Levels of leisure and workplace physical activity have continued to decrease in high-income countries. Reference Foresight1 This increase in sedentary behaviour is known to be reducing physical health, but may also be having a negative impact on the mental health of the population. 2 The majority of studies that have examined the relationship between physical activity and common mental disorders have found lower rates of depression among those who are more active. Reference Teychenne, Ball and Salmon3 However, almost all of the published research on this topic has focused exclusively on intense leisure-time physical activity such as organised sports, jogging and fitness classes. When other types of physical activity such as light leisure activity, domestic chores or workplace activity have been considered, the results have been more mixed. Reference Sexton, Sogaard and Olstad4–Reference Dunn, Trivedi and O'Neal7 Even within studies focusing on leisure-time activity, the importance of exercise intensity in predicting any psychological benefits remains unclear. Although some intervention studies have suggested high-intensity physical activity confers greater antidepressant effects compared with low-intensity activity, Reference Dunn, Trivedi, Kampert, Clark and Chambliss8,Reference Singh, Stavrinos, Scarbek, Galambos, Liber and Fiatarone Singh9 other interventional and observational studies have failed to demonstrate any dose–response relationship. Reference Dunn, Trivedi and O'Neal7,Reference Lindwall, Rennemark, Halling, Berglund and Hassmen10–Reference West, Otte, Geher, Johnson and Mohr12
Significant uncertainties also remain regarding the mechanisms underlying any relationship between physical activity and common mental disorders. Reference Zoeller13 Physical activity is associated with a number of biological changes that could have an impact on mental health. A combination of human and animal experiments have demonstrated that exercise can stimulate serotonin release, Reference Meeusen, Thorre, Chaouloff, Sarre, De Meirleir and Ebinger14 increase expression of neuronal growth factors such as brain-derived neurotrophic factor, Reference Neeper, Gomez-Pinilla, Choi and Cotman15 promote release of beta-endorphins, Reference Angelopoulos16 and provoke hypothalamic–pituitary–adrenal axis stimulation. Reference Hill, Zack, Battaglini, Viru, Viru and Hackney17 These changes may have direct mood-enhancing effects or may stimulate other pathways such as hippocampal neurogenesis. Reference Ernst, Olson, Pinel, Lam and Christie18 A further purported biological mechanism that has attracted particular attention is alterations in the activity of the autonomic nervous system. Reference Zoeller13,Reference Rottenberg19 Regular physical activity increases parasympathetic vagal activity, leading to physiological changes such as resting bradycardia. Reference Seals and Chase20 Vagal nerve stimulation has been used to treat depression, although the effectiveness of this treatment and the role of cardiac vagal tone in depression remains unclear. Reference Rottenberg19 Other, less biological explanations for any association between exercise and common mental disorders focus on concepts of self-esteem, social support and perception of control and mastery. Reference Paluska and Schwenk21 Any relationship may also be bidirectional, with the symptoms of common mental disorders contributing to lower levels of physical activity. Depression is associated with a number of changes that may lead to reduced activity such as a reduction in exercise capacity, fatigue, social isolation and poor motivation. Reference Harvey, Wessely, Kuh and Hotopf22,Reference Ruo, Rumsfeld, Pipkin and Whooley23
This study used a large (n = 40 401) population-based sample to examine the association between physical activity and common mental disorders. We aimed to test the following hypotheses: (a) whether there is an inverse relationship between leisure-time physical activity and symptoms of depression and anxiety; (b) whether there is a dose–response effect according to the intensity of physical activity such that higher-intensity activity has a stronger inverse relationship with symptoms of anxiety and depression; and (c) whether there is a similar inverse relationship between workplace physical activity and depression and anxiety. We also hoped to explore the relative importance of biological v. social factors in understanding the bidirectional relationship between physical activity and common mental disorders. In particular, to test the hypotheses that increased parasympathetic vagal tone, alterations in metabolic markers and/or increased social engagement account for any relationship between physical activity and symptoms of depression and anxiety.
Method
Sample
The Health Study of Nord-Trøndelag County (HUNT-2) took place in Norway between August 1995 and June 1997. All inhabitants of the Nord-Trøndelag County aged 20–89 years (n = 92 936) were invited to a clinical examination as part of a general health screening. The overall objectives, methods and contents of the HUNT-2 study have been described in detail elsewhere. Reference Holmen, Midthjell, Kruger, Langhammer, Holmen and Bratberg24
Measurement of physical activity
All participants were asked how often they engaged in both light and intense leisure-time physical activity. Light activity was defined as an activity that did not lead to being sweaty or out of breath, while intense activity was any leisure-time activity that did result in sweating or breathlessness. Participants were given four options for each question: none, less than 1 h per week, 1–2 h per week or 3 h or more per week. They were also asked about how physically active they were at work. Participants were given four options in relation to their work activity: mostly sedentary, required to walk a lot, walk and lift a lot, or intense physical work. A number of examples were given to help define each of these categories.
Assessment of depression and anxiety
All participants were asked to complete the Hospital Anxiety and Depression Scale (HADS). Reference Zigmond and Snaith25 The HADS is a self-report questionnaire comprising 14 four-point Likert-scaled items covering anxiety and depression over the past 2 weeks. It was designed to avoid false-positive cases among individuals with somatic illness. It contains no questions on somatic symptoms, sleep or appetite disturbance, focusing instead on the psychological and cognitive symptoms relevant to the two disorders. A cut-off score of 8 in each subscale has been found to be optimal for case finding, with sensitivity and specificity estimates of about 0.80. Reference Bjelland, Dahl, Haug and Neckelmann26
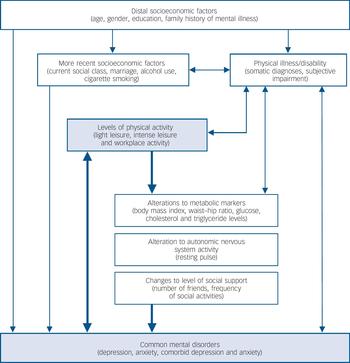
Fig. 1 Proposed hierarchical model of how other factors may confound and/or mediate any relationship between physical activity and common mental disorders.
Mediating and confounding variables
A wide range of potential confounding or mediating factors were also considered. Based on existing knowledge, a conceptual hierarchical framework was constructed to demonstrate how each of these factors may interact with both levels of activity and risk of common mental disorder. Reference Victora, Huttly, Fuchs and Olinto27 This is shown in Fig. 1.
Socioeconomic factors
Information on participants' age, gender and marital status was obtained from the Norwegian National Population Registry. Participants were asked to record their highest completed education level, with responses coded on a three-point ordinal scale of compulsory (primary), secondary school and university. An index of social class based on individuals' current occupation was calculated according to the International Erikson–Goldthorpe–Portocarero classification. Reference Krokstad and Westin28 Participants were asked if they smoked cigarettes and were requested to complete the CAGE questionnaire (a four-question screening instrument for alcohol problems). Reference Ewing29 Participants were also asked how many of their immediate relatives (mother, father, sibling or child) had a mental illness.
Physical illness/disability
Many somatic diseases limit the amount of physical activity that is possible, while also independently increasing the risk of common mental disorders. Reference Harvey and Ismail30 Therefore they are potentially an important confounder in the relationship between physical activity and common mental disorders. The number of somatic diagnoses reported by each participant (from the list of angina pectoris, myocardial infarction, asthma, cancer, diabetes, epilepsy, hypertension, arthritis, osteoporosis, respiratory disease, stroke and thyroid disease) was considered as a continuous variable in regression models. Participants were also asked to rate their level of functional impairment owing to any physical diseases (on a four-point scale: none, slightly, moderately or severely). They were also asked if their movement was limited (for any reason), and if so, to what degree.
Metabolic markers
A trained nurse obtained measurements of participants' height, weight, hip and waist circumference. This allowed participants' body mass index (BMI) and waist–hip ratio to be calculated. Both BMI and waist–hip ratio have been associated with depression, Reference Rivenes, Harvey and Mykletun31 suggesting that obesity may be an important mediator in any relationship between physical activity and common mental disorders. Non-fasting blood samples were also taken and analysed to measure total cholesterol, triglyceride and glucose levels. Each of these is known to alter according to levels of physical activity and to be associated with common mental disorders. Reference Harvey and Ismail30,Reference Shin, Suls and Martin32,Reference Winokur, Maislin, Phillips and Amsterdam33
Measurement of autonomic nervous system activity
Clinically trained nurses or technicians measured participants' blood pressure and heart rate on three occasions. The first measurement was taken after the participant had been seated for 2 min, with subsequent measurements occurring at 1 min intervals. The mean value of the second and third pulse measurement was considered to be the resting heart rate. The exact mechanism for exercise-induced bradycardia has been the subject of some debate, although there is reasonable evidence to suggest it is due to increased parasympathetic vagal tone. Reference Seals and Chase20 The use of resting heart rate as a measure of autonomic nervous system activity is further supported by studies showing a close association between resting pulse and more validated measures of autonomic function such as respiratory sinus arrhythmia and baroreflex sensitivity. Reference Dietrich, Riese, Sondeijker, Greaves-Lord, van Roon and Ormel34
Level of social support
The extent of the individual's social network and support was assessed via two separate questions. Participants were asked ‘How many good friends do you have?’ with the instruction to only count those who they could confidentially talk to and who would help them when they were in need. They were also asked how often they took part in social activities. The estimated number of friends was considered as a continuous variable, and the frequency of social activities was recorded on a four-point scale (never or only a few times a year, once a week, once or twice a month, or more than once a week).
Statistical analysis
The sample used in this analysis was selected on the basis of participants having answered both questions on leisure-time physical activity and the full HADS questionnaire. The associations between different types of physical activity and both depression and anxiety was assessed using multivariate logistic regression with competing risk analysis. The following factors were entered as covariates: age, gender, family history of mental illness, current social class, education, marriage status, cigarette use, alcohol problems, somatic diagnoses and subjective impairment owing to physical illness. The relative confounding effect of each of these variables was examined. Interactions by gender and age were tested for using maximum likelihood. Although age was entered as a continuous variable in the main multivariable models, age tertiles were used when examining for interactions. Alterations to metabolic markers (BMI, waist–hip ratio, glucose, cholesterol and triglyceride levels), vagal tone (measured by resting pulse) and measures of social engagement and support were hypothesised a priori to be more likely mediating than confounding factors. The effect of including these factors in each model was therefore examined separately. All analyses were conducted using STATA version 10.1 statistical software for Windows.
Ethics
The HUNT-2 study was approved by the National Data Inspectorate and the Board of Research Ethics in Health Region IV of Norway. All participants gave their informed consent to participate in this study.
Results
Description of the sample
Of the 92 936 individuals invited to participate in HUNT-2, 66 140 (71.2%) participated in some way. The representativeness of the sample and the main reasons for non-participation have been described in a previous paper. Reference Holmen, Midthjell, Kruger, Langhammer, Holmen and Bratberg35 Rates of participation were lowest among men, young adults and the elderly. Reference Holmen, Midthjell, Kruger, Langhammer, Holmen and Bratberg35 Of the 66 140 residents who participated, 41 668 (63.0%) answered questions on both light and intense leisure-time activity. Those who only answered the question of intense activity (n = 3778, 6.2%) tended to be more active than those who answered both questions (P<0.001). However, levels of common mental disorders among those who answered one question compared with those who answered both questions on physical activity were very similar (20.3% v. 19.7%). In order to allow comparisons between different types of activity, analyses presented in this paper will focus on those individuals who answered both questions on leisure-time physical activity.
Of the 41 668 individuals who answered both exercise questions, 40 401 (97.0%) completed a HADS questionnaire and form the sample for this paper. Case-level symptoms of depression were reported in 4080 (10.1%) individuals, case-level symptoms of anxiety in 6129 (15.2%), and comorbid depression and anxiety in 2258 (5.6%) individuals.
The sociodemographics of the sample used in this study are described in Table 1. Only individuals currently in employment were able to answer the questions relating to their workplace physical activity. The number of individuals reporting different levels of physical activity is also displayed in Table 1.
Table 1 Description of the sociodemographic characteristics of the study population (n=40 401)

| Variable | n (%) |
|---|---|
| Gender | |
| Male | 19 828 (49.1) |
| Female | 20 573 (50.9) |
| Age, years: mean (s.d.) | 45.9 (16.2) |
| 19–30 | 7943 (19.7) |
| 31–40 | 8891 (22.0) |
| 41–50 | 9182 (22.7) |
| 51–60 | 6272 (15.5) |
| 61–70 | 4330 (10.7) |
| 71 and over | 3783 (9.4) |
| Marital status | |
| Married/regular partnership | 23 760 (58.8) |
| Unmarried | 11 651 (28.8) |
| Divorced/separated | 2712 (6.7) |
| Widowed | 2278 (5.6) |
| Highest education | |
| Compulsory (primary) | 12 307 (30.5) |
| Secondary school | 18 629 (46.1) |
| University | 9465 (23.4) |
| Social classa | |
| Higher-grade professionals | 2915 (7.2) |
| Lower-grade professionals | 5264 (13.0) |
| Non-manual employees | 5779 (14.3) |
| Small proprietors/farmers | 4722 (11.7) |
| Lower-grade technicians | 3463 (8.6) |
| Manual workers | 4903 (12.1) |
| Not classified | 13 355 (33.1) |
| Amount of light leisure-time physical activity | |
| None | 3724 (9.2) |
| Less than 1 h/week | 7716 (17.6) |
| 1–2 h/week | 1461 (36.3) |
| 3 h or more/week | 14 890 (36.9) |
| Amount of intense leisure-time physical activity | |
| None | 16 976 (42.0) |
| Less than 1 h/week | 11 307 (28.0) |
| 1–2 h/week | 8275 (20.5) |
| 3 h or more/week | 3840 (9.5) |
| Amount of workplace physical activityb | |
| Mostly sedentary | 10 835 (31.9) |
| A lot of walking | 10 586 (31.2) |
| Walk and lift a lot | 8410 (24.8) |
| Intense physical work | 4094 (12.1) |
Physical activity and common mental disorders
The prevalence of common mental disorders according to the amount of physical activity reported is shown in Fig. 2. Individuals who engage in more light and intense leisure activities have lower rates of case-level depression and comorbid depression and anxiety. Individuals who engage in lighter leisure-time physical activities also have a slightly lower prevalence of case-level anxiety. These associations are also demonstrated in the logistic regression models summarised in Table 2. The odds ratios shown in this table once again demonstrate the inverse relationship between light and intense physical activity and depression, both with and without comorbid anxiety (P<0.001). These associations remained even after accounting for the effects of age, gender, family history of mental illness, current social class, education, marriage status, cigarette use, alcohol problems, somatic diagnoses and subjective impairment owing to physical illness. The covariates with the most confounding effect in the relationship between leisure activity and case-level depression were subjective impairment owing to physical illness, lower social class and lower educational attainment. There was no association between intense leisure-time activity and anxiety, although a negative association with relatively small effect sizes is present between light leisure activity and anxiety (P = 0.003). There is no evidence of any linear association between workplace activity and either depression or anxiety. There is an increased level of depression symptoms among those undertaking intense physical work, although the strength of this association was substantially reduced once the effect of other covariates (in particular socioeconomic status and education) were accounted for.
Table 2 Associations between various types of physical activity and common mental disordersa

| Amount of physical activity | Anxiety alone OR (95% CI) (n = 3871) | Depression alone OR (95% CI) (n =1822) | Comorbid depression and anxiety OR (95% CI) (n = 2258) |
|---|---|---|---|
| Light leisure activity | |||
| Model 1b | |||
| None | 1.39 (1.23–1.57) | 2.50 (2.16–2.91) | 2.28 (1.98–2.62) |
| Less than 1 h/week | 1.17 (1.07–1.29) | 2.03 (1.77–2.33) | 1.81 (1.60–2.04) |
| 1–2 h/week | 1.01 (0.94–1.10) | 1.37 (1.20–1.55) | 1.15 (1.04–1.29) |
| 3 h or more/week | 1.00 | 1.00 | 1.00 |
| Model 2c | |||
| None | 1.18 (1.04–1.34) | 2.04 (1.74–2.38) | 1.69 (1.46–1.96 |
| Less than 1 h/week | 1.12 (1.01–1.23) | 1.88 (1.64–2.17) | 1.65 (1.45–1.86) |
| 1–2 h/week | 1.02 (0.94–1.10) | 1.35 (1.19–1.53) | 1.13 (1.01–1.26) |
| 3 h or more/week | 1.00 | 1.00 | 1.00 |
| P for linear trend | 0.003 | < 0.001 | < 0.001 |
| Intense leisure activity | |||
| Model 1b | |||
| None | 1.06 (0.94–1.20) | 2.22 (1.78–2.77) | 1.58 (1.33–1.88) |
| Less than 1 h/week | 0.98 (0.87–1.12) | 1.58 (1.26–2.00) | 1.10 (0.92–1.32) |
| 1–2 h/week | 0.98 (0.86–1.12) | 1.13 (0.88–1.46) | 0.86 (0.71–1.04) |
| 3 h or more/week | 1.00 | 1.00 | 1.00 |
| Model 2c | |||
| None | 0.96 (0.85–1.10) | 1.98 (1.58–2.48) | 1.29 (1.08–1.54) |
| Less than 1 h/week | 0.97 (0.85–1.11) | 1.61 (1.28–2.04) | 1.07 (0.89–1.29) |
| 1–2 h/week | 0.99 (0.87–1.13) | 1.19 (0.93–1.53) | 0.89 (0.73–1.09) |
| 3 h or more/week | 1.00 | 1.00 | 1.00 |
| P for linear trend | 0.48 | < 0.001 | < 0.001 |
| Workplace activity | |||
| Model 1b | |||
| Mostly sedentary | 0.95 (0.84–1.09) | 0.68 (0.57–0.80) | 0.79 (0.70–0.93) |
| A lot of walking | 0.90 (0.79–1.03) | 0.59 (0.49–0.71) | 0.71 (0.59–0.84) |
| Walk and lift a lot | 0.92 (0.80–1.06) | 0.65 (0.54–0.79) | 0.73 (0.62–0.88) |
| Intense physical work | 1.00 | 1.00 | 1.00 |
| Model 2c | |||
| Mostly sedentary | 0.99 (0.85–1.14) | 0.80 (0.66–0.97) | 0.83 (0.68–1.00) |
| A lot of walking | 0.95 (0.82–1.10) | 0.69 (0.57–0.83) | 0.77 (0.64–0.93) |
| Walk and lift a lot | 0.95 (0.82–1.09) | 0.71 (0.58–0.86) | 0.76 (0.63–0.92) |
| Intense physical work | 1.00 | 1.00 | 1.00 |
| P for linear trend | 0.81 | 0.16 | 0.35 |

Fig. 2 Prevalence of common mental disorders according to the amount of physical activity.
Of the eighteen different interactions tested for (age and gender for all permutations between light leisure, intense leisure and workplace activity and depression, anxiety and comorbid depression and anxiety), three reached statistical significance: interaction by age (P = 0.002) and gender (P = 0.0001) in the association between light leisure activity and depression, and an interaction by gender (P = 0.01) in the association between light leisure activity and comorbid anxiety and depression. These associations were re-examined stratified by both age (tertiles) and gender (not shown). This demonstrated only a minor difference in effect sizes between different strata, with no overall differences in the pattern of associations seen.
To examine the associations between activity levels and symptoms of depression in more detail, all cases of depression, both with and without comorbid anxiety, were combined. The adjusted odds ratio for case-level depression according to different levels of physical activity is displayed in Fig. 3. Although there is a clear dose–response effect in terms of overall time of activity reported, the intensity of leisure activity (light v. intense) does not appear to be important, with similar effect sizes seen for both categories. The differences between leisure-time physical activity and physical activity in the workplace is once again demonstrated.
Possible mediating factors
Three categories of factors had been identified a priori as possible mediators in any relationship between physical activity and common mental disorders: alterations in metabolic markers, changes to parasympathetic vagal tone and fluctuations in levels of social support. In the second phase of this study we focused on the possible role that each of these factors may play in the relationship that had been identified between leisure-time physical activity and depression. Associations between both light and intense physical activity and each of these factors were confirmed using linear regression (P<0.001 for all associations, data not shown). Associations between each group of potential mediating variables and depression were also demonstrated (P<0.05 for all associations except glucose and depression (P = 0.16), data not shown). Table 3 demonstrates how the addition of each group of potential mediating factors alters the associations between leisure-time physical activity and depression. The addition of metabolic markers or resting heart rate (a measure of vagal tone) to multivariate models had virtually no impact on the relationship between leisure activity and depression, suggesting that neither vagal tone (as measured by resting heart rate) nor metabolic markers mediate this relationship. In contrast, the addition of social factors (number of friends and social activities) as covariates reduced the observed effect sizes moderately, suggesting social support is a potential factor in understanding why leisure-time physical activity is associated with lower levels of depression.
Table 3 Additional multivariate models to investigate the role of metabolic changes, vagal tone (resting pulse) and social support in explaining the relationship between leisure-time physical activity and case-level depression.a

| OR (95% CI) for case-level depression | ||
|---|---|---|
| Amount of leisure time physical activity | Light activity (e.g. not sweating or out of breath) | Intense activity (e.g. sweating or out of breath) |
| Model 1b | ||
| None | 1.83 (1.64–2.04) | 1.57 (1.37–1.81) |
| Less than 1 h/week | 1.72 (1.56–1.89) | 1.29 (1.11–1.49) |
| 1–2 h/week | 1.22 (1.12–1.33) | 1.01 (0.86–1.18) |
| 3 h or more/week | 1.00 | 1.00 |
| P for linear trend | < 0.001 | < 0.001 |
| Model 2c | ||
| None | 1.79 (1.60–2.00) | 1.54 (1.34–1.78) |
| Less than 1 h/week | 1.70 (1.54–1.87) | 1.26 (1.09–1.47) |
| 1–2 h/week | 1.21 (1.11–1.32) | 0.99 (0.84–1.16) |
| 3 h or more/week | 1.00 | 1.00 |
| P for linear trend | < 0.001 | < 0.001 |
| Model 3d | ||
| None | 1.82 (1.63–2.04) | 1.57 (1.36–1.81) |
| Less than 1 h/week | 1.71 (1.55–1.88) | 1.28 (1.10–1.48) |
| 1–2 h/week | 1.22 (1.12–1.32) | 1.00 (0.85–1.18) |
| 3 h or more/week | 1.00 | 1.00 |
| P for linear trend | < 0.001 | < 0.001 |
| Model 4e | ||
| None | 1.64 (1.44–1.88) | 1.43 (1.21–1.69) |
| Less than 1 h/week | 1.58 (1.41–1.76) | 1.15 (0.97–1.37) |
| 1–2 h/week | 1.20 (1.09–1.32) | 0.99 (0.82–1.18) |
| 3 h or more/week | 1.00 | 1.00 |
| P for linear trend | < 0.001 | < 0.001 |
Discussion
Key findings
Using a large community-based sample we revealed an inverse association between the amount of leisure-time physical activity and depression. Individuals who reported no leisure-time physical activity were more likely than active individuals to have case-level symptoms of depression. This association was only present with leisure-time (as opposed to workplace) activity and was not dependent on the intensity of the activity undertaken. We found evidence that social factors such as social support and social engagement may partially explain this relationship. We did not find any evidence that biological changes associated with exercise such as alterations to parasympathetic vagal tone and metabolic markers could account for the association between physical activity and lower levels of depression.

Fig. 3 Comparison of the relative associations between different types of physical activity and case-level depression.
All odds ratios (with 95% confidence intervals) compare with most active group for that type of activity and are adjusted for age, gender, family history of mental illness, current social class, education, marriage status, cigarette use, alcohol problems, somatic diagnoses and subjective impairment owing to physical illness. a. For light and intense leisure activity ‘low’ = less than 1 h/week, ‘medium’ = 1–2 h per week and ‘high’ = 3 h or more/week. For workplace activity ‘none’ = sedentary, ‘low’ = a lot of walking, ‘medium’ = walk and lift a lot, and ‘high’ = heavy physical work.
Our findings in relation to anxiety were more mixed. There was evidence that regular light leisure activity was associated with lower reporting of anxiety, but the size of this effect was relatively small and similar associations were not seen with intense activity. Workplace activity was not associated with levels of anxiety.
Strengths and limitations
The large size of this community-based sample and detailed information available on both biological and social factors are key strengths of this study. Previous studies have tended to focus only on intense leisure-time physical activity such as sport or organised training despite evidence suggesting that such activities make up only a small proportion of total energy expenditure. Reference Lagerros, Bellocco, Adami and Nyren36 Our inclusion of light leisure activity (physical activity not resulting in sweating or breathlessness) and workplace-based physical activity is therefore an important feature of this study.
The main limitations of our study are its reliance on self-reported activity levels and the cross-sectional nature of our data collection. We are not able to make any firm conclusions on the direction of causation in any of the associations described as it is likely that there may be some reverse causation. For example, individuals with depression may be more socially isolated and less motivated to engage in physical activity. There may also be ‘up stream’ factors present from a young age such as personality and temperament, which independently influence levels of physical activity and risk of common mental disorders. Although the use of a validated outcome measure such as the HADS is an important strength of this study, the operationalisation of depression and anxiety via a self-report screening tool gives rise to additional limitations. The HADS cannot provide a clinical diagnosis of either depression or anxiety and may misclassify those whose mental disorder presents with prominent somatic symptoms. However, published reviews have concluded that the HADS performs at least as well as other more comprehensive assessments in identifying symptoms of anxiety disorders and depression, Reference Bjelland, Dahl, Haug and Neckelmann26 and better than the clinical global impression of general practitioners. Reference Olsson, Mykletun and Dahl37 In addition, the focus on cognitive and psychological symptoms of mental disorders should have reduced the confounding from symptoms independently associated with physical activity such as poor energy levels and sleep disturbance. Finally, the sample used in this study was drawn from the population of Nord-Trøndelag County of Norway in the late 1990s. This area is mostly rural where individuals may be more likely to be active and engaged in outdoor pursuits than those who live in more urban environments. Such concerns may limit the generalisability of our findings. However, the reported levels of physical activity within our sample are very similar to previous estimates in other high-income countries. 2 Norway also has longer and colder winters than many countries; however, the popularity of both winter and summer sporting activities suggests that levels of activity should remain relatively constant throughout the year, and therefore seasonal factors should not be a major concern in this sample.
The importance of context
These results provide further strong evidence for an inverse association between physical activity and depression. Regular exercise, undertaken as a leisure-time activity, is associated with lower levels of case-level depression. However, no such association was seen with activity in the workplace. Only two other studies have examined the association between workplace-based physical activity and depression, and both also failed to find any significant associations. Reference Sexton, Sogaard and Olstad4,Reference Wiles, Haase, Gallacher, Lawlor and Lewis5 Given the size of our sample it is unlikely that the lack of an association with workplace activity was due to type 2 error. Therefore, we propose four possible explanations for why leisure activity but not workplace activity is associated with lower levels of depression. First, there may be residual confounding in one of the models. For example, unmeasured workplace or social factors associated with manual occupations (e.g. uncomfortable work conditions or poor remuneration) may independently increase the risk of depression, thereby confounding any association between workplace activity and depression. Second, workplace activity may be less physically intensive than leisure activities and may not produce the same physiological benefits. Third, there may be varying degrees of reverse causation operating, with depression more likely to result in lower levels of leisure activity than any alteration in workplace activity, although previous prospective studies suggest this is unlikely to explain all of the associations observed. Reference Wiles, Haase, Gallacher, Lawlor and Lewis5 Finally, and perhaps most compellingly, it may be that the context of physical activity is important. Leisure activities are different from non-recreational activities because of the associated enjoyment and social contact. Therefore, the ‘leisure’ component of leisure activities may be the most important factor in defining the association with common mental disorders. This conclusion is in keeping with findings from a recent twin study, which found that regular exercise was associated with reduced symptoms of mental disorders, but that exercise itself did not seem to be the causal factor in the relationship. Reference De Moor, Boomsma, Stubbe, Willemsen and de Geus38
Biological v. social models
Numerous biological models have been proposed as explanations for the link between exercise and depression. Reference Meeusen, Thorre, Chaouloff, Sarre, De Meirleir and Ebinger14,Reference Ernst, Olson, Pinel, Lam and Christie18,Reference Rottenberg19 Given the nature of our data we were limited in our ability to directly test models of causation. However, we were able to investigate whether increased parasympathetic vagal tone or changes in metabolism could potentially be mediators in the associations between physical activity and depression. We did not find any evidence to support these hypotheses. Other methods of measuring vagal tone such as heart rate variability are usually considered as purer measures than resting heart rate. One study has measured heart rate variability in a group of individuals (n = 40) before and after randomised withdrawal of regular exercise. Reference Weinstein, Deuster and Kop39 Although exercise withdrawal was associated with increased negative mood, this did not appear to be mediated by any change in the heart rate variability. Reference Weinstein, Deuster and Kop39 This is consistent with our findings and suggests parasympathetic vagal tone is unlikely to be an important mediator in the association between physical activity and depression. In contrast, we did find evidence that social support and social engagement were important and could explain a considerable amount of the effect size observed. However, even after controlling for this effect there was still a significant association between leisure activities and depression, suggesting other unmeasured pathways are important. It may be that other biological systems we were not able to test are involved in the relationship between physical activity and depression, or that different causal pathways operate depending on the type of intensity of activity undertaken. The complexity of these associations is further highlighted by our observation that much of the apparent psychological benefit of physical activity is seen in those doing at least 1 h of activity each week. This is in keeping with previous studies, Reference Teychenne, Ball and Salmon3 but implies that the biological or social systems involved in this relationship can be altered by relatively low levels of activity.
Previous research on the links between physical activity and mental health has focused on the possible antidepressant effects of exercise; however, less is known about the association between exercise and anxiety. Reference Dunn, Trivedi and O'Neal7 When anxiety symptoms have been considered, it has often been as part of a general measure of psychopathology such as the General Health Questionnaire. Reference Wiles, Haase, Gallacher, Lawlor and Lewis5,Reference Hamer, Stamatakis and Steptoe6 We were able to extend beyond this approach and look at symptoms of anxiety separately. We have replicated an earlier finding of a negative association between physical activity and anxiety disorders, Reference Goodwin40 although the effect size we describe was relatively small and this association was only seen with light leisure activity. This suggests the association between exercise and improved levels of mental health may be specific to certain symptom types.
Implications
This study demonstrates that individuals who engage in regular leisure-time activity of any intensity are less likely to have symptoms of depression. The context of any physical activity is vital, with workplace activity having no association with lower levels of common mental disorders. The social benefits associated with exercise appear to be more important than biological changes in explaining the associations between physical activity and symptoms of depression.
Acknowledgements
The HUNT Study is a collaboration between the HUNT Research Centre, Faculty of Medicine, Norwegian University of Science and Technology (NTNU, Verdal), Norwegian Institute of Public Health and Nord-Trøndelag County Council. We are grateful to Erlend Bergesen who was initially the principal investigator in this project, but who sadly died too young on the 20 August 2006. We also wish to thank Kari Eriksen for her technical support and translating skills.









eLetters
No eLetters have been published for this article.