The most common reason for the prescription of antipsychotic medication to people with learning disability is the management of behavioural problems (Reference Wressell, Tyrer and BerneyWressell et al, 1990; Reference Molyneaux, Emerson and CaineMolyneaux et al, 2000). The effectiveness of antipsychotic drugs in reducing maladaptive behaviour is questionable (Reference Brylewski and DugganBrylewski & Duggan, 1998). Concerns about side-effects and dubious efficacy have led to litigation in the USA, where prescription rates have fallen (Reference BriggsBriggs, 1989; Reference PoindexterPoindexter, 1989), by contrast with the apparent increase found in a longitudinal cohort studied in England (Reference Emerson, Alborz and KiernanEmerson et al, 1997). In addition, it has been found that many individuals can be taken off antipsychotic drugs completely with positive results or at least no deterioration. However, a worsening of behaviour problems, leading to the reinstatement of medication (Reference Fielding, Murphy and ReaganFielding et al, 1980; Reference BriggsBriggs, 1989), has also been noted, as has an increase in the risk of irreversible tardive dyskinesia (Reference Baumeister, Sevin and KingBaumeister et al, 1998). A number of drug withdrawal studies have investigated predictors of successful withdrawal (Reference Luchins, Dojka and HanrahanLuchins et al, 1993; Reference BranfordBranford, 1996), but studies suffer from being retrospective, or inadequately rigorous.
This study was designed as a prospective randomised controlled trial to investigate the feasibility of antipsychotic drug reduction in such people with learning disability, and the factors influencing the outcome.
METHOD
Subjects
Participants were recruited in south and mid-Wales and north-west England. Clinical consultants were asked to volunteer people who were currently taking antipsychotic medication for a behavioural problem and who did not have a psychotic illness, were living in institutions or community residential homes and were 18 or more years of age. Families, care staff and (where possible) potential participants were consulted, the nature of the study was explained and written consent to participation was sought. Potential participants were screened to exclude people with psychotic illness by examining clinical notes and by using the Psychiatric Assessment Schedule for Adults with a Developmental Disability (PAS-ADD; Reference Moss, Patel and ProsserMoss et al, 1993) and the Psychopathology Instrument for Mentally Retarded Adults (Reference MatsonMatson, 1988). In total, 67 people were volunteered to the study, of whom two were found to have a psychotic illness, two refused to participate and seven had consent refused by family or care staff. Twenty-seven (48%) of the remaining 56 participants were men, 25 (45%) lived in National Health Service (NHS) hospitals, five (9%) lived in a small NHS community unit and 26 (46%) lived in community residential homes, 10 of which (38%) were run by the NHS, 10 (38%) by the voluntary sector, 3 (12%) by social services departments and 3 (12%) by private proprietors. The mean age of the sample was 43 years (range 20-78).
In total, participants received 12 different antipsychotic drugs, most frequently thioridazine (18 people, 12%), haloperidol (13, 23%) and chlorpromazine (8, 14%). Eleven participants (20%) were prescribed two antipsychotic drugs and five (9%) were prescribed three, not including p.r.n. medications. Five subjects were prescribed depot antipsychotic drugs. Forty-five participants (79%) had been prescribed the same drugs for five years or more. The mean daily chlorpromazine equivalent dose per participant was 372 mg (range 20-4067 mg, s.d.=613).
Instrumentation
Adaptive and maladaptive behaviour and uncontrolled movements were assessed for each participant by using the Adaptive Behaviour Scale (ABS; Reference Nihira, Leland and LambertNihira et al, 1993), the Aberrant Behavior Checklist (ABC; Reference Aman and SinghAman & Singh, 1986) and the Dyskinesia Identification System: Condensed User Scale (DISCUS; Reference Sprague, Kalachnik and ShawSprague et al, 1989). We measured weight by using standard weighing scales. Medication history was abstracted from notes; subsequent medication was monitored, with dosages converted to chlorpromazine equivalents. In addition, the behaviour of each participant was observed directly. Observers used palm-top Psion 3a portable computers (Psion plc, London) programmed for multiple-category, real-time data capture (details available from the first author upon request). Behavioural categories (and constituent codes) included ‘activity’ (engaged in an appropriate social activity, engaged in an appropriate non-social activity, disengaged but active, disengaged and not active), ‘maladaptive behaviour’ (self-injury, aggression to others, damage to the environment, other), ‘stereotypy’, and ‘staff involvement’ (assistance, praise, restraint, processing, i.e. doing something to a person without their involvement, or other interaction).
Information on each participant's place of residence was collected by using a setting questionnaire designed for the study. This covered the following: the nature of the facility, building design, physical characteristics and building adaptations; the number, gender, age and general capabilities of residents; numbers, types and qualifications of staff; professional support; and working arrangements including budgetary control, arrangements for gaining assistance with behavioural difficulties, administration of p.r.n. medication, and guidelines and training for behavioural intervention, restraint and break-away strategies.
Design
Participants were allocated randomly to experimental (n=36) and control groups (n=20). The two groups did not differ in age, but the control group had fewer males (30% v. 58%; χ2=4.1, P<0.05) and a lower proportion living in hospital (25% v. 56%; χ2-4.9, P<0.05). The proportions of the two groups receiving various antipsychotic drugs at baseline differed by less than 10% in 10 out of the 12 types prescribed. The exceptions, which were still not statistically significant, were thioridazine (experimental 25%, control 45%) and haloperidol (experimental 28%, control 15%). The experimental group received a significantly higher initial mean daily dose (460 mg, range 34-4067 mg (s.d.=717) v. 213 mg, range 20-1260 mg (s.d.=314), Mann-Whitney U=208.5, P<0.01). The groups did not differ significantly in weight, baseline ABS, ABC or DISCUS scores or in observed behaviour, with the exception that the experimental group spent more time disengaged and inactive (Mann-Whitney U=233, P<0.01). The only significant difference in setting characteristics was that the average size of the residential group for the experimental participants (ten) was significantly greater than that for control participants (five) (Mann-Whitney U=200, P<0.01).
Each participant was studied for six months. Baseline assessments (month 1) were followed by four, monthly drug reduction stages of 25% of the baseline chlorpromazine equivalent dose (months 2-5). The final month (month 6) was included to allow for any delayed changes in behaviour after drug cessation. There were no planned drug changes for the control group. Clinical consultants remained in charge of drug treatment for all participants throughout the study. Consultants were free to change medication at any time, including stopping drug reduction or reinstating baseline drug dosage.
The ABS, ABC, DISCUS and setting questionnaire were administered at baseline, and weight and medication regimen were recorded. In addition, behaviour was directly observed for a randomly selected 1.5-hour period during the second, third and fourth weeks of the month. In each subsequent month (months 2-6), behavioural observations were repeated at equivalent times. The ABC was also repeated at these times. The DISCUS and weight measurement were repeated in the fourth week of each month.
Analysis
Observational data were transferred to an IBM-compatible computer, and the three periods of observation per participant for each month were arranged as a single file set. Constituent codes for summary behavioural categories were combined in order to eliminate concurrency of behaviours within the categories but not between them. For example, ‘total engagement in activity’ was a combination of social and non-social activity, and ‘total staff involvement’ was a combination of all codes referring to any form of attention received by participants from staff. The cumulative percentage durations of occurrence of these categories and of the individual behavioural codes were calculated for each participant for each month.
Given the sequential nature of the experimental protocol, it was important to analyse all dependent variables for sequence effects across the six time periods. A measure of linear trend in each variable was achieved by calculating regression coefficients for each participant across the data for months 1-5. (Month 6 was omitted from the analysis because of missing data caused by delays in identifying the last experimental and control participants taken into the study.) Between-group differences in these regression coefficients were compared by using t-tests. This approach was preferred to analysis of variance because it is sensitive to the temporal order of data.
Reliability
Inter-observer reliability for the direct observations was assessed by two researchers observing simultaneously for nine hours (i.e. for about 11% of the time). The level of agreement of the two observers was calculated for each code by using a modified form of Cohen's κ (Reference ReevesReeves, 1994). Summary values of κ across observation sessions were calculated as an average weighted for the occurrence of the behavioural category in question. Values of κ for social engagement, non-social engagement, maladaptive behaviour, stereotypy, disengaged but active, disengaged inactive and staff involvement were 0.63, 0.79, 0.75, 0.72, 0.61, 0.62 and 0.88 respectively. Suen & Ary (Reference Suen and Ary1989) suggest that a value of κ of 0.6 or higher is acceptable in observational research.
RESULTS
Drug reduction
Twelve of the 36 experimental participants (33%) completed the full withdrawal programme. A further seven (19%) followed the reduction protocol until medication had been reduced to at least 50% of baseline dosage and were maintained on the reduced dosage throughout the study. These 19 participants are subsequently referred to as the “success” group. The remaining 17 participants (48%), subsequently referred to as the ‘fail’ group, had their medication reinstated to baseline levels - ten of them after the initial 25% reduction, one after 50% reduction and six after 100% reduction.
The effects of drug reduction
The impact of drug reduction was explored through the comparison of the ‘success’ (n=19) and control (n=20) groups (Table 1). Drug reduction was associated with increased DISCUS scores and higher activity engagement, but not with increased maladaptive behaviour. Staff contact with participants and participant weight were unaffected. However, there were a number of significant differences between the ‘success’ and control groups at baseline; the former had greater numbers of members who were the following: men (χ2=4.3, d.f.=1, P<0.05); living in hospital (χ2=5.8, d.f.=1, P<0.05); living in a setting with a specialist mental health orientation (χ2=3.9, d.f.=1, P<0.05); living in settings with lower staff to resident ratios (Mann-Whitney U=94, P<0.02); receiving neuroleptic medication for more than five years (χ2=4.4, d.f.=1, P<0.05); receiving higher chlorpromazine equivalent dosages of neuroleptic medication (Mann-Whitney U=93, P<0.01).
Table 1 Effects of drug dosage reduction

| Variable | Measure | ‘Success’ group v. ‘control’ group |
|---|---|---|
| Weight | Direct assessment | No difference |
| Dyskinesia | DISCUS | Increase: t=3.0, d.f.=28.4, P<0.01 |
| Stereotypy | Direct observation | No difference |
| Maladaptive behaviour | ABC | No difference |
| ABC sub-domains | No difference | |
| Direct observation | No difference | |
| Engagement: total | Direct observation | Increase: t=2.3, d.f.=32.0, P<0.05 |
| Engagement: social | Direct observation | No difference |
| Engagement: non-social | Direct observation | Increase: t=2.5, d.f.=32.0, P<0.05 |
| Disengagement | Direct observation | No difference |
| Staff contact | Direct observation | No difference |
Predictors of drug reinstatement
Baseline comparisons between the ‘success’ (n=19) and ‘fail’ (n=17) groups, and between the ten participants who failed to progress beyond the first stage of drug reduction (nine immediately reinstated to baseline dosages and one maintained at 75% baseline level) and the 26 participants who proceeded to the 50% reduction stage, revealed a number of significant differences between the settings in which participants lived (Table 2). Variables concerning restrictiveness of the setting and the policy and training of staff in relation to use of physical restraint and break-away techniques were consistently implicated. In neither analysis were there significant differences on any measure of participant ability, maladaptive behaviour or medication status other than the ‘failure to progress’ group in the second analysis being less able in physical development (Mann-Whitney U=63, P<0.05) and vocational activity (U=75.5, P<0.05) on the ABS and being less likely to receive depot neuroleptics (χ2=4.6, d.f.=1, P<0.05).
Table 2 Setting characteristics predictive of drug reinstatement
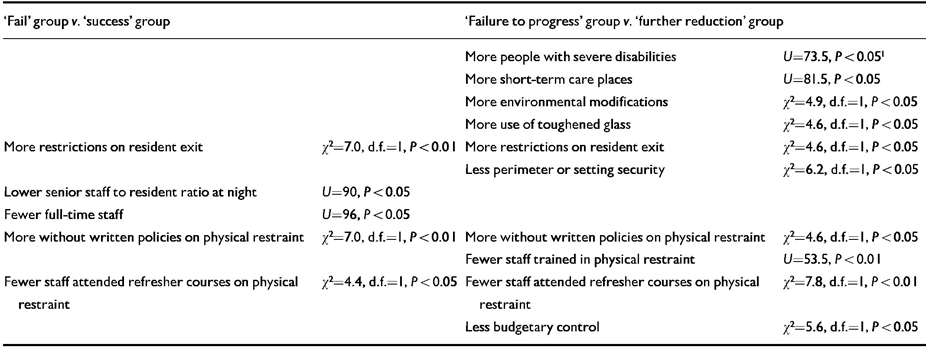
| ‘Fail’ group v. ‘success’ group | ‘Failure to progress’ group v. ‘further reduction’ group | ||
|---|---|---|---|
| More people with severe disabilities | U=73.5, P<0.051 | ||
| More short-term care places | U=81.5, P<0.05 | ||
| More environmental modifications | χ2=4.9, d.f.=1, P<0.05 | ||
| More use of toughened glass | χ2=4.6, d.f.=1, P<0.05 | ||
| More restrictions on resident exit | χ2=7.0, d.f.=1, P<0.01 | More restrictions on resident exit | χ2=4.6, d.f.=1, P<0.05 |
| Less perimeter or setting security | χ2=6.2, d.f.=1, P<0.05 | ||
| Lower senior staff to resident ratio at night | U=90, P<0.05 | ||
| Fewer full-time staff | U=96, P<0.05 | ||
| More without written policies on physical restraint | χ2=7.0, d.f.=1, P<0.01 | More without written policies on physical restraint | χ2=4.6, d.f.=1, P<0.05 |
| Fewer staff trained in physical restraint | U=53.5, P<0.01 | ||
| Fewer staff attended refresher courses on physical restraint | χ2=4.4, d.f.=1, P<0.05 | Fewer staff attended refresher courses on physical restraint | χ2=7.8, d.f.=1, P<0.01 |
| Less budgetary control | χ2=5.6, d.f.=1, P<0.05 | ||
Finally, analyses examined changes between baseline and the first drug reduction stage for the ‘failure to progress’ and ‘further progress’ (i.e. from 100% to 75% of baseline dosage) groups. The only significant change on any dependent variable for the ‘failure to progress’ group was a reduction in score on the inappropriate speech sub-scale of the ABC (t=2.5, d.f.=9, P<0.05). The ‘further progress’ group showed a significant reduction in score on the hyperactivity sub-scale of the ABC (t=2.3, d.f.=25, P<0.05). Combined analyses using a multivariate analysis of variance (MANOVA) model did not identify significant interaction effects.
DISCUSSION
Impact of drug reduction
Just over half of the experimental group had stopped taking or were taking substantially reduced medication by the end of the study. Best practice guidance suggests that the lowest ‘optimum effective dose’ should be used if an individual cannot be drug-free (Reference Kalachnik, Levethal and JamesKalachnik et al, 1998); this dose is defined as the lowest amount of an antipsychotic drug that will improve or stabilise behaviour. Therefore, all of the ‘success’ group including the seven people who remained on substantially reduced dosages, may be considered to have benefited in terms of health gain. It is important to emphasise the absence of increase in maladaptive behaviour following drug reduction. Possible change in maladaptive behaviour was monitored repeatedly (three times per month) and by complementary methodologies which together reflected carer perceptions of behaviour and independent objective assessment. People with psychotic illness were excluded from the study; neuroleptic medications were therefore prescribed for treatment of maladaptive behaviour. Neither the questionnaire nor observational indicators used in the study showed behavioural deterioration with medication reduction. The findings of the study reinforce concerns that antipsychotic medication for maladaptive behaviour reduction is often ineffective and inappropriate.
Drug reduction was associated with increased DISCUS scores, a finding consistent with the literature on tardive dyskinesia emerging upon withdrawal of neuroleptic medication (Reference Baumeister, Sevin and KingBaumeister et al, 1998). The increase in movement disorder became significant at month 4, at which time 18 out of 19 people in the group were down to 25% of the baseline drug dosage (i.e. dosage was reduced by 75%). Movement disorder reached its maximum level at month 5, when 13 out of 19 people in the group had ceased to take antipsychotic drugs. There was a decline in DISCUS scores at month 6 relative to month 5, but scores remained higher than those at baseline.
Drug reduction was also associated with significantly higher engagement in activity. This is an important outcome, because engagement in activity has been a widely used indicator of quality of life, particularly in studies of residential settings for people with severe learning disabilities (Reference Emerson and HattonEmerson & Hatton, 1994). As well as indicating greater purpose and productivity, increased engagement also signifies greater alertness and environmental responsiveness. Increased engagement following drug reduction would be consistent with reduced sedative effects and increased coordination and general physical well-being. An impression of greater alertness was confirmed by anecdotal staff accounts. However, increased engagement in activity among the ‘success’ group was only marginal, while a slight reduction in engagement over time in the control group also contributed to the difference between the groups. The consequences of drug reduction for responsiveness and quality of activity require further research.
There were indications of an anticipated weight loss among the ‘success’ group at months 5 and 6, with average weight losses of 1.6 kg and 2.3 kg respectively. Weight loss was seen as a benefit in some participants because they were overweight, possibly as a result of prolonged antipsychotic medication. Weight loss was problematic in only one participant, a member of the ‘fail’ group who had a 4 kg loss of weight after 75% drug reduction; she also showed a decrease in appetite and behavioural deterioration. Appetite and weight returned after drug reinstatement; it is possible that this individual experienced non-dyskinetic withdrawal syndrome (Reference Gualtieri, Schroeder and HicksGualtieri et al, 1986).
Predictors of drug reinstatement
Analysis of differences between the ‘success’ and ‘fail’ groups and between the ‘failure to progress’ and ‘further progress’ groups consistently failed to implicate level of problem behaviour or prior medication status as predictive factors. Reinstatement was not associated with deterioration of behaviour derived from either staff report measures or independent observations. That is not to say that on a case-by-case basis specific incidents may not have occasioned drug reinstatement. Clearly, participants continued to exhibit maladaptive behaviours. Instances of such behaviour among those having their medications reduced may have been attributed to drug reduction and caused a decision to reinstate original dose levels. However, what is shown is that drug reinstatement was not associated with either a higher level of maladaptive behaviour or a worsening of maladaptive behaviour overall.
Clinical attitudes and influences
The issue of attribution is one example of the possible impact that clinical approach, staffing and environmental characteristics may have on the likelihood of drug treatment for behavioural problems being pursued and maintained. Where differences on the variables measured in this study were apparent between participants for whom drug reduction was successful or unsuccessful, they were mainly in staff and environmental characteristics. In particular, drug reinstatement was associated with greater restriction and adaptation of the setting, less conducive staffing arrangements in certain respects and less well developed policies and poorer staff training concerning responding to difficult behaviour. The confidence of clinicians and carers to cope with possibly transient fluctuations in behaviour seems to be an important factor in the determination necessary to see whether any initial adverse reactions to withdrawal can be tolerated and drug reduction sustained. Staff attitudes and apprehension may play important roles in determining drug reduction outcome. Training staff in how to diffuse and cope with problem behaviour when it occurs can increase staff confidence (Reference McDonnellMcDonnell, 1997). The following two examples of the reasons for drug reinstatement illustrate the importance of the position that staff take.
A woman who lived in a staffed community residence showed an increase in stereotypy, aggression and irritability upon withdrawal of antipsychotic medication. Initially, care staff coped with this behaviour, hoping that it might prove temporary. However, although her behaviour had not deteriorated further, she kicked the pet dog during a period of excitement and it sustained fatal internal injuries. This had a marked effect on the emotional state of the carers, and her antipsychotic medication was reinstated to baseline dose. In the second example, another four participants allocated to the experimental group all lived in one hospital unit. Staff were anxious about drug reduction. After the first 25% drug reduction stage, one of the four was felt to have deteriorated behaviourally. Consequently, all four participants were put back on their baseline dosages even though no deterioration in behaviour was considered to have occurred in the other three participants.
Lessons for practice
Despite case reports of pharmacological management of behavioural symptoms among people with learning disabilities, an international consensus conference recently re-emphasised the scarcity of systematic research on pharmacotherapy, and pointed to the persistent problem of over-prescription of neuroleptic medications for behavioural management (Reference Reiss and AmanReiss & Aman, 1998). The present study has confirmed that a substantial proportion of people with learning disability prescribed antipsychotic medications for behavioural purposes rather than psychotic illness can have their drugs reduced or withdrawn. It is likely that this proportion can be increased if favourable clinical approaches and environmental conditions can be made more common. This study suggests that these improvements should include more rigorous behavioural assessment and causal attribution of behaviour change, more attention being given to the proportion of experienced and full-time staff, and an investment in initial and refresher staff training courses on responding to difficult behaviour, rather than an undue reliance on environmental restriction or adaptation.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ An encouragingly large proportion of people with learning disability can have their antipsychotic drug dosages decreased.
-
▪ Dosage reduction can be achieved without extra support in ordinary clinical practice.
-
▪ If a drug is reinstated, any deterioration in behaviour is reversed; behaviour returns to that at baseline (i.e. prior to drug reduction).
LIMITATIONS
-
▪ The small numbers involved in analysis increased the risk of type 2 statistical error, hence significant factors which may influence outcome may have been assessed statistically as non-significant.
-
▪ The time scale for drug reduction may have been too rapid. A balance had to be reached between best clinical practice and research limitations.
-
▪ A double-blind study design was not used. This study was designed to reflect the factors that come into play in a real-life situation when drug reduction is implemented.
ACKNOWLEDGEMENTS
We thank the following for their kind assistance: Drs V. R. Anness, S. Deb, M. Ferguson, S. Ganeshanetham, L.J. Haines, R. Jacques, T.J. O'Hare, R. Patel, R. Porter, S. Rowe, U. Sivagamasundari, P.V. Ramachandran, and H. M. Verma.





eLetters
No eLetters have been published for this article.