Hyperprolactinaemia is a frequent adverse effect of antipsychotic medication (Reference PettyPetty, 1999), and is the result of D2 dopamine receptor drug binding (Reference Markianos, Hatzimanolis and LykourasMarkianos et al, 2001). Hyperprolactinaemia may result in depression, sexual dysfunction, amenorrhoea, galactorrhoea, breast cancer and osteoporosis (Reference MaguireMaguire, 2002; Reference Halbreich, Kinon and GilmoreHalbreich et al, 2003). Antipsychotic drugs vary widely in their binding affinity for the D2 receptor. Clozapine and quetiapine, with a lower binding affinity than dopamine (Remington et al, 2000), have not been associated with hyperprolactinaemia (Reference Markianos, Hatzimanolis and LykourasMarkianos et al, 2002). Hyperprolactinaemia is typically associated with tighter-binding agents such as risperidone, an atypical antipsychotic, whereas olanzapine, an antipsychotic with intermediate binding, is associated with a modest increase in prolactin levels (Reference David, Taylor and KinonDavid et al, 2000). The A1 allele of the D2 dopamine receptor gene (DRD2) is associated with significantly reduced density of D2 receptors (Reference Noble, Blum and RitchieNoble et al, 1991) and thus may influence D2 receptor antagonism. This study reports the effect of DRD2 polymorphism on prolactin response to a variety of antipsychotic medications.
METHOD
Sample
Patients were recruited at the Fortitude Valley Community Mental Health Centre, the Royal Brisbane Mental Health Unit and the Park Psychiatric Hospital. Inclusion criteria were age 18-65 years and meeting DSM-IV criteria for schizophrenia (American Psychiatric Association, 1994). Potential participants were excluded if they had schizoaffective disorder, bipolar disorder, dementia, organic brain syndrome, major depressive disorder with delusions or epilepsy. Those who were pregnant were excluded. As a range of psychoactive agents can influence prolactin levels (Reference Jarvinen, Rago and MannistoJarvinen et al, 1992; Reference Hugues, Gourlot and Le JeunneHugues et al, 2000; Reference Basturk, Karaaslan and EselBasturk et al, 2001; Reference Keltner, McAfee and TaylorKeltner et al, 2002), patients regularly taking antidepressant, opiate, anxiolytic or mood-stabilising medication were excluded from the study. Potential participants who nursing or medical staff believed were not adhering to their medication regimen were also excluded. All participants provided informed consent and were able to terminate participation without prejudice. Institutional ethics approval was obtained from the clinics and hospitals involved.
Assessments
A total of 144 unrelated White patients (123 men, 21 women), attending various psychiatric units for the treatment of their schizophrenia, were enrolled in the study. Their average age was 36.4 years (s.d.= 12.0); 61 participants were in-patients and 83 were out-patients. A clinical history was taken by either a psychiatrist (S.B., B.L., M.B., W.W.) or a clinical psychologist (R.Y.). Demographic details including ethnic background data were obtained. The Positive and Negative Syndrome Scale (PANSS; Reference KayKay, 1990) was used to assess psychotic symptoms. All raters were trained to a criterion of 90% agreement using PANSS training videos. Interrater reliability was obtained through random checks of the PANSS by independent raters of the same patient. These reliabilities were sound.
Medications
All participants received their prescribed antipsychotic medication for at least 1 month at a stable dosage. Thirty-one patients were prescribed clozapine, 31 olanzapine, 33 typical antipsychotics (12 flupentixol, 2 fluphenazine decanoate, 13 zuclopenthixol, 3 haloperidol decanoate, 1 thioridazine, 1 thiothixene and 1 trifluoperazine) and 49 risperidone. Antipsychotic dosage was transformed to chlorpromazine equivalents per kilogram. The mean chlorpromazine equivalent dosages in the four medication groups were clozapine 5.14 mg/kg (s.d.=2.94), olanzapine 5.37 mg/kg (s.d.=2.62), typicals 5.60 mg/kg (s.d.=3.70) and risperidone 4.82 mg/kg (s.d.=2.00). There was no significant difference in dosage among the four drug groups (F(3,128)=0.51, P=0.67). Adherence by the in-patients was sound as all medication was administered by nursing staff; outpatient adherence was estimated by self-report and assessment by the treating psychiatrist. Thirty of the 33 patients receiving typical medication were treated with nurse-administered depot preparations.
Prolactin levels and DRD2 alleles
A 10 ml blood sample was drawn from each participant for DNA extraction and prolactin determination. The DNA was sent to the University of California Los Angeles for genotyping, and prolactin determination was conducted at the Royal Brisbane Hospital. Serum prolactin level was determined by a heterogeneous sandwich magnetic separation assay (Immuno 1 system; Bayer Diagnostics, Newbury, Berkshire, UK) which was standardised against the World Health Organization 3rd IRP 84/500 Reference Manual.
DNA was extracted from leucocytes using standard techniques and subsequently used as a template for determination of Taq1A DRD2 alleles by the polymerase chain reaction (Reference Grandy, Zhang and CivelliGrandy et al, 1993). As previously described (Reference Noble, Noble and RitchieNoble et al, 1994), the amplification of DNA was carried out using a Perkin Elmer GeneAmp 9600 thermocycler (Perkin Elmer, Boston, MA, USA). Approximately 500 ng of amplified DNA was then digested with 5 units of Taq1 restriction enzyme (Gibco/BRL, Grand Island, NY, USA) at 65°C overnight. The resulting products were analysed by electrophoresis in a 2.5% agarose gel containing ethidium bromide and visualised under ultraviolet light. The A1/A2 genotype is revealed by three fragments (310 bp, 180 bp and 130 bp) and the A2/A2 genotype by two fragments (180 bp and 130 bp); the A1/A1 genotype is shown by the uncleaved 310 bp fragment. Participants with A1/A1 and A1/A2 genotypes were considered to have A1 +allelic status and those with the A2/A2 genotype were considered to have A1 - allelic status.
Data analysis
Information coded from interview proformas was entered into a computer database along with prolactin results. The Taq1A DRD2 allelic data were entered last. Chi-squared tests (Yates' corrected) were employed to compare differences in categorical variables between A1 + and A1 - allelic groups. Analysis of variance (ANOVA) was used to compare differences in prolactin levels among the various drug groups. Similarly, one-way ANOVA was employed to examine differences in prolactin levels between the A1 + and A1 - allelic groups. A P value of ≥ 0.05 was considered to be statistically significant.
RESULTS
The results confirmed a significant gender effect in prolactin levels of patients treated with antipsychotic medications. Female participants had significantly higher prolactin levels than males (F(1,142)=25.19, P<0.0001), with the mean prolactin level for females being 1146 mIU/l (s.d.=1136) and for males being 420 mIU/l (s.d.=374). Men and women were equally represented across the four medication groups.
Analysis of variance revealed significant differences in prolactin levels among the four medication groups (F(3,140)=19.0, P<0.0001). Figure 1 shows prolactin levels in each of the treatment groups. Post hoc pairwise comparisons were undertaken to test differences between groups. Olanzapine compared with clozapine treatment resulted in significantly higher prolactin levels (F(1,60)=4.76, P=0.033). Patients treated with typical antipsychotics had significantly higher prolactin levels than their olanzapine-treated counterparts (F(1,62)=7.60, P=0.007). Finally, significantly higher levels of prolactin were evident in patients treated with risperidone compared with those treated with typical antipsychotics (F(1,80)=8.97, P=0.004).
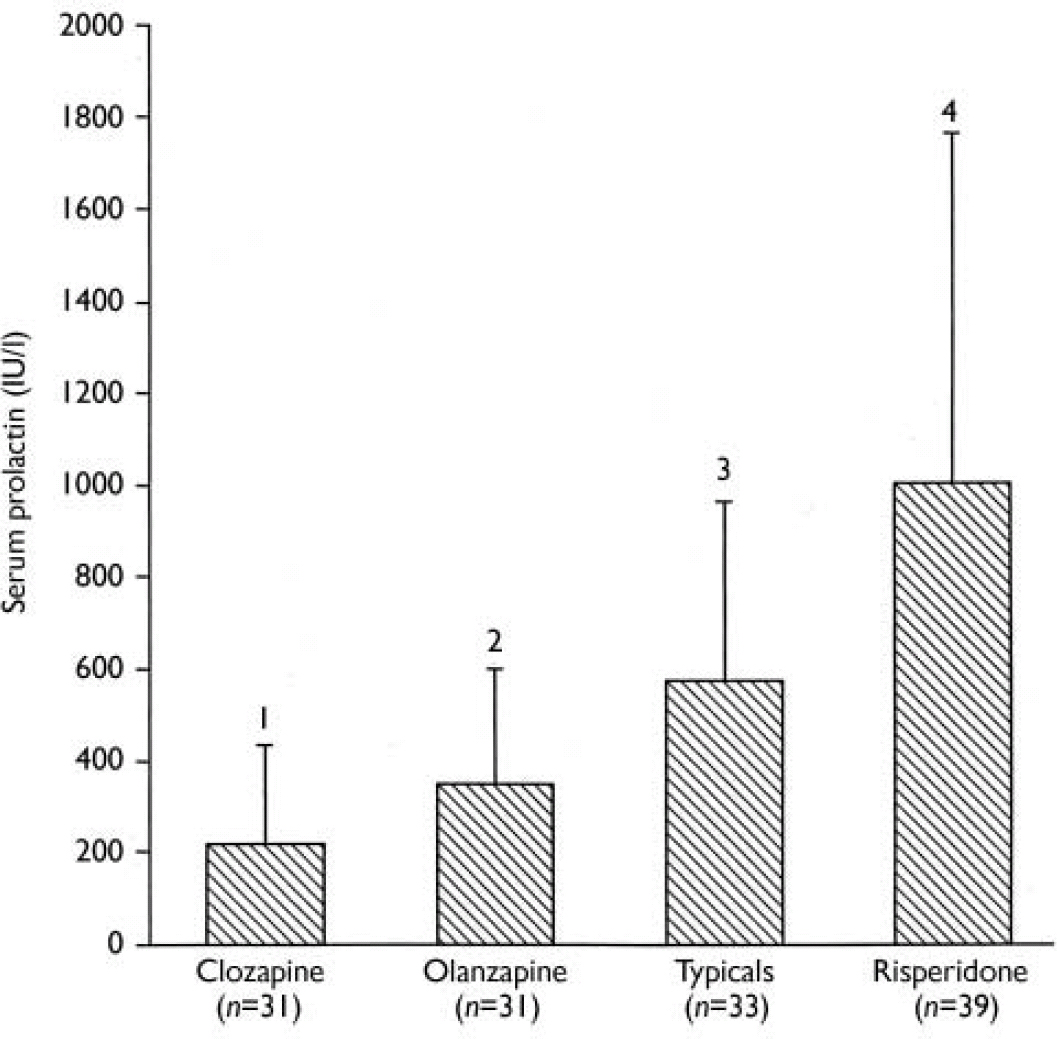
Fig.1 Serum prolactin levels in patients with schizophrenia treated with different antipsychotic medications. 1 v. 2, P=0.033; 2 v. 3, P=0.007; 3 v. 4, P=0.004.
The genotypes of the 144 patients were as follows: A1/A1 (n=7), A1/A2 (n=55) and A2/A2 (n=82). The mean age of the A1 + group (35.5 years, s.d.=12.7) was not significantly different from that of the A1 - group (37.1 years, s.d.=11.5) (F(1,141)=0.66, P=0.42). There was no significant difference between the A1 + and A1 7 groups according to gender (χ2(1)=0.048, P=0.83), in-patient or outpatient status (χ2(1)=0.00, P>0.99), family history of schizophrenia (χ2(1)=0.00, P>0.99), criminality (χ2(1)=0.02, P=0.90), binge drinking (χ2(1)=0.08, P=0.78) or suicide attempts (χ2(1)=2.03, P=0.16). There was also no significant difference in markers of psychosis severity, as measured by number of admissions (F(1,140)=1.45, P=0.23), PANSS positive symptoms (F(1,140)=0.06, P=0.81) and PANSS negative symptoms (F(1,139)=2.60, P=0.11). There was no difference in antipsychotic chlorpromazine equivalent dosage between A1 + and A1 - patients taking clozapine (F(1,128)=2.36, P=0.14), olanzapine (F(1,27)=1.0, P=0.33), typical antipsychotics (F(1,24)=0.33, P=0.57) and risperidone (F(1,45)=0.47, P=0.50).
Table 1 shows the serum prolactin levels of A1 + and A1 - group patients treated with antipsychotic medications. Analysis of variance of the total sample of patients indicated that those carrying the A1 allele had about a 40% higher prolactin level than patients without this allele (F(1,142)=4.50, P=0.04).
Table 1 Serum prolactin levels in patients with or without the DRD2*AI allele receiving antipsychotic medication for schizophrenia

| Antipsychotic group | AI allele present | AI allele absent | P | ||
|---|---|---|---|---|---|
| Prolactin level, IU/I mean (s.d.) | n | Prolactin level, IU/I mean (s.d.) | n | ||
| All antipsychotics (n=144) | 708 (622) | 62 | 499 (561) | 82 | 0.04 |
| Clozapine (n=31) | 310 (318) | 12 | 147 (78) | 19 | 0.04 |
| Olanzapine (n=31) | 327 (107) | 9 | 346 (293) | 22 | 0.85 |
| Typical antipsychotics (n=33) | 622 (350) | 14 | 530 (422) | 19 | 0.52 |
| Risperidone (n=49) | 1058 (736) | 27 | 928 (800) | 22 | 0.56 |
Clozapine, the loosest-binding antipsychotic drug, showed a significant difference across allelic groups, with a prolactin level that was twice as high in A1 + patients compared with A1 - patients (F(1,29)=4.63, P=0.04). When the analysis was conducted for male patients only (there were too few women patients for analysis), significance remained (F(1,26)4.58, P=0.04). There was no significant difference in prolactin levels between A1 + and A1 - patients in the other antipsychotic drug groups.
Hyperprolactinaemia is defined using community sample cut-off levels set at a 95% reference range: ≥430 mU/l in men and ≥560 mU/l in women (Reference Vanderpump, French and AppletonVanderpump et al, 1998). In total, 64 patients, 44% of the sample, exceeded these levels. Only 7 of these patients were prescribed lower-binding agents (clozapine and olanzapine) confirming that these medications are rarely associated with hyperprolactinaemia (5% of the sample). Forty of the patients on risperidone exceeded these prolactin levels, indicating that 81% of patients on this medication were in the hyperprolactinaemic range. A Yates'-corrected χ2 analysis conducted to compare allelic status in the group with prolactin levels in the normal range and those with hyperprolactinaemia was significant (χ2(1)=5.52, P=0.02). Those with A1 + allelic status were significantly overrepresented in the group of patients with clinical hyperprolactinaemia.
DISCUSSION
Prolactin response to antipsychotic medication was greatest in patients prescribed risperidone. Prolactin response was successively lower in patients administered typical antipsychotics, olanzapine and clozapine. This order across medications has also been found in positron emission tomography binding studies (e.g. Reference Markianos, Hatzimanolis and LykourasMarkianos et al, 2001) where D2 dopamine receptor occupancy corresponded to serum prolactin levels. Prolactin levels were higher in women than in men.
Individuals with the A1 allele and higher prolactin levels when treated with antipsychotic medication and were overrepresented among patients with clinical hyperprolactinaemia. The A1 + participants also had significantly higher prolactin levels than A1 - participants when treated with the loosely binding agent clozapine. The greater prolactin response to antipsychotics observed in A1 + patients with schizophrenia in this and other studies (Mihara et al, Reference Mihara, Kondo and Suzuki2000, Reference Mihara, Suzuki and Kondo2001) may be the result of A1 + individuals having fewer unbound dopamine receptors at any given antipsychotic dose. Our results indicate that in addition to the D2 receptor binding dissociation constant of antipsychotic medications, individual D2 receptor density is also important in determining prolactin response to antipsychotic agents.
Individuals with the A1 allele have a reduced density of brain D2 receptors (Reference Noble, Blum and RitchieNoble et al, 1991; Reference Thompson, Thomas and SingletonThompson et al, 1997; Phjalainen et al, 1998; Reference Jonsson, Nothen and GrunhageJonsson et al, 1999). An early brain autopsy study (Reference Noble, Blum and RitchieNoble et al, 1991) found a significant reduction of approximately 30% in the number of D2 dopamine receptors (B max) in the caudate nucleus of A1 + compared with A1 - subjects; there was no difference in D2 binding affinity (K d) between the two allelic groups. Thompson et al (Reference Thompson, Thomas and Singleton1997) also reported a 30-40% reduction in D2 receptor density in the striatum of A1 + compared with A1 - individuals. An in vivo study of healthy Finnish volunteers (Reference Pohjalainen, Rinne and NagrenPohjalainen et al, 1998) showed significantly decreased D2 receptor density in the striatum of A1 + compared with A1 - individuals, with no difference in K d between the two groups. In another positron emission tomography study of healthy humans using [11C]-labelled raclopride (Reference Jonsson, Nothen and GrunhageJonsson et al, 1999), a significant association of the A1 allele was found with low D2 receptor density. Taq1A DRD2 variants are now known to be in linkage disequilibrium with C957T, a synonymous mutation in the human DRD2 (Reference Duan, Wainwright and ComeronDuan et al, 2003). Furthermore, C957T affects messenger ribonucleic acid (mRNA) folding, leading to a decrease in mRNA stability and a 50% decrease in D2 dopamine receptor proteins. These effects dramatically diminish dopamine-induced upregulation of D2 receptors. As a result of fewer D2 receptors at any dose of antipsychotic medication, A1 + individuals may have a lower density of free, unbound D2 receptors, and consequently an enhanced prolactin response.
Dopamine receptor drug occupancy and consequent receptor blockade are necessary for both clinical antipsychotic action (Reference Kapur and RemingtonKapur & Remington, 2001) and a variety of other effects. Studies with conventional antipsychotic drugs report that approximately 70% occupancy results in maximal therapeutic efficacy (Reference Nordstrom, Farde and WieselNordstrom et al, 1993). A trend towards improved efficacy in patients with treatment-resistant schizophrenia was found when doses of olanzapine were increased to an average of 30.4 mg (Reference Volavka, Czobor and SheitmanVolavka et al, 2002). Preliminary investigations have been undertaken to increase the efficacy of clozapine, an agent with a high D2 receptor dissociation constant or loose binding, by adding haloperidol, an agent with a low dissociation constant (Reference Kapur, Roy and DaskalakisKapur et al, 2001). Individuals lacking the A1 allele may be more likely to benefit from these approaches to improve drug D2 receptor occupancy given that they may have relatively more free, unbound, D2 receptors. Conversely, A1 + individuals are unlikely to derive as much improvement from this approach, with optimal therapeutic effect being likely at lower dosages in these patients. Patients lacking the A1 allele may require higher doses for maximal antipsychotic effect, particularly when prescribed a loosely binding antipsychotic such as clozapine or quetiapine.
According to the rapid dissociation model (Reference Kapur and SeemanKapur & Seeman, 2001), loose-binding atypical agents are hypothesised to have an antipsychotic action without causing other effects of dopamine blockade such as raised prolactin levels or extrapyramidal side-effects (Reference Kapur and SeemanKapur & Seeman, 2001). Our data are not consistent with this, because clozapine has definite effects on serum prolactin levels, with A1 + individuals having significantly raised prolactin levels compared with A1 - patients. The D2 blockade effect of clozapine in A1 + patients is not limited to an antipsychotic effect alone.
Other clinical parameters influenced by D2 receptors require investigation. For example, D2 receptor occupancy correlates with liability to extrapyramidal adverse effects in patients treated with risperidone (Reference Yamada, Ohno and NakashimaYamada et al, 2002) and a variety of antipsychotic drugs, including clozapine (Reference Broich, Grunwald and KasperBroich et al, 1998), haloperidol (Reference Kapur, Zipursky and JonesKapur et al, 2000) and olanzapine (Reference Jauss, Schroder and PantelJauss et al, 1998). Individuals with the A1 allele treated with antipsychotic medication may experience extrapyramidal adverse effects at lower dose than A1- patients, as these patients have decreased nigrostriatal D2 receptor density (Reference Thompson, Thomas and SingletonThompson et al, 1997).
Limitations of the study
Although the total number of patients investigated is adequate, one of the limitations of this study is the relatively small number of patients in each medication subgroup. Further research involving larger numbers of patients with each individual medication is indicated in order to ascertain whether or not this association occurs with specific antipsychotic agents. Prospective studies examining the changes in prolactin levels over time are also recommended.
Implications of the study
Our study implicates the D2 receptor dissociation constant of the antipsychotic agent as well as DRD 2 variants as important determinants of D2 receptor blockade induced by antipsychotic medications. Patients carrying the A1 allele generally display higher prolactin levels, probably as a result of lower density of free, unbound, D2 receptors, and may be at increased risk of adverse effects associated with hyperprolactinaemia. The results demonstrate that this association is most evident with the loose D2 receptor binding antipsychotic agent, clozapine. Future research should employ this pharmacogenetic approach to investigate clinical parameters other than prolactin response. This may result in clinicians being able to optimise antipsychotic treatment with regard to drug selection, dose and possible adverse effects.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ In patients with schizophrenia, serum prolactin levels increased in proportion to tightness of antipsychotic medication D2 dopamine receptor binding.
-
▪ Patients with the DRD2*A1 allele treated with antipsychotic medications had higher prolactin levels than patients without this allele, and a higher percentage of patients with the DRD2*A1 allele compared with patients without this allele had hyperprolactinaemia.
-
▪ The A1 allele of DRD2 may be a useful clinical marker for identification for those at risk of hyperprolactinaemia and associated adverse effects.
LIMITATIONS
-
▪ Studies with a larger number of people with schizophrenia in each medication subgroup are recommended.
-
▪ Prospective studies examining changes in prolactin levels over time are indicated.
-
▪ Clinical correlation of hyperprolactinaemia in patients with the DRD2*A1 allele were not assessed.
Acknowledgements
The excellent editorial assistance of Ian Howland and Robyn Johnston in the preparation of the manuscript is gratefully acknowledged. The laboratory assistance of Leanne Arnold is also noted with gratitude. The patients involved in the study are thanked for their time and involvement. This study was made possible by a competitive grant from the Risperidal Foundation, Janssen-Cilag.





eLetters
No eLetters have been published for this article.