Affective problems, such as depressive and anxiety symptoms and disorders, are important predictors of poorer cognitive functionReference James, Davis, O'Hare, Sharma, John and Gaysina1 and faster cognitive declineReference John, Patel, Rusted, Richards and Gaysina2 and constitute a risk factor for dementiaReference da Silva, Gonçalves-Pereira, Xavier and Mukaetova-Ladinska3 in older age. Understanding the pathways linking affective problems to later cognitive decline and dementia is a key public health priority, with important implications for dementia prevention.Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames4 Various biological mechanisms have been proposed to underlie associations between affective symptoms and cognitive ageing,Reference Butters, Young, Lopez, Aizenstein, Mulsant and Reynolds5 but to date, few of these have been systematically tested. One plausible biological mechanism is the cardiometabolic pathway.Reference Butters, Young, Lopez, Aizenstein, Mulsant and Reynolds5 Cardiometabolic risk is an umbrella term that encompasses a cluster of cardiovascular and metabolic conditions, including insulin resistance, obesity, hypertension, dyslipidaemia and atherosclerosis. A dose–response relationship has been found between severity of depression and cardiometabolic risk,Reference Butters, Young, Lopez, Aizenstein, Mulsant and Reynolds5,Reference Thomas, Kalaria and O'Brien6 and persistence of affective symptoms over the life course has been shown to be a predictor of mid-life cardiometabolic risk in the National Child Development Study (NCDS).Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky7 There is also increasing evidence that cardiometabolic risk factors are associated with accelerated cognitive ageing.Reference Bhat8 Despite this, no research has systematically investigated whether cardiometabolic pathway contributes to the association between affective symptoms and cognitive ageing and precisely how this association may operate. In particular, it is unknown whether cardiometabolic risk may be on the pathway between affective symptoms and cognitive ageing, or alternatively as a common cause contributing to both affective symptoms and cognitive outcomes. Consequently, the aim of this research was to test whether and how cardiometabolic risk may contribute to associations between affective symptoms and cognitive outcomes. Specifically, this study aims to test: (a) if there is an indirect path between accumulating affective symptoms and cognitive outcomes via cardiometabolic risk, something which would be suggestive of a mediational role; and (b) whether cardiometabolic risk predicts both affective symptoms and cognitive function at age 50, suggestive of common cause.
Method
Participants
Data were from the National Child Development Study (NCDS), a sample of 17 415 people born in England, Scotland and Wales during one week in March 1958. Data have been collected from cohort members a total of 11 times, at ages 0, 7, 11, 16, 23, 33, 42, 44, 46, 50 and 55 years. Comprehensive details about the sample, data collection methods and attrition rates are published elsewhere and are freely available online.Reference Power and Elliott9 Data were made available for the present study by METADAC, who manage genetic and biomedical data for the NCDS.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval for the biomedical sweep in NCDS was obtained from South East Multicentre Research Ethics Committee. Further ethical approval for the secondary analysis of NCDS data for this study was provided by the University of Sussex (ER/AJ316/2).
Measures
Cognitive outcomes
Measures of immediate memory, delayed memory, verbal fluency, information processing speed and information processing accuracy were available at age 50. Memory was assessed using a word-list recall test, in which 10 words were presented visually and cohort members listed the words they could recall immediately after presentation and then again after a delay. Verbal fluency was assessed using the Animal Naming Task, in which cohort members listed as many animals as they could within a timed period. Information processing speed and accuracy were assessed using a letter cancellation task. The number of letters scanned within a timed period represented information processing speed, with number of errors made used as a measure of processing accuracy. Higher scores represent better performance for all cognitive outcomes, with the exception of information processing accuracy, for which higher scores indicate more errors. These cognitive measures have been described in detail and are published elsewhere.Reference Brown and Dodgeon10
Affective symptoms
Affective symptoms in adulthood were measured at age 23, 33 and 42 using the Malaise Inventory, a 24-item self-completion questionnaire.Reference Rodgers, Pickles, Power, Collishaw and Maughan11 This is a measure of psychological distress, including emotional disturbance and related physical symptoms. There is a validated cut-off for clinical relevance (used in this study). Cohort members scoring 8 and above out of 24, the validated threshold for clinical relevance,Reference Rodgers, Pickles, Power, Collishaw and Maughan11 were coded as having case-level affective symptoms (i.e. a high risk of depression) and those scoring 7 and below were coded as having no symptoms. The Malaise Inventory has been used frequently in previous researchReference Winning, Glymour, McCormick, Gilsanz and Kubzansky7,Reference John, James, Patel, Rusted, Richards and Gaysina12 and its internal consistency is acceptable.Reference Rodgers, Pickles, Power, Collishaw and Maughan11 In line with previous research,Reference John, James, Patel, Rusted, Richards and Gaysina12 a measure of accumulating affective symptoms was derived by summing the binary Malaise Inventory score at age 23, 33 and 42 to represent the number of time points through adulthood with case-level affective symptoms (score range 0–3).
Cardiometabolic risk
At age 44, nine measures of cardiometabolic risk were assessed: total cholesterol; high-density lipoprotein (HDL) cholesterol; triglycerides; glycosylated haemoglobin (HbA1c); systolic blood pressure; diastolic blood pressure; C-reactive protein (CRP) (excluding values >10 mg/L, which may be indicative of recent infection); fibrinogen; resting heart rate.
HDL cholesterol was reverse scored, so that for all biomarkers higher scores represent greater cardiometabolic dysfunction. These biomarkers were selected on the basis of the previous research examining cardiometabolic risk in the NCDS.Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky7 Specifically, each of these nine variables is a component of cardiometabolic risk. Biochemical analyses of blood samples used to collect information on HbA1c, triglycerides, total and HDL cholesterol, fibrinogen and CRP are published elsewhere and are described in depth in the NCDS biomedical user guide and the technical report.Reference Elliott, Johnson and Shepherd13,Reference Fuller, Power, Shepherd and Strachan14
These measures were used both as individual variables and to derive a composite measure of cardiometabolic risk. Measures of CRP and triglycerides were skewed in the direction of low risk and therefore were log transformed. Standardised z-scores were derived for all biomarkers and then used in all subsequent analyses. As in previous work,Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky7 z-scores for each biomarker were summed to create an overall composite measure of cardiometabolic risk score. Higher scores on this derived measure represent greater cardiometabolic dysfunction.
Covariates
Covariates were selected on the basis of variables known to be associated with cognitive function. Specifically, models were adjusted for gender,Reference da Silva, Gonçalves-Pereira, Xavier and Mukaetova-Ladinska3 educational attainment,Reference Hatch, Feinstein, Link, Wadsworth and Richards15 childhood socioeconomic position,Reference Kaplan, Turrell, Lynch, Everson, Helkala and Salonen16 childhood cognitive functionReference Richards, James, Sizer, Sharma, Rawle and Davis17 and affective symptoms contemporaneous with measurement of cognitive function (at age 50). Education was measured as the highest academic achievement attained by age 50. This was classified into three categories: no education; GCSE to A-level (or Scottish equivalent); higher education. Childhood socioeconomic position was derived according to guidelines from the Centre for Longitudinal Studies,Reference Elliott and Lawrence18 and was based on parental occupation and household tenure. This was coded into three categories: working class; intermediate class; middle class. Childhood cognitive function at age 11 was assessed using a general ability test,Reference Douglas19 which cohort members completed at school. Affective symptoms at age 50 were assessed using the short form of the Malaise Inventory, which encompassed nine items,Reference Bowling, Pikhartova and Dodgeon20 as the 24-item measure is not available at this age. This variable was included as a continuous measure (i.e. the number of items on the short form of the Malaise Inventory that the cohort member endorsed).
Analytical procedure
For main analyses, path models were run, estimating direct associations between accumulating affective symptoms and mid-life cognitive function and indirect associations through the composite measure of cardiometabolic risk. The use of this composite score allows the model to take into account that combined small variations across multiple biomarkers can confer meaningful change in disease risk, regardless of the signal for any individual biomarker.Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky21
Next, an additional analysis was run to test whether associations between affective symptoms and cognitive function are driven by cardiometabolic risk as a common cause mechanism. To test this, a path model was conducted using a cardiometabolic risk score to predict cognitive function and affective symptoms at age 50.
Initial models were unadjusted and subsequent models were adjusted for the covariates. All cognitive measures were included together in the models to account for covariances between different cognitive domains. Non-significant covariances were removed from the model to improve fit. Stratifying analyses by gender did not significantly improve model fit (supplementary Table 1, available at https://doi.org/10.1192/bjp.2020.123), therefore gender was adjusted for in all subsequent models rather than used as a stratifying variable. Model fit was tested using standard fit statistics: chi-squared goodness-of-fit test, comparative fit index (CFI), Tucker–Lewis index (TLI) and root mean square error of approximation (RMSEA). Missing data were accounted for using full information maximum likelihood (FIML) methods, in which model parameters and s.e. are estimated using all available data. All analyses were conducted in Mplus V8 (for Windows).Reference Muthén and Muthén22
To maximise the analytic sample size, main models were rerun using multiple imputation to impute all covariate data. In total, 21 variables were imputed over five sweeps using the MICE package in R (for Windows). Main models were re-run on the imputed data-set (n = 9377).
Supplementary analyses
To check whether indirect associations between affective symptoms and cognitive outcomes were driven primarily by any particular biomarker, an additional model was run in which the nine biomarkers were included in the model individually. This approach allows the effects of each biomarker to be estimated, while simultaneously accounting for covariances between these markers. Following relevant work in the NCDS, supplementary analyses were conducted with models additionally adjusted for cardiovascular medication use.Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky7
Results
Missing data and descriptive statistics
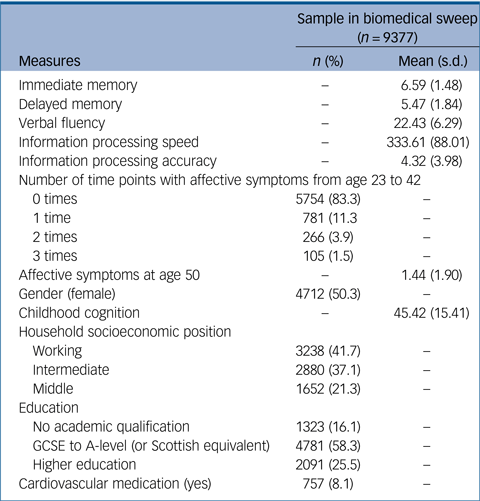
The biomedical sweep at age 44 included 9377 participants. Of this sample, 3730 people had complete information for all key variables and covariates (supplementary Fig. 1). This subsample was compared with the sample with missing data on key variables (supplementary Table 2). This revealed that people with missing data had significantly poorer scores for all cognitive tests (including those in childhood) except processing speed; had reported more episodes of affective symptoms from age 23 to 42 and higher affective symptoms at age 50; were more likely to be categorised as working class, have lower education and to take cardiovascular medication; and showed significantly higher levels of all cardiometabolic biomarkers. However, there were no gender differences in probability of missing data. Missing data were addressed using FIML, resulting in an analytic sample of 6405 people in fully adjusted models. Main models were also conducted on the imputed sample, resulting in a sample of 9377 in fully adjusted models. Demographic information for this sample is presented in Table 1.
Table 1 Demographic information of the sample who took part in the biomedical sweep of the National Child Development Study (NCDS)
Path models to test the role of cardiometabolic risk in the association between affective symptoms and cognitive function
Both the unadjusted and adjusted FIML models fit the data well (unadjusted: n = 6682; χ2(1) = 5.91, P = 0.02; CFI = 1.00; TLI = 0.99; RMSEA = 0.03; adjusted: n = 6403; χ2(2) = 1.24, P = 0.54; CFI = 1.00; TLI = 1.00; RMSEA = 0.00). The model conducted on the imputed sample also fit to the data well (n = 9377; χ2(2) = 6.22, P = 0.04; CFI = 1.00; TLI = 0.99; RMSEA = 0.02).
Direct effects
Unadjusted FIML models showed significant direct effects of accumulating affective symptoms across adulthood on mid-life immediate memory (β = −0.09, s.e. = 0.01, P < 0.001), delayed memory (β = −0.08, s.e. = 0.01, P < 0.001), verbal fluency (β = −0.09, s.e. = 0.01, P < 0.001), and information processing accuracy (β = 0.05, s.e. = 0.01, P < 0.001), but not for information processing speed. However, there were no significant direct effects of affective symptoms on any cognitive domain after adjustment for all covariates (Table 2; Fig. 1). Similarly, the fully adjusted model conducted on the imputed sample revealed no significant direct effects of affective symptoms on any cognitive domain (Table 2; Fig. 1).

Fig. 1 Path models: fully adjusted models showing direct, indirect and total associations between accumulating affective symptoms from age 23 to 42 and mid-life cognitive outcomes at age 50, via cardiometabolic risk.
FIML, full information maximum likelihood. Significant pathways are denoted by black arrows. Non-significant pathways are denoted by blue arrows.
Table 2 Adjusted path models testing direct, indirect and total associations between accumulating affective symptoms from age 23 to 42 and mid-life cognitive outcomes at age 50, via cardiometabolic risk

FIML, full information maximum likelihood; χ2, goodness-of-fit test; CFI, comparative fit index; TLI, Tucker–Lewis index; RMSEA, root mean square error of approximation.
a. Total effects include direct effects of affective symptoms on cognitive outcomes and indirect effects through cardiometabolic biomarkers and affective symptoms at age 50.
Indirect effects
In the unadjusted FIML model, there were significant indirect effects of affective symptoms on immediate memory, delayed memory, verbal fluency and information processing speed operating through composite cardiometabolic risk score (immediate memory: β = −0.01, s.e. = 0.002, P = 0.008; delayed memory: β = −0.01, s.e. = 0.002 P = 0.008; verbal fluency: β = −0.003, s.e. = 0.001, P = 0.01; information processing speed: β = −0.003, s.e. = 0.001, P = 0.02). There was no effect for information processing accuracy. After adjustment for covariates, there was still a significant indirect effect of accumulating affective symptoms on immediate memory (β = −0.002, s.e. = 0.001, P = 0.02), accounting for 4% of the total effect (Table 2; Fig. 1). The fully adjusted model run on the imputed sample revealed significant indirect effects of accumulating affective symptoms on immediate memory (β = −0.002, s.e. = 0.001, P = 0.009), delayed memory (β = −0.002, s.e. = 0.001, P = 0.02) and verbal fluency (β = −0.002, s.e. = 0.001, P = 0.045), through the composite cardiometabolic risk score. This accounted for 4%, 4% and 5% of the total effect respectively. There were no significant indirect effects on information processing speed or accuracy (Table 2, Fig. 1).
Total effects
In the unadjusted FIML model, there were significant total effects of accumulating affective symptoms on immediate memory (β = −0.09, s.e. = 0.01, P < 0.001), delayed memory (β = −0.08, s.e. = 0.01, P < 0.001), verbal fluency (β = −0.09, s.e. = 0.01, P < 0.001), and information processing accuracy (β = 0.05, s.e. = 0.01, P < 0.001), but not information processing speed. After adjusting for covariates, significant total effects remained for immediate memory (β = −0.05, s.e. = 0.01, P = 0.001), delayed memory (β = −0.05, s.e. = 0.01, P < 0.001), and information processing accuracy (β = 0.04, s.e. = 0.01, P = 0.01), but not for verbal fluency or information processing speed (Table 2; Fig. 1). The adjusted model conducted on the imputed sample revealed significant total effects of accumulating affective symptoms on immediate memory (β = −0.05, s.e. = 0.01, P < 0.001), delayed memory (β =−0.05, s.e. = 0.01, P < 0.001), verbal fluency (β = 0.04, s.e. = 0.01, P = 0.003) and information processing accuracy (β =−0.04, s.e. = 0.01, P = 0.01), but not on information processing speed (Table 2; Fig. 1).
Next, a separate model was run using cardiometabolic risk score at age 44 as a predictor of affective symptoms and cognitive function at age 50 to test cardiometabolic risk as a potential common cause. The model fit the data well (n = 3847; χ2(18) = 34.27, P = 0.01; CFI = 1.00; TLI = 0.99; RMSEA = 0.02). The fully adjusted FIML model revealed that cardiometabolic risk significantly predicted immediate memory (β = −0.04, s.e. = 0.02, P = 0.008), but not other cognitive outcomes. Cardiometabolic risk did not significantly predict affective symptoms at age 50 (β = 0.002, s.e. = 0.01, P = 0.90) (Table 3). The adjusted model conducted on the imputed sample fit the data well (n = 5288; χ2(18) = 36.23, P = 0.007; CFI = 1.00; TLI = 0.99; RMSEA = 0.01). The analysis on imputed data revealed that cardiometabolic risk score at age 44 was significantly associated with poorer scores of immediate memory (β = −0.04, s.e. = 0.02, P = 0.003), delayed memory (β = −0.03, s.e. = 0.02, P = 0.048) and verbal fluency (β = −0.03, s.e. = 0.02, P = 0.03) at age 50. However, cardiometabolic risk at age 44 was not significantly associated with affective symptoms at age 50 (β = 0.02, s.e. = 0.01, P = 0.24) (Table 3).
Table 3 Path model using cardiometabolic risk score to predict affective symptoms and cognitive function at age 50

FIML, full information maximum likelihood; χ2, goodness-of-fit test; CFI, comparative fit index; TLI, Tucker–Lewis index; RMSEA, root mean square error of approximation.
Supplementary analysis
The model including nine individual biomarkers as predictors fit the data well (n = 6405; χ2(12) = 33.29, P < 0.001; CFI = 1.00; TLI = 0.99; RMSEA = 0.02). In fully adjusted models including all covariates and individual cardiometabolic biomarkers, there were no significant direct effects of accumulating affective symptoms on cognitive outcomes. There was a significant indirect path between accumulating affective symptoms and delayed memory through fibrinogen (β = −0.003, s.e. = 0.001, P = 0.03), accounting for 6% of the total effect. All other indirect paths were not significant at the 5% level in adjusted models. There were significant total effects of accumulating affective symptoms on immediate memory (β = −0.05, s.e. = 0.01, P = 0.001), delayed memory (β = −0.05, s.e. = 0.01, P < 0.001) and information processing accuracy (β = 0.04, s.e. = 0.01, P = 0.01), but not on verbal fluency or information processing speed (supplementary Table 3).
Further analyses were conducted additionally adjusting for cardiovascular medication use. The model fit the data well (n = 6370; χ2(3) = 12.12, P = 0.007; CFI = 1.00; TLI = 0.98; RMSEA = 0.02). Results were largely identical to those reported in main models (supplementary Table 4).
Discussion
Summary of findings
Results across models using two missing data methods (FIML and multiple imputation) both revealed significant indirect associations between accumulation of affective symptoms and immediate memory through a composite cardiometabolic risk score. Additionally, models using multiple imputation revealed significant indirect associations with delayed memory and verbal fluency. These associations may have been revealed in the model using imputed data owing to extra power associated with increasing the sample size. There were no significant direct or indirect paths observed between symptoms and other cognitive domains (information processing). These results build on previous research that showed associations between affective problems and cardiometabolic risk, and also between cardiometabolic risk and cognitive outcomes,Reference Winning, Glymour, McCormick, Gilsanz and Kubzansky7,Reference Raffaitin, Gin, Empana, Helmer, Berr and Tzourio23 but had not investigated the role that cardiometabolic risk might play in the known relationship between affective problems and later cognition. These results do not support the common cause hypothesis that cardiometabolic risk may precede both development of affective symptoms and cognitive dysfunction, because in these data the cardiometabolic risk score did not predict subsequent level of affective symptoms.
There are several plausible mechanisms that could account for the observed indirect associations. For example, accumulated affective symptoms may be associated with cardiometabolic risk because of behavioural/lifestyle factors with known associations with dementia and cognitive ageing (e.g. smoking, alcohol, physical inactivity).Reference Bonnet, Irving, Terra, Nony, Berthezène and Moulin24 Additionally, affective symptoms may be linked with cardiometabolic risk through other biological pathways (e.g. through dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, inflammatory processes, sympathetic nervous system activity),Reference Dhar and Barton25 which can also be associated with subsequently poorer cognitive function. The mechanisms that underlie these observed associations are likely to be complex and multifaceted.
It should be noted that effect sizes for indirect pathways are small and account for only small amounts of the total effects. This suggests that cardiometabolic risk may be one of multiple underlying pathways and that other lifestyle and biomedical factors are likely to play an important role within the association between affective symptoms and cognitive ageing. Further understanding of these relationships are important for appropriate targeting of intervention and advice for amelioration of cognitive risk in the context of high levels of affective symptoms.
Strengths and limitations
Strengths of this research include the use of a large nationally representative cohort, with prospective data available from birth through to mid-life over a period of five decades. Additionally, the repeated use of the same instrument to capture affective symptoms over time is a strength of this study. Affective symptoms contemporaneous with cognitive function were included in the models, reducing the possibility that associations between affective symptoms and later cognitive function are simply due to cross-sectional associations at age 50.
Missing data is a limitation of most long running cohort studies. In this study, the sample with missing data differed significantly from the sample with complete information on a range of key variables, including cognitive scores, levels of affective symptoms and demographic characteristics. The sample with missing data showed higher affective symptoms, poorer cognitive function and raised cardiometabolic symptoms, and associations may therefore be underestimated in this analysis. To address this issue in these analyses, multiple imputation and FIML were used. It is important to note, however, that if data are missing not at random (MNAR), these methods may not be appropriate. A further limitation of the study is that the number of individuals with case-level affective symptoms is relatively small, which may lead to a possible underestimation of effects.
Additionally, cardiometabolic biomarkers were measured at only one time point (age 44). Therefore, the possibility of reverse causality cannot be ruled out. However, we ran a supplementary analysis to partially address this using cardiometabolic risk score as a predictor of later affective symptoms at age 50. These results showed that the association did not operate in the opposite direction and cardiometabolic risk did not predict subsequent affective symptoms. Furthermore, although we adjusted for cardiovascular medication use in a supplementary analysis, there was no measure of psychotropic medication use, which was consequently not accounted for in the present analyses. Additionally, cognitive data were available at only one time point in this data-set (at age 50), so it was not possible to model cognitive trajectories. Related to this, the extent to which mid-life cognitive function in this cohort is relevant to dementia risk decades later is currently unknown. This can be tested in this cohort in the future with repeated follow-up assessment of cognitive function and dementia status as participants transition into older adulthood. Affective symptoms in late life are a recognised risk factor for dementia. However, it remains unclear whether this link represents a flare-up of earlier depressive symptoms and associated cardiometabolic risk, or whether affective symptoms later in the life course also have direct effects on dementia risk. Finally, the cognitive assessments were limited to those available in NCDS, meaning that conclusions cannot be drawn for other cognitive domains not measured (e.g. executive functions).
Future research and implications
Overall, these findings provide strong evidence that cardiometabolic risk may contribute to the relationship between accumulating affective symptoms over adulthood and mid-life immediate memory. In addition, the findings suggest that cardiometabolic risk may also contribute to associations between affective symptoms and delayed memory and verbal fluency. Only a small amount of the total effect of affective symptoms on cognitive function was accounted for by the indirect effect through cardiometabolic risk. This suggests that cardiometabolic risk is likely to be one of multiple pathways which are important for this association. Other potential pathways may involve complex interactions between lifestyle and biological factors. Future research should aim to identify and test these mechanisms.
These findings have important implications for prevention efforts, specifically the possibility that early intervention to improve cardiometabolic health in people with affective symptoms may help to improve poorer cognitive outcomes later in the life course. Future research should focus on testing this clinically relevant hypothesis.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2020.123.
Funding
Economic and Social Research Council (ESRC) (D.G., A.J.: grant number: ES/J500173/1). Alzheimer's Society (J.S., R.D., A.J., M.R.: MODIFY Project, grant number AS-PG-18-013). Data governance was provided by the METADAC data access committee, funded by the ESRC, Wellcome Trust and Medical Research Council (MRC) (2015–2018: grant number MR/N01104X/1; 2018–2020: grant number ES/S008349/1). Data and samples generated by the NCDS 1958 Birth Cohort is managed by the Centre for Longitudinal Studies at the UCL Institute of Education, funded by the ESRC (grant number ES/M001660/1). Access to these resources was enabled via the Wellcome Trust and MRC (58FORWARDS grant 108439/Z/15/Z). Before 2015 biomedical resources were maintained under the Wellcome Trust and Medical Research Council 58READIE Project (grant numbers WT095219MA and G1001799). Funders were not involved in the study design; collection, analysis, and interpretation of data; writing the report; or in the decision to submit the article for publication.
Acknowledgements
We thank the Economic and Social Research Council (ESRC) for supporting this project and the Centre for Longitudinal Studies for allowing use of the data. We also acknowledge the Alzheimer's Society for supporting this work and thank the 1958 Birth Cohort members who have dedicated their time to participating in the National Child Development Study (NCDS). Data governance was provided by the METADAC Data Access Committee. This work made use of data and samples generated by the NCDS, which is managed by the Centre for Longitudinal Studies at the UCL Institute of Education. Access to these resources was enabled via the Wellcome Trust and Medical Research Council.
Data availability
Data are available on application to METADAC.
Author contributions
A.J.: study design, statistical analysis, interpretation of results, drafting and editing the article. R.D., M.R., D.G. and J.S.: interpretation of data and critical evaluation of the article for important intellectual content.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjp.2020.123.








eLetters
No eLetters have been published for this article.