The comorbidity of non-affective psychoses and eating disorders has received little attention in the literature. Nevertheless, both early accountsReference Striegel-Moore, Garvin, Dohm and Rosenheck1 and more recent investigationsReference Kouidrat, Amad, Lalau and Loas2 suggest that disordered eating attitudes are common in individuals with schizophrenia, particularly binge eating and night-eating syndrome.Reference Kouidrat, Amad, Lalau and Loas2 Although some of these behaviours might be a consequence of pharmacological treatments,Reference Theisen, Linden, König, Martin, Remschmidt and Hebebrand3 they have also been observed in drug-naïve individuals.Reference Fawzi and Fawzi4 A recent, large multi-country study has shown that participants with bulimia nervosa, binge eating disorder and anorexia nervosa had up to three times the odds of developing psychotic symptoms, and that those with psychotic symptoms have twice the odds of developing bulimia nervosa.Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet5 Other studies have shown cross-sectionalReference Koyanagi, Stickley and Haro6 and longitudinalReference Solmi, Melamed, Lewis and Kirkbride7 associations between psychotic experiences and disordered eating behaviours, including fasting, purging and binge eating, in both adults and adolescents. In line with these clinical and epidemiological findings, recent genome-wide association studies (GWAS) have identified a positive genetic correlation between schizophrenia and anorexia nervosa.Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh8 Both conditions also share a negative genetic correlation with body mass index (BMI),Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh8 reflecting low BMI as a clinical feature of anorexia nervosa and a risk factor for schizophrenia.Reference Sørensen, Gamborg, Sørensen, Baker and Mortensen9 Although large GWAS of bulimia nervosa and binge eating disorder do not yet exist, positive genetic correlations between schizophrenia and bipolar disorder and depression (both highly comorbid with bulimia nervosa and binge eating disorder) have been observed.Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh8 Because disordered eating behaviours such as binge eating and purging can also occur in people with anorexia nervosa and studies have shown that eating disorder diagnoses are unstable,Reference Schaumberg, Jangmo, Thornton, Birgegård, Almqvist and Norring10 a shared genetic aetiology between schizophrenia and the entire eating disorder phenotypical spectrum is plausible. To date, however, no epidemiological study has directly investigated this hypothesis in the general population. Evidence of a shared genetic association between schizophrenia and eating disorders would support observational findings showing the co-occurrence of psychotic and eating disorders in the general population and highlight the need for clinical approaches that account for this possible comorbidity. It could also raise novel hypotheses for early-life intermediate phenotypes, which might be common across disorders and could provide potential targets for intervention. The aim of this study was therefore to investigate the presence of a common genetic liability across these disorders. To do this, we tested whether polygenic risk scores (PRS) for schizophrenia – a composite score capturing genetic susceptibility to the disorder – were associated with disordered eating behaviours frequently observed across eating disorder diagnoses (binge eating, purging, fasting and excessive exercise) and BMI, as low BMI is a distinct clinical feature of anorexia nervosa.
Method
Sample
We used data from the Avon Longitudinal Study of Parents and Children (ALSPAC), an ongoing birth cohort study of children born in Avon (Bristol, UK) between 1 April 1991 and 31 December 1992, who were alive at 1 year of age (n = 13 988, 99.5% of all live births). More details on the ALSPAC sample is available in previous publicationsReference Boyd, Golding, Macleod, Lawlor, Fraser and Henderson11 and on the study website (www.bristol.ac.uk/alspac), which also contains details of all the available data in a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). This study includes children with available genetic data who had information on disordered eating behaviours on at least one time point across ages 14, 16 and 18 years. For twins, although it was not possible to distinguish whether they were monozygotic or dizygotic, we only retained the first born (twin A), as twins are non-independent observations. As sensitivity analyses, we further re-run our models including the second-born twin (twin ‘B’, N = 202) to explore if this resulted in any changes. We further restricted analyses to participants of White ethnicity as current PRS have sufficient discriminant validity in individuals of European ancestry.Reference Vassos, Di Forti, Coleman, Iyegbe, Prata and Euesden12 All participants gave their informed consent to participate in the study. The ALSPAC ethics and law committee and the local research ethics committees approved this study.
Exposure
PRS are a continuous variable capturing the cumulative effect of risk alleles for a disorder in a given individual. Although PRS are not yet able to predict clinical presentations of disorders, they have increasingly been recognised as valid biomarkers and used to identify intermediate behavioural phenotypes or correlates of clinical disorders.Reference Sullivan, Agrawal, Bulik, Andreassen, Børglum and Breen13 The derivation of PRS for schizophrenia in this sample has been previously described in detail.Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 Briefly, PRS were calculated using training set data from the second schizophrenia GWAS of the Psychiatric Genomics Consortium,15 the largest genetics consortium devoted to the study of psychiatric disorders.Reference Sullivan, Agrawal, Bulik, Andreassen, Børglum and Breen13
For each ALSPAC child, a total score was derived by adding the number of risk alleles in each single nucleotide polymorphism (SNP) (range, 0–2) weighted by the logarithm of its odds ratio for schizophrenia in the Psychiatric Genomics Consortium sample.Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 In our main analyses, we used PRS derived from a set of SNPs selected using a P-value threshold of 0.05, as in previous ALSPAC studies,Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 as this threshold best discriminates schizophrenia liability. To account for the need to balance true and false associations, in sensitivity analyses, we used scores derived from a broad range of P-value thresholds ranging from 1 × 10−8 to 0.5, as in previous investigations.Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 PRS were standardised to achieve a continuous variable with a mean of 0 and an s.d. of 1. Squared PRS terms were also used to test for non-linear associations as well as quintiles of PRS.
Outcomes
Data on disordered eating behaviours (binge eating, fasting, purging and excessive exercise) in the previous 12 months was self-reported in postal questionnaires by adolescents at approximately 14, 16 and 18 years of age, using a modified version of the Youth Risk Behaviour Survey.Reference Kann, Warren, Harris, Collins, Williams and Ross16 We considered each of the disordered eating behaviours under investigation as present (yes/no) if they had occurred at least once in the previous year. Binge eating was defined as having ever eaten large amounts of food in a short period of time while usually experiencing a sense of loss of control over the amount of food eating. We defined purging adolescents as those who had ever self-induced vomit or used laxatives for weight loss, and fasting adolescents as those who had ever fasted for an entire day to lose weight. We considered excessive exercise as exercising frequently for weight loss even when sick, or finding it difficult to do school work (age 14 years) or feeling guilty when not exercising (age 16 and 18 years), as these dimensions (as opposed to amount of exercise undertaken, for instance) have been shown to better capture eating disorder psychopathology.Reference Mond, Hay, Rodgers and Owen17 Objective measurements of height and weight were collected in clinic visits when adolescents were approximately 13, 15 and 17 years of age. From these measures we derived a continuous standardised measure of BMI, which was standardised at ages 13 and 15 years by age- and gender-specific z-scores derived from the updated Stata package zanthro,Reference Vidmar, Cole and Pan18 as recommended in the literature.Reference Cole, Bellizzi, Flegal and Dietz19
Confounders
In our analyses, we accounted for population stratification by conducting principal component analyses and adjusting our models for population principal components as covariates. We also described our sample in relation to a number of child and family characteristics, including child's gender, maternal age, marital status (single/married/divorced, widowed or separated) and education (compulsory/non-compulsory education).
Data analysis
To take in to account the repeated nature of our outcome measurements, we used random-effects logistic and linear multilevel regressions (Stata commands xtlogit and xtreg, respectively), which allowed us to model repeated follow-up time points nested within individuals. In our models, we further added a linear variable for age (age 14, 16 and 18 years) to model the effect of time, as well as population principal components to account for population stratification. We tested for non-linear associations with a quadratic term for PRS. To further explore non-linearity and dose-response patterns of these associations, we divided PRS scores in quintiles and re-run our models with this variable as our main exposure. As sensitivity analyses, we tested for these associations using continuous measures of PRS derived from risk alleles associated with schizophrenia at different P-value thresholds, as previously done.Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 We conducted all analyses on Stata version 13 for Windows.20
Results
Sample
In total, 6920 children had available genetic data. Of these, 4473 (64.6%) and 5069 (73.3%) had data available at least at one time point on all disordered eating variables and one BMI outcome measurement, respectively. Across outcome measurements, between 26.8% (n = 1851, BMI) and 34.5% (n = 2387, binge eating) did not have any outcome data available, and between 24.8% (n = 1718, excessive exercise) and 42.8% (n = 2961, BMI) had data at all three time points. Proportions of children with two time points of data available varied between 16.6% (n = 1151, BMI) and 21.0% (n = 1454, excessive exercise), and that of children with only one time point available ranged between 13.8% (n = 957, BMI) and 19.4% (n = 1345, fasting and excessive exercise; data available from authors).
As shown in Table 1, just over half of our sample were girls (54.9%) and most children had a married mother (84.3%) who had completed compulsory education (52.2%) and was, on average, 29.4 years of age.
Table 1 Sample characteristics and polygenic risk score distribution
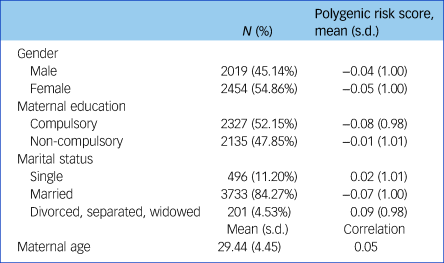
Table 2 shows the prevalence of disordered eating behaviours at age 14, 16 and 18 years. In line with the known epidemiology of eating disorders, prevalence of all behaviours was relatively low, but increased over time. All disordered eating behaviours were also more prevalent in girls compared with boys at all time points (data shown in Supplementary Table 1 available at https://doi.org/10.1192/bjp.2019.39).
Table 2 Twelve-month prevalence of disordered eating behaviours at ages 14, 16 and 18 years
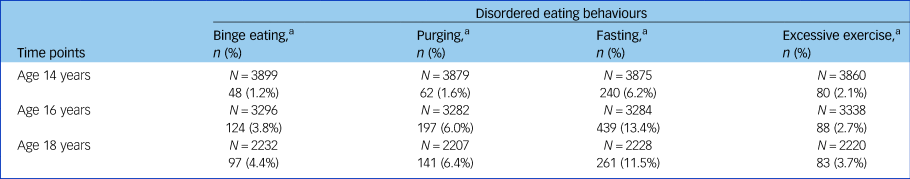
a Sample sizes vary slightly across outcomes at each time point because of different patterns of missing data across questions used to derive them.
Schizophrenia PRS and disordered eating behaviours
As shown in Table 3, in logistic regression models, we found strong evidence that a 1 s.d. increase in PRS for schizophrenia was associated with a 1.36 increase in the odds of binge eating (95% CI 1.16–1.60, P < 0.0001). Greater PRS were not associated with purging behaviours (odds ratio, 1.03; 95% CI 0.88–1.21; P = 0.71), fasting (odds ratio, 1.02; 95% CI 0.91–1.15; P = 0.73) or excessive exercise (odds ratio, 1.09; 95% CI 0.95–1.26; P = 0.19). Finally, an s.d. increase in PRS was associated with a −0.03 s.d. decrease in BMI (95% CI −0.06 to −0.01; P = 0.02), although evidence of this association was moderate to weak. We did not find any evidence of non-linear relationships when using quadratic PRS terms.
Table 3 Random-effects logistic and linear regression model results
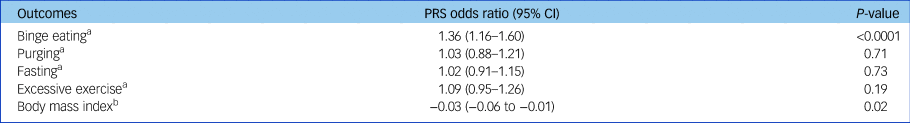
PRS, polygenic risk score.
a N = 4473.
b N = 5069.
When we investigated these associations using quintiles of PRS, we observed that children in the top three quintiles had the highest odds of binge eating and lower BMI (see Table 4).
Table 4 Multilevel logistic and linear regression models for the association between quintiles of polygenic risk scores for schizophrenia and disordered eating and body mass index
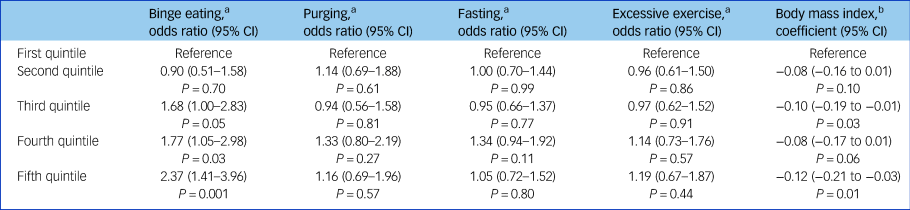
a N = 4473.
b N = 5069.
Sensitivity analyses
When we used PRS derived from SNPs with different P-value thresholds in the GWAS sample, our findings were overall consistent, although associations became more prominent at larger P-value thresholds (findings shown in Supplementary Figs 1 and 2). Including the other twin (twin B) in our sample did not alter our findings in relation to binge eating (odds ratio, 1.38; 95% CI 1.17–1.62; P < 0.0001) or BMI (coefficient, −0.03; 95% CI −0.06 to −0.01; P = 0.026; data available from authors).
Discussion
Using a large prospective cohort of British children, this study showed an association between genetic risk of schizophrenia and disordered eating behaviours. In particular, we found that higher schizophrenia PRS were associated with greater odds of binge eating, a behaviour that is central to the diagnoses of bulimia nervosa and binge eating disorder, but that can also occur in individuals with anorexia nervosa of the binge-purge type. We also observed some evidence of an association between greater schizophrenia PRS and lower BMI. These patterns of associations were largely consistent across a range of PRS training-set P-value thresholds.
Strengths and limitations
This study has a number of strengths. We used a large general population sample of adolescents whose disordered behaviours were self-reported across adolescence, thus avoiding sampling biases often associated with clinically diagnosed cases. The PRS we used were derived using findings from the largest schizophrenia GWAS to date.15 Our BMI measure was obtained from objective measurements, thus avoiding reporting biases, and standardised using age and gender growth curves. The detailed characterisation of the ALSPAC sample allowed us to investigate which specific disordered eating phenotypes might be underpinned by shared genetic liability with psychotic disorders. The use of repeated measurements across adolescence also allowed us to fully exploit the richness of the data-set, minimise the role of missing data and account for outcome clustering within individuals.
Several limitations should nevertheless be noted. Our disordered eating definitions were broad and some of the participants might have experienced these behaviours at a very low threshold. However, previous evidence in this sample has shown that even adolescents with broad disordered eating presentations experienced comorbidity and long-term outcomesReference Micali, Solmi, Horton, Crosby, Eddy and Calzo21 comparable with those observed in full diagnoses. This finding is consistent with the idea that eating disorders, like other psychiatric disorders, occur on a continuum of severity and suggest that these definitions can be useful for future studies investigating the aetiology of and outcomes associated with these conditions. Our sample was also characterised by a notable degree of attrition, which was predicted by greater schizophrenia PRS. It is possible that collider (i.e. selection) bias, which occurs if selection into the study is conditional on the exposure and outcome measured, could have induced or prevented the observation of some of the associations under study. The PRS measure that we used was derived in an adult population and might not capture genetic liability to early-onset cases of schizophrenia. However, this measure has been extensively used to explore intermediate phenotypes in general population samples, hence our approach aligns with that of previous investigations.Reference Sullivan, Agrawal, Bulik, Andreassen, Børglum and Breen13, Reference Jones, Stergiakouli, Tansey, Hubbard, Heron and Cannon14 Finally, although they were derived from a large discovery data-set, it has been shown that the PRS measure we used only explain around 7% of the variance in schizophrenia.15 Hence, we cannot exclude that some of the associations that we did not observe were due to the limited predictive value of our exposure measure.
Comparisons with previous literature and interpretation of the findings
Although this is the first study to show an association between schizophrenia PRS and binge eating, this finding is consistent with those of previous epidemiological investigations in clinical and general population samples.Reference Striegel-Moore, Garvin, Dohm and Rosenheck1, Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet5, Reference Koyanagi, Stickley and Haro6 For instance, McGrath et al have recently shown that individuals with lifetime psychotic symptoms had two to four times the odds of also experiencing bulimia nervosa and binge eating disorder, respectively, two eating disorders for whose diagnosis binge eating is a central criterion.Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet5 A cross-sectional study of UK adults found that those who experience loss of control over eating (a symptom of binge eating) were more likely to report auditory hallucinations and other psychotic experiences.Reference Koyanagi, Stickley and Haro6 In this sample, psychotic experiences at age 13 years were associated with greater disordered eating behaviours, including fasting and binge eating, at age 18 years.Reference Solmi, Melamed, Lewis and Kirkbride7 Clinical studies also found that individuals with non-affective psychoses had greater disordered eating, including binge eating,Reference Fawzi and Fawzi4 and that individuals with eating disorders, and in particular those who binge eat,Reference Aragona22 display greater comorbidity with psychotic illnessesReference Striegel-Moore, Garvin, Dohm and Rosenheck1 or psychotic symptoms.Reference Aragona22
Shared genetic liability between schizophrenia, bipolar disorder and major depressive disorderReference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh8 could explain our findings. Epidemiological studies in clinicalReference McElroy, Frye, Hellemann, Altshuler, Leverich and Suppes23 and general populationReference Hudson, Hiripi, Pope and Kessler24 samples have highlighted a high comorbidity between bipolar disorder and bulimia nervosa and binge eating disorder. However, the cross-sectional or retrospective designs of these studies have so far limited aetiological inferences about the temporality of these associations or presence of shared risk factors. By investigating the association between schizophrenia PRS and binge eating in our longitudinal birth cohort, our findings suggest that the observed comorbidity between these conditions could be partially explained by shared genetic liability, although potential risk pathways remain unclear.
Previous studies have also shown that higher schizophrenia PRS are associated with poorer social understanding and greater irritability,Reference Riglin, Collishaw, Richards, Thapar, Maughan and O'Donovan25 domains that appear to be impaired in individuals with eating disordersReference Christensen, Bentz, Clemmensen, Strandberg-Larsen and Olsen26, Reference Raffi, Rondini, Grandi and Fava27 and could therefore represent intermediate phenotypes shared by these conditions. Although the majority of the literature has focused on anorexia nervosa,Reference Westwood, Eisler, Mandy, Leppanen, Treasure and Tchanturia28 a recent systematic review has shown that those with broader disordered eating behavioursReference Christensen, Bentz, Clemmensen, Strandberg-Larsen and Olsen26 also show deficits in social cognition, and greater autistic traits. To date, these studies have been solely conducted cross-sectionally,Reference Christensen, Bentz, Clemmensen, Strandberg-Larsen and Olsen26 making it difficult to infer whether these cognitive deficits traits emerge before or after the onset of eating disorders. However, a recent study has shown that children of mothers with binge-purge behaviours had poorer social communication and emotion recognition skills, which the authors suggested could represent intermediate phenotypes in the intergenerational transmission of eating disorders.Reference Kothari, Barona, Treasure and Micali29
Irritability in early life, shown to be associated in this sample with greater genetic risk of schizophrenia,Reference Riglin, Collishaw, Richards, Thapar, Maughan and O'Donovan25 could also represent an early risk factor for binge eating. Literature on irritability in childhood as a risk factor for eating disorders is limited, although clinical accounts report that individuals with eating disorders show greater irritability.Reference Raffi, Rondini, Grandi and Fava27 Difficulties in regulating negative emotions have also been cross-sectionally associated with loss-of-control eating in adolescence,Reference Goldschmidt, Lavender, Hipwell, Stepp and Keenan30 mirroring findings of adult populations showing that dysregulated eating behaviours can serve to regulate immediate emotional states.Reference Macht and Simons31 Longitudinal studies investigating the association between social cognition and irritability in childhood and disordered eating in the general population are therefore warranted, as these could be transdiagnostic markers of risk across multiple phenotypes.
The observed association between PRS for schizophrenia and binge eating also raises a number of hypotheses regarding the observed presence of similar metabolic abnormalities shared by people with psychotic illnesses and binge eating disorder. In people with psychotic disorders, metabolic abnormalities may arise for several reasons, including use of antipsychotics. Nevertheless, impaired glycaemic control and insulin resistance have also been shown in adolescents with non-clinical psychotic experiences,Reference Perry, Upthegrove, Thompson, Marwaha, Zammit and Singh32 as well as in drug-naïve individuals with schizophrenia and their first-degree relatives.Reference Chadda, Ramshankar, Deb and Sood33 Similar metabolic abnormalities are observed among individuals with binge eating disorder.Reference Succurro, Segura-Garcia, Ruffo, Caroleo, Rania and Aloi34 Although more research is needed to disentangle temporal associations, our findings lend themselves to two possible interpretations, which will require further investigation. First, it is possible that metabolic abnormalities could represent shared risk factors for both of these phenotypes. Second, binge eating could represent a mediator in the association between psychotic experiences and dysglycaemia. This hypothesis is supported by recent findings showing that insulin resistance measured at age 9 years was not associated with greater psychotic experiences at age 18 years, but those with psychotic experiences at 18 years had, cross-sectionally, greater insulin resistance.Reference Perry, Upthegrove, Thompson, Marwaha, Zammit and Singh32 In turn, another study found that psychotic experiences at age 13 years predicted greater binge eating behaviours at age 18 years,Reference Solmi, Melamed, Lewis and Kirkbride7 suggesting that the latter could be a contributing factor in the onset of metabolic abnormalities.
In light of the observed association between PRS and binge eating, often associated with greater BMI, our finding that schizophrenia PRS predict lower BMI is difficult to interpret. This association, however, has been previously observed,Reference So, Chau, Ao, Mo and Sham35 and is in line with findings of genetic studies showing an inverse correlation between schizophrenia and BMI,Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day and Loh8 and with those of epidemiological investigations showing that low BMI is a risk factor for schizophrenia.Reference Sørensen, Gamborg, Sørensen, Baker and Mortensen9 The pathophysiological mechanisms underpinning the association between genetic liability for schizophrenia and low BMI are unclear, particularly as clinically, overweight and obesity are more frequently observed in individuals with psychotic illnesses.Reference Manu, Dima, Shulman, Vancampfort, De Hert and Correll36 Environmental factors associated with increased genetic risk (i.e. deprivation) could explain low BMI before disease onset, and greater BMI could be a result of medication use after disease onset. It is also possible that binge eating behaviours could contribute to greater BMI through the life course, so that emergence of binge eating in adolescence could partially contribute to minimising any existing differences in BMI. Another hypothesis is that genetic risk for schizophrenia leads to both binge eating and low BMI via different risk pathways.
To our knowledge, this is the first study to show an association between polygenic risk for schizophrenia and disordered eating behaviours. Although replication of these results is needed, we find that PRS are associated with greater risk of binge eating behaviours. Future research should investigate the presence of shared intermediate phenotypes across these disorders, including the role of metabolic abnormalities, to improve early identification of at-risk children. From a clinical viewpoint, these findings further highlight the need to consider the co-occurrence of disordered eating behaviours, particularly binge eating, in individuals with psychotic illnesses, as this might account for the weight gain observed in this population.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.39.
Funding
Dr James B. Kirkbride and Dr Francesca Solmi are supported by a Sir Henry Dale Fellowship to Dr Kirkbride, jointly funded by the Wellcome Trust and the Royal Society (grant no. 101272/Z/13/Z). Dr Francesca Solmi is supported by a Sir Henry Wellcome Fellowship funded by the Wellcome Trust (grant no. 209196/Z/17/Z). Prof. Stanley Zammit is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at the University Hospitals Bristol National Health Service Foundation Trust and the University of Bristol. This research was further supported by the University College London Hospitals NIHR Biomedical Research Centre. The views expressed in this paper are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health. The UK Medical Research Council and Wellcome (grant no. 102215/2/13/2) and the University of Bristol provide core support for the ALSPAC. This publication is the work of the authors and all authors will serve as guarantors of the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). The collection of exposure and outcome measures used in this study was funded by the following grants: National Institutes of Health (grant no. MH087786-01), NIHR (grant no. CS/01/2008/014), the Wellcome Trust and Medical Research Council (grant no. 076467/Z/05/Z), the Wellcome Trust (grant no. WT091310) and Medical Research Council grant no. MR/M006727/1.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We would also like to thank Dr Hannah Jones for sharing the findings from her previous analyses related to the schizophrenia polygenic risk scores.







eLetters
No eLetters have been published for this article.