 (1.46) | 8.48 (2.04) | 7.95 (2.81) | 8.22 (2.07) | 7.61 (1.71) | <0.001 | |||||
| Second follow-up | 11.24 (0.66) | 11.25 (1.01) | 9.11 (2.03) | 8.81 (2.09) | 8.51 (2.50) | 8.50 (2.30) | <0.001 | |||||
| BADS rule | ||||||||||||
| First follow-up | 3.38 (0.87) | 3.72 (0.46) | 2.52 (1.15) | 2.53 (1.25) | 2.56 (1.12) | 2.52 (1.57) | <0.001 | |||||
| Second follow-up | 3.48 (0.62) | 3.68 (0.48) | 2.91 (1.10) | 2.86 (1.06) | 2.73 (1.39) | 2.68 (1.25) | 0.001 | |||||
| BADS key | ||||||||||||
| First follow-up | 2.70 (1.08) | 2.91 (1.25) | 1.79 (1.39) | 1.84 (1.52) | 1.71 (1.24) | 1.68 (1.30) | <0.001 | |||||
| Second follow-up | 3.15 (1.09) | 3.57 (0.69) | 1.66 (1.45) | 1.76 (1.55) | 1.71 (1.29) | 1.95 (1.43) | <0.001 | |||||
| BADS temp | ||||||||||||
| First follow-up | 2.11 (0.79) | 2.44 (0.91) | 1.64 (0.88) | 1.76 (0.97) | 1.56 (0.83) | 1.42 (1.15) | <0.001 | |||||
| Second follow-up | 2.18 (0.85) | 2.29 (0.81) | 1.66 (0.97) | 1.62 (0.81) | 1.76 (0.96) | 1.86 (1.08) | 0.010 | |||||
| BADS zoo | ||||||||||||
| First follow-up | 2.94 (1.05) | 2.91 (1.15) | 1.78 (1.33) | 2.05 (1.18) | 1.89 (1.18) | 1.84 (1.21) | <0.001 | |||||
| Second follow-up | 2.94 (1.06) | 3.07 (0.90) | 2.18 (1.27) | 2.29 (1.15) | 1.98 (1.20) | 1.95 (1.36) | <0.001 | |||||
CBCL, Child Behavior Checklist; SIS, Structured Inventory for Schizotypy; RBMT, Rivermead Behavioural Memory Test; BADS, Behavioural Assessment of Dysexecutive Syndrome
1. Kruskal–Wallis test (d.f.=5) was used to analyse test performance across all six participant groups
2. χ2=8.25, d.f.=3, P=0.041 for comparison across groups with intellectual disability
3. χ2=9.51, d.f.=3, P=0.023 for comparison across groups with intellectual disability
As we were principally interested in how neuropsychological performance related to schizophrenia, we divided participants into high (above the cut-off) and low (below the cut-off) SIS groups. Table 5 shows the mean IQ scores and mean scores for tests where significant differences were found between participants divided according to SIS category.
Table 5 IQ scores and scores on sub-tests from the neuropsychological battery according to category on the Structured Inventory for Schizotypy

| Assessment | Above SIS cut-off, mean (s.d.) | Below SIS cut-off, mean (s.d.) | ||||
|---|---|---|---|---|---|---|
| First follow-up (n=86) | Second follow-up (n=67) | First follow-up (n=81) | Second follow-up (n=56) | |||
| Full-scale IQ | 74.63 (17.14) | 78.64 (16.26) | 72.99 (17.13) | 79.95 (16.69) | ||
| Verbal IQ | 75.84 (17.00) | 78.48 (15.57) | 74.05 (16.55) | 78.32 (15.63) | ||
| Performance IQ | 77.84 (17.10) | 82.30 (17.76) | 76.37 (18.04) | 83.32 (18.28) | ||
| RBMT | ||||||
| Immediate route recall | 10.60 (1.24) | 10.58 (1.35) | 10.68 (1.05) | 11 (0.00) † | ||
| Orientation | 7.98 (1.52) * | 8.10 (1.40) | 7.52 (1.67) * | 7.96 (1.51) | ||
| Recall of appointment | 1.47 (0.63) * | 1.52 (0.53) | 1.67 (0.52) * | 1.69 (0.54) † | ||
SIS, Structured Inventory for Schizotypy; RBMT, Rivermead Behavioural Memory Test
* P<0.05 for groups at first follow-up
† P<0.05 for groups at second follow-up
There was no significant difference between participants above and below the cut-off for SIS in terms of verbal, performance and full-scale IQ measures at either the first or second follow-up assessments.
In the RBMT, participants below the cut-off for SIS performed better, although overall profile and screening scores did not significantly differ between groups at either assessment. There were, however, some significant differences between SIS high and SIS low groups on individual sub-tests of the RBMT with SIS high groups performing less well. Participants below the cut-off for SIS were significantly better than those above on recalling an appointment at both the first (Z=–2.17, P=0.03) and second (Z=–2.06, P=0.04) assessments. These participants also showed significantly better immediate route recall (Z=–2.48, P=0.013) at the second follow-up assessment and show trends to significantly better performance on several other RBMT sub-tests at both first and second follow-up assessments. High SIS participants were significantly better than low SIS participants on the orientation sub-test of the RBMT (Z=–2.06, P=0.04) at the first follow-up assessment. This is against our prediction, but it was not sustained at the second follow-up and it could be argued that orientation is more a test of general knowledge than of memory.
Performance scores on the BADS sub-tests did not significantly differ between the two SIS groups at either the first or second follow-up assessment, although the tendency was for higher scores in those below the SIS cut-off.
As full-scale IQ correlated significantly in all participant groups with RBMT profile and screening scores and with some individual sub-tests of the BADS, and in view of the very wide range of IQ scores in the participants with mild intellectual disability, we divided these into two subgroups. The performance of those with an IQ above the mean for the entire group (which was 74.68) was analysed separately from that of those below the mean. Each subgroup was then divided according to SIS category. We considered that this would control to an extent for the confounding effects of general intelligence on memory and executive measures.
We also wished to examine whether any cognitive decline had occurred over time which might manifest at the second follow-up assessment. We therefore included in this analysis only participants for whom there were baseline data and who had also returned for the second follow-up. In the immediate route recall sub-test of the RBMT, performance was significantly poorer at the second follow-up assessment in participants above the cut-off for SIS who were below the group mean IQ (Z=–2.17, P=0.03). In the group of participants above the group mean IQ (also at the second follow-up), SIS high participants were significantly worse than SIS low participants at recalling an appointment (Z=–2.01, P=0.04) and also on the BADS rule shift test profile score based on the numbers of errors made on the second trial (Z=–2.22, P=0.026) and on the time taken to complete the second (test) trial (Z=–2.04, P=0.04). Further details of the mean IQs and mean scores for these participants on sub-tests where significant differences were found is provided in Table DS1 of the online data supplement.
DISCUSSION
High rates of symptomatology were found among these young people at first follow-up (time 1) and these were higher among those with mild intellectual disability than among controls. The participants with mild intellectual disability are a population drawn from educational rather than health services and do not see themselves as medically unwell. Although symptoms are quite widespread at a level that would be considered just morbid, as would be expected from other studies of children and adolescents with intellectual disability, the numbers in whom individual symptoms were at the level 3 or 4 (Reference Goldberg, Cooper and EastwoodGoldberg et al, 1970) were relatively small and the number in whom psychopathology was at such a level that we felt it necessary to discuss with the young person and their family the need for medical attention was very small indeed. The fact that so many of the psychopathological features were more marked among those with mild intellectual disability than controls of higher IQ is not surprising (Reference Hoare, Harris and JacksonHoare et al, 1998; Reference EmersonEmerson, 2003; Reference SimonoffSimonoff, 2005). Emerson (Reference Emerson2003) found increased rates for anxiety disorder as well as conduct disorder, hyperkinesis and pervasive developmental disorders in young people with intellectual disability but perhaps surprisingly, not depression or psychosis. Clearly, however, psychotic symptoms do, in fact, occur in this population, both among controls and, to a much greater extent, among the groups with mild intellectual disability. Their occurrence is not unexpected. A number of studies have demonstrated that between 10 and 20% of the general population may experience isolated psychotic symptoms at some point in their lives, especially during adolescence (Reference Verdoux and van OsVerdoux & van Os, 2002) and in the EHRS we found these in 40% of the sample, even though most did not go on to develop schizophrenia and may now be expected to remain free of that disorder (Reference Johnstone, Ebmeier and MillerJohnstone et al, 2005).
Hypothesis testing
We had predicted that some of the study groups, notably those scoring above our cut-off on the SIS (Reference Kendler, Lieberman and WalshKendler et al, 1989) and to a lesser extent the CBCL (Reference Achenbach, Howell and QuayAchenbach et al, 1991), would be more likely to develop psychotic symptoms. A similar pattern was predicted for the neuropsychological tests.
The findings support these predictions. It had been considered that because of the young age of the participants none might actually develop schizophrenia within the time scale of the study, but at least three have and six more have symptoms highly suggestive of the condition. All are from the high SIS groups. Lesser symptoms, which may however be indicative of the extended phenotype of schizophrenia, were found in a substantial number of participants in these groups. Although the four groups with mild intellectual disability do not differ in general IQ, specific sub-tests of memory and executive function show significant differences between the high and low SIS groups, such that high SIS groups perform less well.
Comparison with EHRS
It therefore appears that the simple methods used to predict those of the EHRS sample at major risk of developing schizophrenia may also be used to predict the illness in those vulnerable to schizophrenia because of mild or borderline intellectual disability. This conclusion is strengthened by the fact that on assessment of the magnetic resonance imaging scans the measure of cortical gyrification which successfully predicted schizophrenia in the EHRS sample shows the same pattern in the high SIS groups in this sample (Reference Stanfield, Moorhead and HarrisStanfield et al, 2007). The EHRS sample did, of course, derive its vulnerability from familial risk, and the genetic causes of the structural and functional abnormalities as well as the psychopathology are becoming increasingly apparent (Reference Hall, Whalley and JobHall et al, 2006; Reference McIntosh, Baig and HallMcIntosh et al, 2007). We cannot at present know to what extent such molecular genetic influences are applicable to the present sample, but the commonalities of psychopathology, neuropsychological impairment and anomalies of brain structure do seem to suggest that we are seeing a final common pathway that leads to schizophrenia.
Implications
This study was not designed as an epidemiological study to provide an accurate population-based assessment of the frequency of symptomatology among young people receiving special educational services, but rather to see whether it is possible to detect vulnerability to schizophrenia in this population by relatively simple means. If this is the case, then it may be possible to make appropriate management available for these people at an early and hopefully more useful stage. We note a potential limitation within this study in terms of differing gender balances between the unrelated control group and the other study groups – however, the significance of gender as a confound is unclear, and this does not affect our main analyses, which are confined to the four groups with mild intellectual disability. In view of the association between intellectual disability and other disorders such as autism, it would be of interest to employ the clinical instruments used in this study in other such samples. The study sample was drawn from a total sample of 394, of whom, assuming a prevalence of schizophrenia in mild intellectual disability of 3% and assuming a similar risk in borderline intellectual disability, about 12 may be expected to develop schizophrenia. The findings we have at present indicate that it may well prove possible to detect most of these in advance of the onset of clinical symptoms sufficient to meet the criteria for schizophrenia. These results indicate that investigation of young people with mild/borderline intellectual disability with simple clinical methods may yield findings both of clinical importance for the individuals concerned and of theoretical value. This is a population which has received little research, perhaps because it falls to some extent between the remit of the educational and health services. It is clear that significant psychiatric morbidity within this population is not uncommon, and further research in people with intellectual disability is merited.
Acknowledgements
The kind cooperation of the young people, relatives and educational services is acknowledged. This study was supported by a programme grant from the UK Medical Research Council. S.M.L. is supported by the Mortimer and Theresa Sackler Institute for Psychobiological Research. The study was conducted under the auspices of the Multicentre Research Ethics Committee for Scotland, from whom it had approval.









eLetters
No eLetters have been published for this article.