Progress in understanding the role of psychological processes in bipolar disorder may facilitate the development of interventions for people with this highly disabling illness and also research on underlying biological mechanisms, for example by identifying candidate endophenotypes or by providing targets for neuroimaging studies. Some studies have identified depression-related psychological abnormalities in people with bipolar disorder, for example low Reference Jones, Scott, Haque, Gordon-Smith, Heron and Caesar1 or unstable self-esteem, Reference Knowles, Tai, Jones, Highfield, Morriss and Bentall2 rumination, Reference Thomas, Knowles, Tai and Bentall3 dysfunctional attitudes to self-evaluation Reference Scott and Pope4 and a pessimistic explanatory style, Reference Lyon, Startup and Bentall5 which have sometimes been interpreted as evidence that mania arises from dysfunctional strategies for avoiding depression. Reference Thomas, Knowles, Tai and Bentall3 Other studies have found that manic episodes are preceded by goal attainment life events, Reference Johnson, Sandow, Meyer, Winters, Miller and Solomon6 suggesting that excessive reward responsiveness Reference Depue and Iacano7 may be important in the condition. It is not clear whether these mechanisms are trait vulnerability factors or related to symptoms and episodes. To answer this question we administered multiple measures of depressogenic cognitive biases and reward responsivity to people with bipolar disorder who had remitted, were currently depressed or were currently manic and healthy controls. We predicted that depressive symptoms would be specifically related to depressogenic cognitive styles and manic symptoms would be specifically related to reward responsivity.
Method
The design was a cross-sectional study with four groups broadly matched for age, gender and premorbid intelligence.
Participants
One hundred and seven people with bipolar disorder were recruited from across the North West of England. Potential in-patients were identified and approached by either their consultant psychiatrist or the senior nurse in charge on participating wards. Potential out-patients were identified and approached by their consultant psychiatrist, community mental health team keyworker or lithium clinic doctor to obtain verbal consent to be approached by the researcher. Adverts were also placed in out-patient waiting areas, day hospitals and Pendulum (the Manic Depression Fellowship quarterly magazine for service users).
The inclusion criteria for the patient sample were: a DSM–IV 8 diagnosis of bipolar disorder; age 18 years or over; ability to read and write English; and willingness to give written informed consent to the study. Diagnoses were confirmed by Structured Clinical Interview for DSM–IV (SCID) Reference First, Spitzer, Gibbon and Williams9 and the intellectual comparability of the groups was assessed using the National Adult Reading Test. Reference Nelson10 Individuals were excluded if they had a clear organic cause for their disorder or medical comorbidity that put the diagnosis of any bipolar episode in the past 24 months in doubt. Thirty-four participants were allocated to the mania group because they met DSM–IV criteria for manic or hypomanic episode (31) or mixed affective state (3); 30 to the depression group because they met DSM–IV criteria for major depressive episode; and 43 to the remitted group because they had not met DSM–IV criteria for a major depressive, hypomanic, mixed affective or manic episode in the past 2 months and also had a Hamilton Rating Scale for Depression (HRSD) Reference Hamilton11 score of eight or less and a score on the Mania Rating Scale (MRS) Reference Bech, Rafaelsen, Kramp and Bolwig12 of three or less.
Forty-one healthy controls were recruited via adverts placed around the Royal Liverpool University Hospital, in local libraries, and on the University of Liverpool staff internet message board. Inclusion criteria were not meeting SCID criteria for any psychiatric disorder within the last 2 years; age 18 years or over; ability to read and write English; and willingness to give written informed consent to the study.
Procedures
The study was approved by a National Health Service Multi-centre Research Ethics Committee in accordance with the Helsinki Declaration of 1975. Once the suitability of participants had been confirmed by SCID, participants were administered the HRSD, the Bech–Rafaelsen Mania Rating Scale Reference Bech, Rafaelsen, Kramp and Bolwig12 and the Cassidy Scale for Manic States. Reference Cassidy, Forest, Murry and Carroll13 Assessments were then conducted within the following 7 days in meetings between 11:00 and 18:00 to control for diurnal mood variation in the following order: Personal Style Inventory (PSI); Reference Robins, Ladd, Welkowitz, Blaney, Diaz and Kutcher14 Behavioural Inhibition System/Behavioural Activation System Scales (BIS/BAS); Reference Carver and White15 Autobiographical Memory; Reference Williams and Broadbent16 Pragmatic Inference Task (PIT); Reference Winters and Neale17 Rosenberg Self-Esteem Scale (RSE); Reference Rosenberg18 Modified Response Style Questionnaire (RSQ); Reference Knowles, Tai, Christensen and Bentall19 Card Arranging Reward Responsivity Objective Test (CARROT); Reference Powell, Al-Adawi and Morgan20 and the diary. Participants were debriefed and paid £20 plus travel expenses.
Psychological measures
The following psychological measures were used.
-
(a) The Personality Style Inventory (PSI) Reference Robins, Ladd, Welkowitz, Blaney, Diaz and Kutcher14 is a 48-item self-schema measure with sub-scales of autonomy and sociotropy.
-
(b) The Behavioural Inhibition System/Behavioural Activation System scale (BIS/BAS) Reference Carver and White15 has 20 items with sub-scales measuring behavioural inhibition and three scores for behavioural activation; drive, reward responsiveness and fun seeking.
-
(c) Autobiographical Memory was assessed using the method of Williams & Broadbent. Reference Williams and Broadbent16 Participants attempted to retrieve specific memories to six positive and six negative cue words. Memories were categorised as general extended (e.g. ‘The last time I was in hospital’); general categorical (e.g. ‘When I go to the park’); specific (a memory linked to a particular occasion) or delusional. Reliability of the classification was good (96% agreement between independent raters on the first 50 participants).
-
(d) The Pragmatic Inference Task (PIT) Reference Winters and Neale17 is an implicit measure of attributional (explanatory) style, giving internality scores for positive and negative events.
-
(e) The Rosenberg Self-Esteem Scale (RSE) Reference Rosenberg18 is a widely used 10-item self-reported measure of self-esteem.
-
(f) Modified Response Style Questionnaire (RSQ) Reference Knowles, Tai, Christensen and Bentall19 is a 38-item questionnaire with sub-scales measuring rumination, adaptive coping (distraction and problem solving), and risk-taking.
-
(g) The Card Arranging Reward Responsivity Objective Test (CARROT) Reference Powell, Al-Adawi and Morgan20 is a three-trial card sorting task. On the third trial participants receive monetary reward for speed and increased speed in this trial is taken as a measure of reward responsivity.
-
(h) Participants completed a self-esteem diary Reference Knowles, Tai, Jones, Highfield, Morriss and Bentall2,Reference Kernis, Cornell, Sun, Berry and Harlow21 twice daily for 4 consecutive days at approximately 10:00 and at 22:00. At each time, participants completed the RSE and the Positive and Negative Affect Scale, Reference Watson, Clark and Tellegren22 a brief measure of mood. Within-participant standard deviations (s.d.'s) were calculated as a measure of variability on these scales.
Statistical analysis
Analyses were carried out using SPSS 14 for Windows. Groups were compared with ANOVA followed by post hoc Tukey honestly significant difference (HSD) tests. Partial correlations were used to examine relationships between psychological measures and symptom scores, controlling for Bech–Rafaelsen scores when analysing relationships with depression, and controlling for HRSD scores when examining relationships with mania. Factor analysis (principal components with varimax rotation) on the psychological measures from the complete sample of 148 was then used to reduce the 17 psychological measures to a minimum number of dimensions, which were then compared between groups. The ratio of sample size to measures, approximately 9:1 exceeded the recommended minimum of 5:1. Reference Gorusch23
Results
Demographic and clinical measures
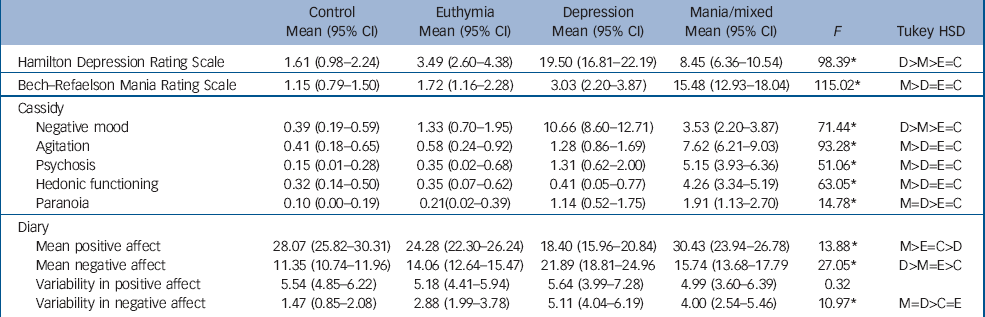
Demographic and clinical data are shown in online Table DS1 and Table 1, which also give the results of univariate tests. No differences were observed between the groups for age, IQ, the proportion of males or educational achievement, but the people in the bipolar groups were less likely to be employed and more likely to be living alone compared with the controls. The mania group scored higher on the Bech–Rafaelsen Scale compared with all other groups (minimum P<0.0001). On the HRSD, the depression group scored higher than all other groups (P at least <0.001) but the mania group scored higher than both the euthymia group and controls (P at least <0.001). An identical pattern was observed on the Cassidy negative mood sub-scale. On the psychomotor agitation, psychosis and increased hedonic functioning Cassidy sub-scales the mania group scored higher than all other groups (P at least <0.001). Finally, on the Cassidy paranoia sub-scale, the mania and depression groups scored higher than the euthymia and control groups (P at least <0.05) but not differently from each other.
Table 1 Mean (95% CI) scores and F-values of participants in the three bipolar disorder groups and the control group on the study instruments
| Control Mean (95% CI) | Euthymia Mean (95% CI) | Depression Mean (95% CI) | Mania/mixed Mean (95% CI) | F | Tukey HSD | |
|---|---|---|---|---|---|---|
| Hamilton Depression Rating Scale | 1.61 (0.98–2.24) | 3.49 (2.60–4.38) | 19.50 (16.81–22.19) | 8.45 (6.36–10.54) | 98.39 * | D>M>E=C |
| Bech–Refaelson Mania Rating Scale | 1.15 (0.79–1.50) | 1.72 (1.16–2.28) | 3.03 (2.20–3.87) | 15.48 (12.93–18.04) | 115.02 * | M>D=E=C |
| Cassidy | ||||||
| Negative mood | 0.39 (0.19–0.59) | 1.33 (0.70–1.95) | 10.66 (8.60–12.71) | 3.53 (2.20–3.87) | 71.44 * | D>M>E=C |
| Agitation | 0.41 (0.18–0.65) | 0.58 (0.24–0.92) | 1.28 (0.86–1.69) | 7.62 (6.21–9.03) | 93.28 * | M>D=E=C |
| Psychosis | 0.15 (0.01–0.28) | 0.35 (0.02–0.68) | 1.31 (0.62–2.00) | 5.15 (3.93–6.36) | 51.06 * | M>=D=E=C |
| Hedonic functioning | 0.32 (0.14–0.50) | 0.35 (0.07–0.62) | 0.41 (0.05–0.77) | 4.26 (3.34–5.19) | 63.05 * | M>D=E=C |
| Paranoia | 0.10 (0.00–0.19) | 0.21(0.02–0.39) | 1.14 (0.52–1.75) | 1.91 (1.13–2.70) | 14.78 * | M=D>E=C |
| Diary | ||||||
| Mean positive affect | 28.07 (25.82–30.31) | 24.28 (22.30–26.24) | 18.40 (15.96–20.84) | 30.43 (23.94–26.78) | 13.88 * | M>E=C>D |
| Mean negative affect | 11.35 (10.74–11.96) | 14.06 (12.64–15.47) | 21.89 (18.81–24.96 | 15.74 (13.68–17.79 | 27.05 * | D>M=E>C |
| Variability in positive affect | 5.54 (4.85–6.22) | 5.18 (4.41–5.94) | 5.64 (3.99–7.28) | 4.99 (3.60–6.39) | 0.32 | |
| Variability in negative affect | 1.47 (0.85–2.08) | 2.88 (1.99–3.78) | 5.11 (4.04–6.19) | 4.00 (2.54–5.46) | 10.97 * | M=D>C=E |
HSD, honestly significant difference; D, depression group; M, mania/mixed group; E, euthymia group; C, control group
a. Significant Tukey between-group comparisons are also indicated (see text for P-values)
* P<0.001, degrees of freedom 3, 143
Diary mood scores are also shown in Table 1. People in the mania group reported more positive affect than those in the euthymia (P<0.01) and depression groups (P<0.001), who reported less positive affect than the euthymia (P<0.01) and control groups (P<0.001). All three bipolar disorder groups reported higher negative affect than the control group (P<0.001 for all comparisons) and the depression group reported more negative affect than both the mania (P<0.001) and euthymia groups (P<0.001), who did not differ on this measure. No differences were observed in variability of positive affect (measured by the within-participant standard deviation of scores) but the depression (P<0.001) and mania groups (P<0.005) had greater variability in negative affect than the control group, and the depression group also showed greater variability in negative affect than the euthymia group (P<0.01).
Depression-related psychological measures
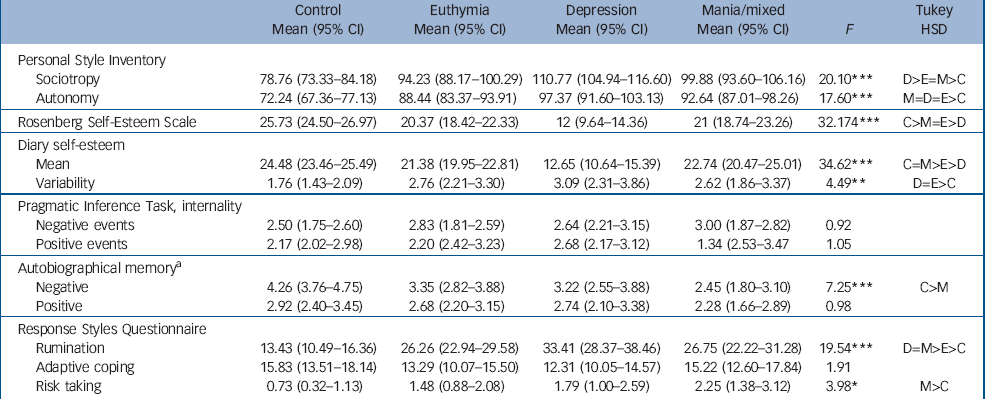
Scores for depressogenic cognitive style are shown in Table 2. Significant main effects were observed on all measures with the exception of the PIT and RSQ adaptive coping. All bipolar groups scored higher than the control group on sociotropy and autonomy (P<0.0001 for each comparison) and the depression group scored higher than the euthymia group on sociotropy (P<0.001). The control group reported higher self-esteem on the RSE than all three patient groups (P at least <0.005) but the mania and euthymia groups scored higher than the depression group (P<0.001 for each comparison). On the twice daily ratings of self-esteem, post hoc analysis revealed that the depression group had a lower mean score than all the other groups (P at least <0.005 level) and the euthymia group had a lower mean score than the control group (P<0.05) but there was no significant difference between the controls and mania groups. The control group also showed less variability (as measured by the within-participant standard deviation of scores) in self-esteem compared with the euthymia (P<0.05) and depression groups (P<0.05). No significant differences were observed on the PIT internality scores. On the autobiographical memory test, the mania group recalled fewer specific negative memories than the control group (P<0.001) but no differences were observed for the recall of positive memories. On the RSQ, all three bipolar groups reported more rumination in response to negative mood than the controls (P<0.001 for all comparisons) and the euthymia group reported less rumination than the mania group (P<0.05). No differences were reported for adaptive coping (distraction and problem-solving) but the mania group reported higher levels of risk-taking than the controls (P<0.01).
Table 2 Mean (95% CI) scores of participants in the three bipolar disorder groups and the control group on the study instruments
| Control Mean (95% CI) | Euthymia Mean (95% CI) | Depression Mean (95% CI) | Mania/mixed Mean (95% CI) | F | Tukey HSD | |
|---|---|---|---|---|---|---|
| Personal Style Inventory | ||||||
| Sociotropy | 78.76 (73.33–84.18) | 94.23 (88.17–100.29) | 110.77 (104.94–116.60) | 99.88 (93.60–106.16) | 20.10 *** | D>E=M>C |
| Autonomy | 72.24 (67.36–77.13) | 88.44 (83.37–93.91) | 97.37 (91.60–103.13) | 92.64 (87.01–98.26) | 17.60 *** | M=D=E>C |
| Rosenberg Self-Esteem Scale | 25.73 (24.50–26.97) | 20.37 (18.42–22.33) | 12 (9.64–14.36) | 21 (18.74–23.26) | 32.174 *** | C>M=E>D |
| Diary self-esteem | ||||||
| Mean | 24.48 (23.46–25.49) | 21.38 (19.95–22.81) | 12.65 (10.64–15.39) | 22.74 (20.47–25.01) | 34.62 *** | C=M>E>D |
| Variability | 1.76 (1.43–2.09) | 2.76 (2.21–3.30) | 3.09 (2.31–3.86) | 2.62 (1.86–3.37) | 4.49 ** | D=E>C |
| Pragmatic Inference Task, internality | ||||||
| Negative events | 2.50 (1.75–2.60) | 2.83 (1.81–2.59) | 2.64 (2.21–3.15) | 3.00 (1.87–2.82) | 0.92 | |
| Positive events | 2.17 (2.02–2.98) | 2.20 (2.42–3.23) | 2.68 (2.17–3.12) | 1.34 (2.53–3.47 | 1.05 | |
| Autobiographical memory a | ||||||
| Negative | 4.26 (3.76–4.75) | 3.35 (2.82–3.88) | 3.22 (2.55–3.88) | 2.45 (1.80–3.10) | 7.25 *** | C>M |
| Positive | 2.92 (2.40–3.45) | 2.68 (2.20–3.15) | 2.74 (2.10–3.38) | 2.28 (1.66–2.89) | 0.98 | |
| Response Styles Questionnaire | ||||||
| Rumination | 13.43 (10.49–16.36) | 26.26 (22.94–29.58) | 33.41 (28.37–38.46) | 26.75 (22.22–31.28) | 19.54 *** | D=M>E>C |
| Adaptive coping | 15.83 (13.51–18.14) | 13.29 (10.07–15.50) | 12.31 (10.05–14.57) | 15.22 (12.60–17.84) | 1.91 | |
| Risk taking | 0.73 (0.32–1.13) | 1.48 (0.88–2.08) | 1.79 (1.00–2.59) | 2.25 (1.38–3.12) | 3.98 * | M>C |
HSD, honestly significant difference; D, depression group; M, mania/mixed group; E, euthymia group; C, control group
a. Specific memories
* P<0.01
** P<0.005
*** P<0.001
Reward processes
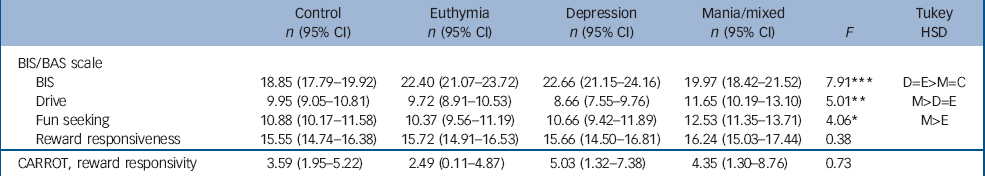
Table 3 shows scores on the BIS/BAS scale and the CARROT. On the BIS (a measurement of sensitivity to punishment-related stimuli) the controls (P<0.001 for each comparison) and mania group (P<0.05 for each comparison) scored lower than the euthymia and depression groups. On BAS drive, the mania group scored higher than those in the depression (P<0.001) and euthymia groups (P<0.05) but not the controls. On BAS fun seeking the mania group scored higher than the euthymia group (P<0.05) but no differences were observed for the reward responsiveness sub-scale. No significant differences were observed on the CARROT.
Table 3 Mean (95% CI) scores for controls, and mania, depression and euthymia bipolar disorder groups on the study instruments
| Control n (95% CI) | Euthymia n (95% CI) | Depression n (95% CI) | Mania/mixed n (95% CI) | F | Tukey HSD | |
|---|---|---|---|---|---|---|
| BIS/BAS scale | ||||||
| BIS | 18.85 (17.79–19.92) | 22.40 (21.07–23.72) | 22.66 (21.15–24.16) | 19.97 (18.42–21.52) | 7.91 *** | D=E>M=C |
| Drive | 9.95 (9.05–10.81) | 9.72 (8.91–10.53) | 8.66 (7.55–9.76) | 11.65 (10.19–13.10) | 5.01 ** | M>D=E |
| Fun seeking | 10.88 (10.17–11.58) | 10.37 (9.56–11.19) | 10.66 (9.42–11.89) | 12.53 (11.35–13.71) | 4.06 * | M>E |
| Reward responsiveness | 15.55 (14.74–16.38) | 15.72 (14.91–16.53) | 15.66 (14.50–16.81) | 16.24 (15.03–17.44) | 0.38 | |
| CARROT, reward responsivity | 3.59 (1.95–5.22) | 2.49 (0.11–4.87) | 5.03 (1.32–7.38) | 4.35 (1.30–8.76) | 0.73 |
BIS/BAS, Behavioural Inhibition System/Behavioural Activation System; CARROT, Card Arranging Reward Responsivity Objective Test; HSD, honestly significant difference; D, depression group; M, mania/mixed group; E, euthymia group; C, control group
* P<0.01
** P<0.005
*** P<0.001
Relationships between psychological measures and symptoms
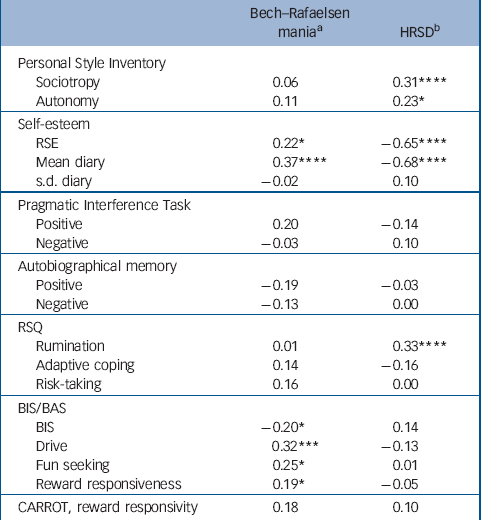
Bech–Rafaelsen and HRSD scores were significantly correlated (r=0.24, P<0.005, d.f.=146). Therefore, partial correlations, shown in Table 4, were used to examine the relationships between psychological variables and mania and depression scores, in each case controlling for the effects of the other mood state. A clear pattern can be seen, with strong correlations between depression and measures of negative cognitive style (sociotropy, autonomy, self-esteem and rumination) and weaker correlations between manic symptoms and reward-related measures (the BAS scale, with a trend towards significance with the CARROT). These observations were confirmed using factor analysis (principal components with varimax rotation, missing data replaced by mean values) on all questionnaire measures and the CARROT, which yielded five easily interpretable factors: negative cognitive style (high loadings for sociotropy, autonomy, BIS and rumination, 22.5% of the variance), excitement (high loadings for the three BAS scores and RSQ risk taking, 18%), PIT pessimism (internality for negative events, 10%), PIT optimism (internality for positive events, 9%) and CARROT scores (9%); total variance accounted for 69.80%. When these factor scores were partially correlated against the Bech–Rafaelsen and HRSD scores, the former robustly correlated with the excitement factor (r=0.36, P<0.001, d.f.=144) but not the negative cognitive style factor (r=–0.01, P=0.64, d.f.=144), whereas the latter robustly correlated with the negative cognitive style factor (r=0.43, P<0.001, d.f.=144) but not the excitement factor (r=–0.09, P=0.25, d.f.=144).
Table 4 Partial correlations between mania and depression scores and the psychological measures (patients only, n=107)
| Bech–Rafaelsen mania a | HRSD b | |
|---|---|---|
| Personal Style Inventory | ||
| Sociotropy | 0.06 | 0.31 **** |
| Autonomy | 0.11 | 0.23 * |
| Self-esteem | ||
| RSE | 0.22 * | –0.65 **** |
| Mean diary | 0.37 **** | –0.68 **** |
| s.d. diary | –0.02 | 0.10 |
| Pragmatic Interference Task | ||
| Positive | 0.20 | –0.14 |
| Negative | –0.03 | 0.10 |
| Autobiographical memory | ||
| Positive | –0.19 | –0.03 |
| Negative | –0.13 | 0.00 |
| RSQ | ||
| Rumination | 0.01 | 0.33 **** |
| Adaptive coping | 0.14 | –0.16 |
| Risk-taking | 0.16 | 0.00 |
| BIS/BAS | ||
| BIS | –0.20 * | 0.14 |
| Drive | 0.32 *** | –0.13 |
| Fun seeking | 0.25 * | 0.01 |
| Reward responsiveness | 0.19 * | –0.05 |
| CARROT, reward responsivity | 0.18 | 0.10 |
HRSD, Hamilton Rating Scale for Depression; RSE, Rosenberg Self-Esteem Scale; s.d., standard deviation; RSQ, Modified Response Style Questionnaire; BIS/BAS, Behavioural Inhibition System/Behavioural Activation System Scales; CARROT, Card Arranging Reward Responsivity Objective Test
a. Controlling for HRSD
b. Controlling for Bech–Rafaelsen mania
* P<0.05
** P<0.01
*** P<0.005
**** P<0.001
Significant differences between the groups were observed for the negative cognitive style (F(3,144)=22.32, P<0.001), excitement (F(3,144)=5.69, P<0.001) and pessimism factors (F(3,1444)=3.87, P=0.01). Interestingly, ANCOVA with Bech–Rafaelsen and HRSD scores as covariates revealed that the differences observed for negative cognitive style remained even after symptom scores were controlled for (F(3,141)=10.08, P<0.001); planned contrasts revealed that both the euthymia (P<0.0001) and depression groups (P<0.0001) scored higher than the controls in this analysis. The group differences in excitement and pessimism did not remain after controlling for symptoms.
Discussion
In this study we aimed to investigate depressogenic cognitive styles and reward responsivity in a well-characterised and large sample of people diagnosed with bipolar disorder. When individuals in different episodes were compared with healthy controls, the results largely replicated those obtained in previous studies, with people with bipolar disorder in all phases showing high levels of sociotropy and autonomy and low self-esteem, Reference Scott and Pope4 self-esteem instability, Reference Knowles, Tai, Jones, Highfield, Morriss and Bentall2 rumination Reference Thomas, Knowles, Tai and Bentall3 and, less clearly, impairment in the ability to recall specific autobiographical memories. Reference Scott and Pope4 On all except the autobiographical memory measure, the euthymia group's results were abnormal compared with the controls. Less robust evidence of abnormal reward responsivity was evident in the group comparisons, but the mania group scored higher than the controls on two of the BAS scores (drive and fun seeking). Only the results from the PIT failed to replicate previous studies, which had indicated a pessimistic attributional style in patients with remitted Reference Knowles, Tai, Jones, Highfield, Morriss and Bentall2,Reference Winters and Neale17 and currently symptomatic bipolar disorder. Reference Lyon, Startup and Bentall5
A crucial issue when interpreting these and previous findings is the relationships between the measures and mood state. For example, previous reports of depressogenic processes during mania, Reference Lyon, Startup and Bentall5 although plausibly reflecting defensive processes, might more simply reflect the coactivation of depressogenic and reward-related processes in mixed episodes. Our factor analysis, which attempted to address this problem, yielded an easily interpretable model that accounted for a very large amount of the variance in the data. The high loadings of poor self-esteem, sociotropy, autonomy, BIS and rumination on a single factor suggest that these are highly related processes and might be considered to form a negative cognitive syndrome. The fact that the PIT and CARROT appeared as separate factors suggests that they are poor indicators of negative cognitive style and reward processing respectively, which probably accounts for their failure to discriminate between the groups.
A clear and specific relationship was observed between negative cognitive style and depression. However, it is notable that the negative cognitive style was still evident in the euthymia group, even after symptoms were controlled for. One possible interpretation of this finding is that negative cognitive style is a vulnerability factor in people with bipolar disorder that, when activated, leads to spiraling negative thoughts about self, rumination and eventually severe depression. By contrast, the factor we have characterised as excitement, consisting of high responsivity to reward signals and excessive risk taking, seems to be clearly state-related and associated with current mania.
The finding that bipolar depression and bipolar mania are related to distinct psychological processes is consistent with recent findings that have suggested that mania is not simply the opposite of depression. Reference Bauer, Simon, Ludman and Unutzer24,Reference Murray, Goldstone and Cunningham25 It is also consistent with the finding that depression and mania are provoked by distinct kinds of life events. Reference Johnson, Sandow, Meyer, Winters, Miller and Solomon6,Reference Johnson, Meyer, Winett and Small26
Limitations
A limitation of this study is that we were unable to take into account the effects of treatment on the psychological processes we were measuring. However, any such effects would reduce the variance in our data and thereby reduce our opportunity to demonstrate significant associations between the measures and psychopathology. A more important limitation is that the study was cross-sectional, and hence could not address the evolution of bipolar symptoms (an inherently dynamic process) over time. It has recently been suggested that neither depressogenic cognitive styles nor reward sensitivity are sufficient to account for the complexity of bipolar phenomena Reference Power27 and that higher level cognitive appraisals play an important role in the ascent into mania. Reference Mansell and Pedley28 More complex longitudinal designs will be required to test these accounts.
Implications
The major clinical implication of the present study is that attention must be given to the two types of psychological processes we have identified when designing interventions for people with bipolar disorder. Given that dopaminergic function is implicated in the anticipatory processing of reward stimuli, Reference Berridge and Robinson29 it is unsurprising that dopamine-blocking drugs are effective in treating manic states. Reference Khanna, Vieta, Lyons, Grossman, Eedekens and Kramer30 It might be useful to consider the effects of other pharmacological interventions, for example mood stabilisers, on negative cognitive styles and reward processing. In humans there is evidence that lithium and carbamazepine normalise self-esteem thereby preventing relapse. Reference Wolf31 In animals, there is evidence that lithium can attenuate some dopamine-induced behaviours thought to be related to reward responsivity. Reference Beaulieu, Sotnikova, Yao, Kockeritz, Woodgett and Gainetdinov32
With respect to psychological interventions, our recent trial of cognitive therapy yielded disappointing results Reference Scott, Paykel, Morriss, Bentall, Kinderman and Johnson33 and it might be argued that other trials Reference Colom, Vieta, Martinez-Aran, Reinares, Goikolea and Benabarre34–Reference Perry, Tarrier, Morriss, McCarthy and Limb36 have been more successful because they have more thoroughly incorporated techniques to prevent excessive responding to reward stimuli. Further advances in the psychological treatment of people with bipolar disorder are likely to be achieved following a more thorough understanding of the processes involved in the evolution of bipolar symptoms.
Acknowledgement
This research was funded by a grant from Mersey Care NHS Trust.







eLetters
No eLetters have been published for this article.