Schizophrenia is associated worldwide with a higher rate of smoking than that observed among the general population or those with other severe mental illnesses (McCreadie, 2002; Reference Llerena, de la Rubia and Penas-LledóLlerena et al, 2003). This association persists after correcting for such confounding factors as antipsychotic medication, institutionalisation or alcohol and drug use (de Leon et al, Reference de Leon, Dadvand and Canuso.1995, Reference de Leon, Tracy and McCann2002a ; Reference Llerena, de la Rubia and Penas-LledóLlerena et al, 2003). Smoking might be a marker of a more severe illness or might have a beneficial effect in schizophrenia by improving its symptoms and/or decreasing extrapyramidal side-effects of antipsychotics – ‘the self-medication hypothesis’ (Reference Lohr and FlynnLohr & Flynn, 1992; Reference Ziedonis, Kosten and GlazerZiedonis et al, 1994; Reference Dalack, Healy and Meador-WoodruffDalack et al, 1998). This study explores both the self-medication hypothesis and the hypothesis that severe forms of schizophrenia are associated with high levels of nicotine dependence.
METHOD
Patients
The study was located at two community mental health centres and their rehabilitation unit, covering the catchment area of the city of Granada (southern Spain). All participants received free psychiatric treatment from the national health system. The sample included the first 250 consecutive patients with a diagnosis of DSM–IV (American Psychiatric Association, 1994) schizophrenia who provided written informed consent after a complete description of the study (18 of 278 patients refused to participate; 10 additional excluded patients had a chart diagnosis of schizophrenia but did not meet the DSM–IV diagnosis). The diagnosis was made with the clinician version of a structured diagnostic interview (Reference First, Spitzer and GibbonFirst et al, 1994). All 250 patients were Caucasians. The mean age was 36.1 (s.d.=9.5) years and the mean age at diagnosis was 21.9 (s.d.=6.0) years. There were 195 males (78%); this male overrepresentation is typical of treatment samples from many countries (Reference Hambrecht, Maurer and HafnerHambrecht et al, 1993), including Granada (Reference Salize, Küstner and Torres-GonzalezSalize et al, 1999).
Twenty per cent had not completed their primary education; 45% had completed primary, 25% secondary and 10% a university education. Most patients (94%, 236/250) were taking antipsychotics, with a mean dose of chlorpromazine equivalents of 550 mg/day (s.d.=459). The frequency of patients taking depot antipsychotics was 45% (113/250); risperidone, 33% (82/250); olanzapine, 6% (14/250); and clozapine, 4% (10/250). There is no reason to believe that the self-reported smoking of these patients was unreliable, because until recently smoking has been socially acceptable in Spain. Moreover, a reliable self-report of smoking or non-smoking status was provided by a subsample of 99 participants (of the 250 studied) whose cotinine in saliva was measured by radioimmunoassay.
Variables
All ratings were conducted by a research psychiatrist (M.C.A.). Table 1 describes the variables used in statistical analyses. In order to avoid bias in the assessment, the clinical evaluation was conducted first, and information concerning medication and substance use, including tobacco and nicotine dependence, was gathered afterwards.
Table 1 Description of variables in 250 patients with schizophrenia, according to their nicotine dependence status: non-smokers (NS), mildly dependent smokers (MDS) and highly dependent smokers (HDS)
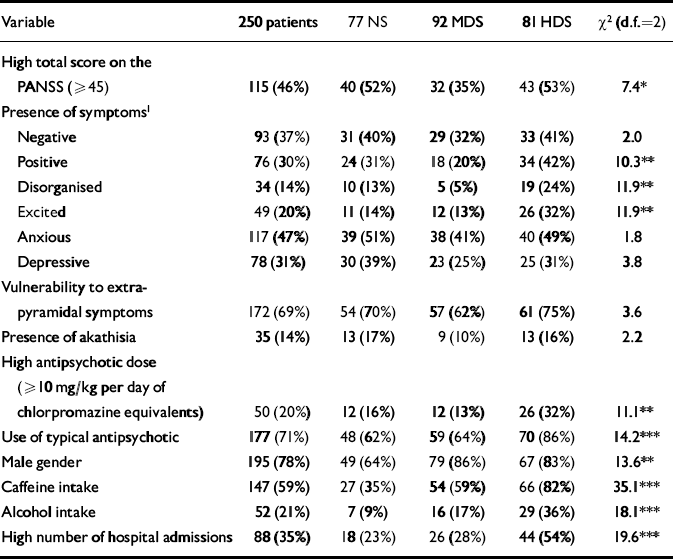
| Variable | 250 patients | 77 NS | 92 MDS | 81 HDS | χ2 (d.f.=2) |
|---|---|---|---|---|---|
| High total score on the PANSS (≥45) | 115 (46%) | 40 (52%) | 32 (35%) | 43 (53%) | 7.4* |
| Presence of symptoms1 | |||||
| Negative | 93 (37%) | 31 (40%) | 29 (32%) | 33 (41%) | 2.0 |
| Positive | 76 (30%) | 24 (31%) | 18 (20%) | 34 (42%) | 10.3** |
| Disorganised | 34 (14%) | 10 (13%) | 5 (5%) | 19 (24%) | 11.9** |
| Excited | 49 (20%) | 11 (14%) | 12 (13%) | 26 (32%) | 11.9** |
| Anxious | 117 (47%) | 39 (51%) | 38 (41%) | 40 (49%) | 1.8 |
| Depressive | 78 (31%) | 30 (39%) | 23 (25%) | 25 (31%) | 3.8 |
| Vulnerability to extrapyramidal symptoms | 172 (69%) | 54 (70%) | 57 (62%) | 61 (75%) | 3.6 |
| Presence of akathisia | 35 (14%) | 13 (17%) | 9 (10%) | 13 (16%) | 2.2 |
| High antipsychotic dose (≥10 mg/kg per day of chlorpromazine equivalents) | 50 (20%) | 12 (16%) | 12 (13%) | 26 (32%) | 11.1** |
| Use of typical antipsychotic | 177 (71%) | 48 (62%) | 59 (64%) | 70 (86%) | 14.2*** |
| Male gender | 195 (78%) | 49 (64%) | 79 (86%) | 67 (83%) | 13.6** |
| Caffeine intake | 147 (59%) | 27 (35%) | 54 (59%) | 66 (82%) | 35.1*** |
| Alcohol intake | 52 (21%) | 7 (9%) | 16 (17%) | 29 (36%) | 18.1*** |
| High number of hospital admissions | 88 (35%) | 18 (23%) | 26 (28%) | 44 (54%) | 19.6*** |
All variables were dichotomised except nicotine dependence, which was given three categories. On the basis of the Fagerström Test for Nicotine Dependence (FTND), smokers were classified as very highly dependent (FTND > 47; smoking a median of 40 cigarettes/day) or not very highly dependent (FTND ≤47; median of 20 cigarettes/day) (Reference Fagerström, Heatherton and KozlowskiFagerström et al, 1990). The three categories will be called highly dependent smokers, mildly dependent smokers and non-smokers. Schizophrenic symptomatology was assessed with the Spanish version of the Positive and Negative Syndrome Scale (PANSS; Reference Peralta and CuestaPeralta & Cuesta, 1994). The PANSS total scores were divided into high (≥45) and low scores. The negative, positive, disorganised, excited, anxious and depressed factors of the PANSS were calculated by adding the scores of the items with a loading higher than 0.50 in the factor (Reference Peralta and CuestaPeralta & Cuesta, 1994), and dividing by the number of those items. Subjects with a score ≥2 for a factor were considered to have clinically significant symptoms (except for the excited factor, see Table 1 footnote). In summary, the presence of symptoms with regard to a factor (e.g. positive symptoms) in these clinically stable out-patients suggests that despite treatment they continue to have sufficient positive symptoms to be identified through a standardised assessment.
The Simpson & Angus (Reference Simpson and Angus1970) Neurological Rating Scale was used to measure parkinsonian side-effects. Akathisia was assessed with the Barnes Akathisia Scale (Reference BarnesBarnes, 1989). Some patients might have extrapyramidal side-effects previously corrected by antiparkinsonian drugs, therefore vulnerability to extrapyramidal side-effects was defined as the occurrence of at least one of the three following conditions: current treatment with antiparkinsonian medication; a score of >0 on the neurological rating scale; or a score of > 0 on the Barnes Akathisia Scale (presence of akathisia).
A high antipsychotic dose was defined as a chlorpromazine equivalent of ≥ 10 mg/kg per day. Current alcohol and caffeine intake was assessed by interview and verified by chart review and collateral information from the family (with whom most patients live in Spain). Owing to the small number of patients using illegal drugs (7%, 17/250), a drug-use variable was not included in the analysis.
Finally, a high number of hospitalisations after correcting for duration of illness was used for the longitudinal definition of the severity of psychiatric symptoms.
Statistics
The Statistical Package for the Social Sciences (version 11.0) was used for calculations (SPSS, 1997). Initially, the three groups were compared by univariate parametric or non-parametric tests, as appropriate. Then, log-linear analyses of the data were performed (Reference AgrestiAgresti, 1990; SPSS, 1997); the log-linear analyses had two main purposes: they tested the hypothesis of a significant association of nicotine dependence with schizophrenic symptomatology, as measured by either the PANSS total score (or each one of its factors) or the number of hospitalisations; and they described the strength and direction of such association across different combinations of levels of potential interacting variables such as gender, antipsychotic dose/type and caffeine and alcohol intake. Strength and direction of associations were measured with odds ratios and their 95% confidence intervals from cross-tabulations.
In a first analysis, the association between nicotine dependence and PANSS total score was tested while controlling for gender, antipsychotic dose/type and caffeine and alcohol intake. This was performed by including the seven variables in a saturated log-linear model. Table 2 shows the significant interactions that were obtained. The significances of interaction were tested using partial χ2 (SPSS, 1997). A significant interaction between two variables was interpreted as evidence that the two variables were associated, even when controlling for the other variables in the model. Analyses similar to that of the PANSS total score were repeated for number of admissions (Table 3) and for negative, positive, disorganised, excited, anxious and depressed PANSS factors (results not presented).
Table 2 Log-linear analysis focusing on the association between the Positive and Negative Syndrome Scale (PANSS) total score and nicotine dependence
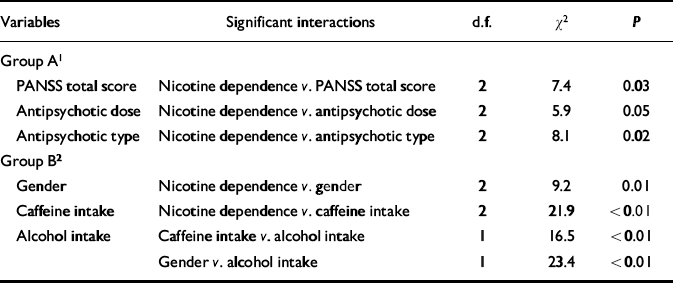
| Variables | Significant interactions | d.f. | χ2 | P |
|---|---|---|---|---|
| Group A1 | ||||
| PANSS total score | Nicotine dependence v. PANSS total score | 2 | 7.4 | 0.03 |
| Antipsychotic dose | Nicotine dependence v. antipsychotic dose | 2 | 5.9 | 0.05 |
| Antipsychotic type | Nicotine dependence v. antipsychotic type | 2 | 8.1 | 0.02 |
| Group B2 | ||||
| Gender | Nicotine dependence v. gender | 2 | 9.2 | 0.01 |
| Caffeine intake | Nicotine dependence v. caffeine intake | 2 | 21.9 | <0.01 |
| Alcohol intake | Caffeine intake v. alcohol intake | 1 | 16.5 | <0.01 |
| Gender v. alcohol intake | 1 | 23.4 | <0.01 |
Table 3 Log-linear analysis focusing on the association between corrected number of hospital admissions and nicotine dependence
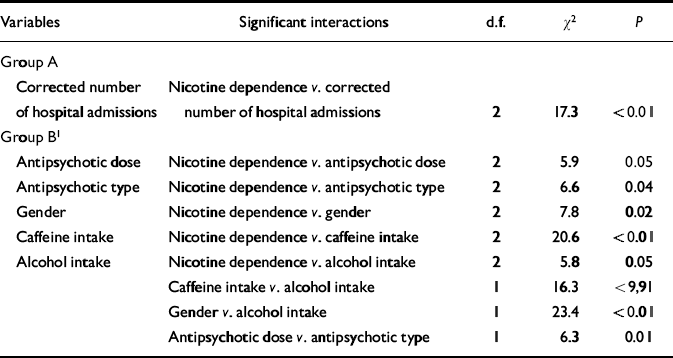
| Variables | Significant interactions | d.f. | χ2 | P |
|---|---|---|---|---|
| Group A | ||||
| Corrected number of hospital admissions | Nicotine dependence v. corrected number of hospital admissions | 2 | 17.3 | <0.01 |
| Group B1 | ||||
| Antipsychotic dose | Nicotine dependence v. antipsychotic dose | 2 | 5.9 | 0.05 |
| Antipsychotic type | Nicotine dependence v. antipsychotic type | 2 | 6.6 | 0.04 |
| Gender | Nicotine dependence v. gender | 2 | 7.8 | 0.02 |
| Caffeine intake | Nicotine dependence v. caffeine intake | 2 | 20.6 | <0.01 |
| Alcohol intake | Nicotine dependence v. alcohol intake | 2 | 5.8 | 0.05 |
| Caffeine intake v. alcohol intake | 1 | 16.3 | <9,91 | |
| Gender v. alcohol intake | 1 | 23.4 | <0.01 | |
| Antipsychotic dose v. antipsychotic type | 1 | 6.3 | 0.01 |
A second purpose of the statistical analyses was to describe the association between nicotine dependence and schizophrenic symptomatology across different combinations of levels of other variables. One difficulty was that the relatively high number of variables considered in this study produced many possible combinations. Some variables represented a small sample size; for instance, the five dichotomous variables (gender, antipsychotic dose and type and caffeine and alcohol intake) produced 25=32 possible combinations of levels. It is not practical or statistically advisable to perform so many cross-tabulations. Because odds ratios are rather inaccurate with small sample sizes, a systematic methodology that discarded irrelevant variables was used (SPSS, 1997). This methodology was based on the collapsibility conditions (Reference AgrestiAgresti, 1990); the rationale behind the collapsibility conditions is that variables that do not affect an association can be excluded from the analysis of that association (group B of variables in Tables 2 and 3), even if those variables have an effect on one of the variables involved in the association. The above analyses were repeated by including vulnerability to extrapyramidal side-effects as an additional variable.
RESULTS
Among the 250 patients there were 173 (69%) current smokers and 77 (31%) non-smokers (including 7 (4%) former smokers and 70 (27%) that had never smoked daily). As expected, the rate of smoking was higher in our sample than among the Spanish general population (Reference Pinilla and GonzálezPinilla & González, 2001) for both males (75% v. 45%) and females (49% v. 27%).
Table 1 shows the variable distribution across the three groups of nicotine dependence. There were no significant differences in current age, age at diagnosis or educational level. The mean (s.d.) PANSS total score was 45.7 (10.7) for non-smokers, 41.7 (9.2) for mildly dependent smokers and 47.9 (14.1) for highly dependent smokers (Kruskal–Wallis χ2=12.0, d.f.=2, P<0.01); had this comparison been made between smokers and non-smokers, no significant difference would have been found: 44.6 (12.1) v. 45.7 (10.7) (Mann–Whitney χ2=1.3, d.f.=1, P=0.25). The mean (s.d.) number of hospital admissions was 2.8 (4.0) for non-smokers, 3.0 (3.0) for mildly dependent smokers and 6.4 (6.3) for highly dependent smokers (Kruskal–Wallis χ2=27.9, d.f.=2, P<0.0001). The levels (median) of cotinine (ng/ml) in saliva in the 99 participants for whom it was determined were: 551 in highly dependent smokers (n=31), 423 in mildly dependent smokers (n=29) and 0.6 in non-smokers (n=29).
Symptom score and nicotine dependence
Table 2 shows results from the log-linear model that included nicotine dependence and the variables listed in the first column. The interaction between nicotine dependence and PANSS total score was significantly different from zero, indicating that these two variables were significantly associated when controlling for the other variables (gender, antipsychotic dose and type, caffeine and alcohol intake). Nicotine dependence was significantly associated with other variables (gender, antipsychotic dose and type, and caffeine intake). Two groups of variables can be identified from Table 2: group A, comprising PANSS total score and antipsychotic dose and type; and group B, comprising gender and caffeine and alcohol intake. Nicotine dependence was associated significantly with all the variables of group A and some variables of group B. However, the variables of group A were not associated with the variables of group B. In fact, no significant interactions simultaneously involving variables of A and variables of B were found. By virtue of the collapsibility conditions, the strength and direction of the association between PANSS total score and nicotine dependence do not vary across the levels of caffeine or alcohol intake, gender or across combinations of those levels. Thus, this association can be studied by controlling only for antipsychotic dose and type.
The association between PANSS total score and nicotine dependence was therefore studied with cross-tabulations for each of the four combinations of antipsychotic doses and types. The association was most significant among those on a low dose of typical antipsychotics (Fig. 1). In these subjects, mildly dependent smokers included the lowest number of subjects with clinically meaningful symptoms in the total PANSS. Among those on a low dose of a typical antipsychotic, non-smokers have an odds ratio of 2.7 of having a high PANSS total score when compared with mildly dependent smokers (Fig. 1). In other words, the percentage of patients with high total scores was significantly lower in mildly dependent smokers than among non-smokers or highly dependent smokers.
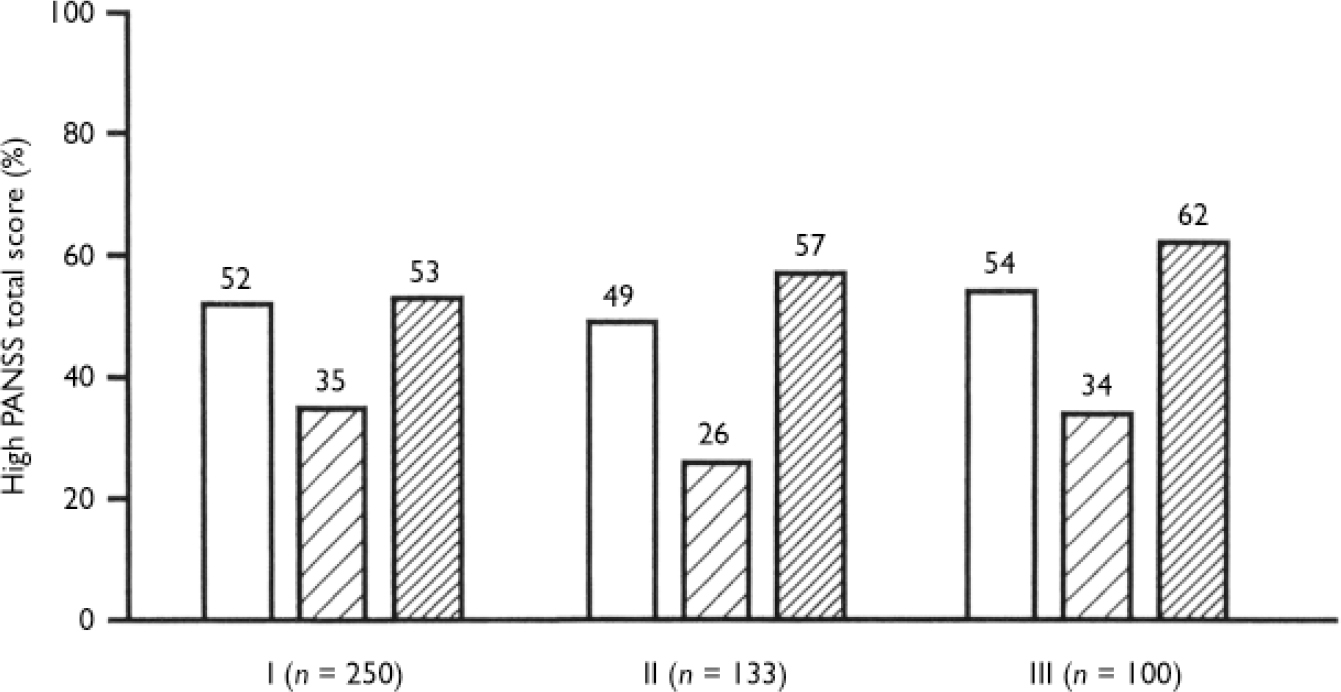
Fig. 1 High Positive and Negative Syndrome Scale (PANSS) total score (≥45, presence of symptoms) among non-smokers (NS, □), mildly dependent smokers (MDS, □) and highly dependent smokers (HDS, □). Numbers above bars indicate percentages. Group 1, all participants. Significance of simultaneous comparisons of the three dependence groups: χ2=7.4, d.f.=2, P<0.02. Odds ratio (95% CI): 2.0 (1.1-8.4) for NS v. MDS; 2.1 (1.9-3.9) for HDS v. MDS. Group II, participants with a low dose of typical antipsychotics: χ2=9.9, d.f.=2, P=0.007. Odds ratio (95% CI) 2.7 (1.1-6.6) for NS v. MDSS; 3.7 (1.6-8.9) for HDS v. MDS. Group III, participants with a low dose of typical antipsychotics and vulnerability to extrapyramidal symptoms: χ2=5.8, d.f.=2, P=0.06.
When vulnerability to extrapyramidal side-effects was also included in the log-linear model, the significant interactions were the same as in Table 2; additionally, a significant interaction between antipsychotic type and vulnerability to extrapyramidal side-effects, and a significant interaction between PANSS total score and vulnerability to extrapyramidal side-effects were found (see footnote to Table 2). The association between PANSS total score and nicotine dependence was close to significant for those on a low dose of typical antipsychotic medication who showed vulnerability to extrapyramidal side-effects (Fig. 1); among these, mildly dependent smokers included the lowest number with clinically meaningful symptoms in the PANSS total score.
The analysis of the positive factor was very similar to the analysis of the PANSS total score and supported the self-medication hypothesis for those on a low dose of typical antipsychotics who are mildly dependent smokers.
Negative, depressive or anxious symptoms were not significantly associated with nicotine dependence (Table 1). Thus, the analyses of these symptoms did not support the self-medication hypothesis. Neither the disorganised nor the excited PANSS factor, after analysis, supported the self-medication hypothesis. Moreover, disorganised residual symptoms were associated with heavy smoking (see next section).
Number of admissions and nicotine dependence
Table 1 shows that highly dependent smokers had the highest proportion of hospital admissions compared with mildly dependent smokers (odds ratio=3.0) and non-smokers (odds ratio=3.9) (see also Fig. 2).
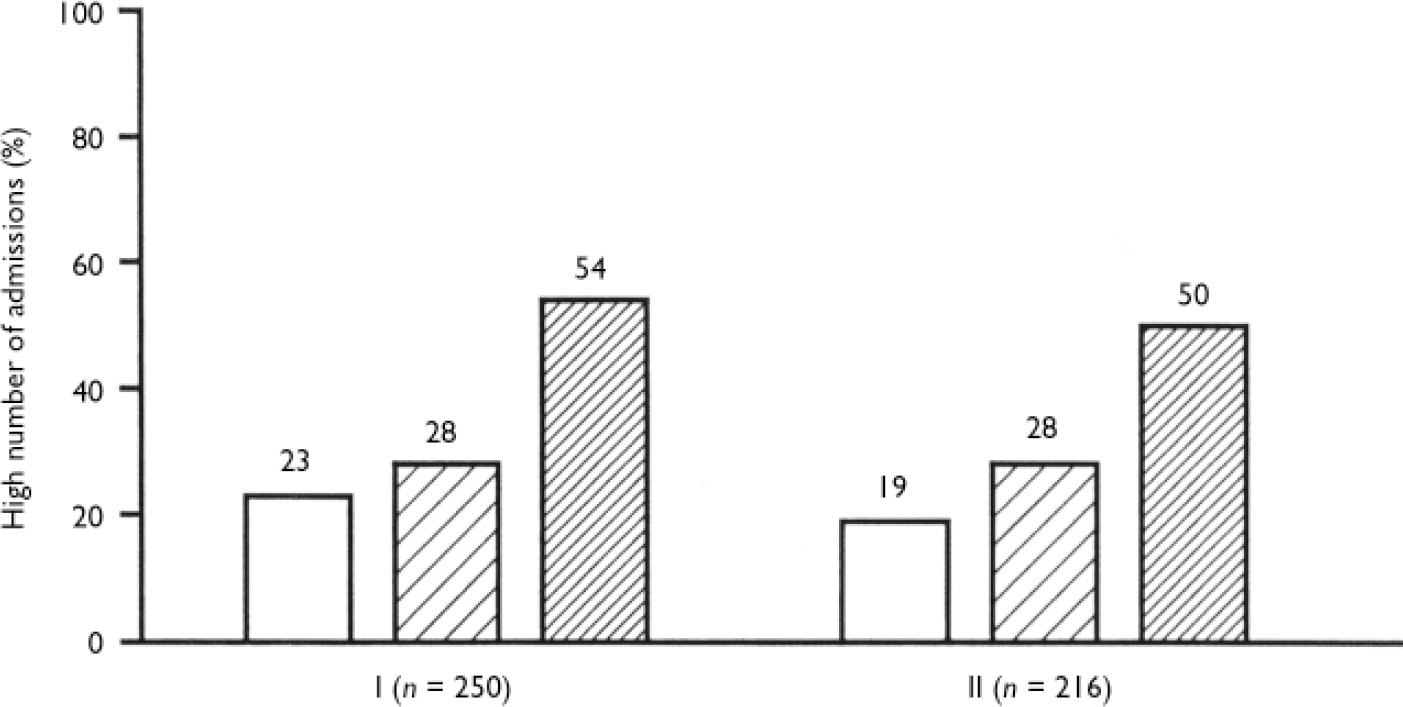
Fig. 2 High number of hospital admissions among non-smokers (NS, □), mildly dependent smokers (MDS, □) and highly dependent smokers(HDS, □). Numbers above bars indicate percentages. Group I, all participants. Significance of simultaneous comparisons of the three dependence groups: χ2=19.6, d.f.=2, P<0.01. Odds ratio (95% CI) 3.9 (2.0-7.7) for HDS v. NS; 3.0 (1.6-5.7) for HDS v. MDS. Group II, participants without disorganised symptoms: χ2=15.0, d.f.=2, P<0.01. Odds ratio (95% CI) 4.2 (1.9-9.1) for HDS v. NS; 2.6 (1.3-5.2) for HDS v. MDS.
When the disorganised symptom variable was considered in the analysis, the association between nicotine dependence and number of admissions was significant only for those without disorganised symptoms (Fig. 2).
DISCUSSION
The self-medication hypothesis of smoking in schizophrenia
There is a discrepancy in the literature, with numerous animal studies suggesting that nicotine should help negative symptoms but scarce clinical data suggesting that this may be true in those with schizophrenia (Reference HughesHughes, 2000). Two main sub-hypotheses are usually included in the self-medication hypothesis: smoking reduces the side-effects of antipsychotics; and nicotine may improve schizophrenic symptoms, particularly the negative, cognitive and/or depressive symptoms (Reference Taiminen, Salokangas and SaarijarviTaiminen et al, 1998).
Two mechanisms have been implicated in the reduction of antipsychotic side-effects: a release of dopamine resulting from the administration of nicotine, a notion supported by both acute administration of nicotine in animal models (Reference Drew, Derbez and WerlingDrew et al, 2000) and in vivo human studies (Reference Salokangas, Vilkman and IlonenSalokangas et al, 2000); and a decrease in antipsychotic blood levels through enzymatic induction. Individuals with schizophrenia who smoke tend to receive consistently higher doses of antipsychotics than non-smokers (Reference Ziedonis, Kosten and GlazerZiedonis et al, 1994; de Leon et al, Reference de Leon, Dadvand and Canuso.1995, Reference de Leon, Tracy and McCann2002a ). The inductive effect of smoking in antipsychotic metabolism therefore is inadvertently corrected by psychiatrists, because smokers tend to be treated with higher daily doses of antipsychotics than non-smokers. When compared with others with severe mental illness in three epidemiological studies in psychiatric hospitals, the effect of antipsychotic medication did not explain the association between schizophrenia and smoking (de Leon et al, Reference de Leon, Dadvand and Canuso.1995, Reference de Leon, Tracy and McCann2002a ; Reference Llerena, de la Rubia and Penas-LledóLlerena et al, 2003). Some cross-sectional studies have suggested that smoking reduces antipsychotic side-effects and others have not (Reference Dalack, Healy and Meador-WoodruffDalack et al, 1998); yet all of these studies are hampered by the lack of control for confounding factors. Longitudinal studies with small samples suggest that, when compared with atypical antipsychotics, typical antipsychotics are associated with increased smoking in some individuals (Reference McEvoy, Freudenreich and LevinMcEvoy et al, 1995) and with a greater difficulty for quitting smoking (Reference George, Ziedonis and FeingoldGeorge et al, 2000). Anticholinergic medication was not associated significantly with smoking in this or in previous studies (de Leon et al, Reference de Leon, Dadvand and Canuso.1995, Reference de Leon, Tracy and McCann2002a , Reference de Leon, Becoña and Gurpegui b ).
In spite of the hypothesis from animal studies (Reference Drew, Derbez and WerlingDrew et al, 2000), very limited clinical data support an association between smoking and a reduction in negative symptoms (Reference Dalack, Healy and Meador-WoodruffDalack et al, 1998). Data indicating that nicotine may improve sensory gating abnormalities and smooth pursuit eye movements in schizophrenia or cognitive abnormalities induced by antipsychotics are somewhat stronger. Nicotine may have antidepressant qualities in individuals with depression (Reference Salin-Pascual, Rosas and Jimenez-GenchiSalin-Pascual et al, 1996), but this is not well established in those with schizophrenia.
The literature appears to suggest that those with severe forms of schizophrenia may smoke more frequently, and more heavily, than those with less severe forms (Reference Lohr and FlynnLohr & Flynn, 1992). The possible beneficial effect of nicotine (and smoking) on schizophrenic symptoms and antipsychotic side-effects may be obscured by this association between smoking and severe forms of schizophrenia. In summary, a critical reading of the literature lends very limited support to the self-medication hypothesis, but this effect may be obscured by the association between severe forms of schizophrenia and heavy smoking.
Limitations and strengths of this study
The limitations of the cross-sectional design make it impossible to prove definitively or to deny that smoking has beneficial effects on schizophrenia. It is not ethical to conduct long-term studies by (ideally) randomising patients to heavy or mild smoking. However, our study – involving a great number of stable out-patients with schizophrenia – like other naturalistic studies, may help to select which individuals are more likely to improve their schizophrenic symptoms and/or extrapyramidal side-effects using nicotine patches or other nicotine agonists. Because experimental designs with randomisation (to different levels of smoking and lack of smoking) are not admissible, other naturalistic studies with large samples, refined assessments and sophisticated statistical techniques to control for confounders are needed to confirm these findings.
Our findings suggest that nicotine dependence and schizophrenic symptomatology might be statistically dependent in out-patients with schizophenia, but the data imply a complex interaction between these two variables. Our large sample size and the use of a sophisticated statistical technique, log-linear analysis, made it possible to control for potential confounding and interacting variables (Reference AgrestiAgresti, 1990). In contrast with other statistical techniques such as multiple linear or logistic regression, the log-linear methodology provides a clearer way to identify and deal with multiple interactions among several variables. In addition, as explained in the statistics section, the methodology used allows the systematic identification of variables affecting the association between nicotine dependence and schizophrenic symptoms, and the identification of subgroups where this association exists. Finally, the log-linear methodology does not imply the assumption of linear relationships among the variables analysed. The results of this study in stable out-patients with low levels of symptoms suggest that such an assumption is unsustainable. One apparent drawback of log-linear methodology is the need to transform continuous variables into categorical ones. Nonetheless, the amount of information that a log-linear analysis produces compensates for the possible loss of some information in the transformation (Reference AgrestiAgresti, 1990).
One may argue that the association between increased frequency of hospitalisation and high nicotine dependence may be partly explained by institutionalisation. However, this is not likely. Our cross-cultural studies suggest that, although the prevalence of current smoking in the general population is influenced by social pressure, high nicotine dependence among smokers with severe psychiatric illness appears to be similar across countries and remarkably resistant to social pressure (Reference de Leon, Becoña and Gurpeguide Leon et al, 2002b ).
Support for the self-medication hypothesis in mildly dependent smokers
The analysis of the PANSS total score, as well as the analysis of its positive symptom factor, supported the self-medication hypothesis for mildly dependent smokers, especially for those taking low doses of typical antipychotics. Those with low levels of total symptoms are overrepresented among mildly dependent smokers compared with non-smokers. It may be that mild levels of smoking were associated with a reduction of schizophrenic symptoms, especially among those taking a low dose of typical antipsychotics (and particularly those showing vulnerability to extrapyramidal side-effects). The differential effect of typical and atypical antipsychotics on smoking behaviour is consistent with other studies with different designs suggesting that those with schizophrenia on typical antipsychotics may smoke more (Reference McEvoy, Freudenreich and LevinMcEvoy et al, 1995) and have more difficulties in quitting smoking (Reference George, Ziedonis and FeingoldGeorge et al, 2000).
The possible alleviation of positive symptoms by chronic nicotine administration could be explained by a potential correction of the cortical–subcortical dissociation of dopamine activity, which may be associated with schizophrenia (Reference Dalack, Healy and Meador-WoodruffDalack et al, 1998). However, in certain cases of schizophrenia (perhaps the most severe), ‘self-medication’, even with higher amounts of nicotine – as in our highly dependent smokers – would not be effective.
In summary, if there is any beneficial effect of nicotine it may be restricted to mildly dependent smokers, and particularly to those on low dosages of typical antipsychotics who are sensitive to the extrapyramidal side-effects. Such a benefit appears to affect only certain symptoms. Our study does not support the self-medication hypothesis for highly dependent smokers, who have poorer outcomes despite their heavy smoking.
Other symptom differences do not support the self-medication hypothesis
In contrast to the positive symptoms, the analyses of negative, anxious and depressive symptoms in our sample do not support the self-medication hypothesis. This does not necessarily refute the hypothesis; the assessment may not have been sensitive enough or the effect size too small to be apparent with the statistical power in our sample. Taiminen et al (Reference Taiminen, Salokangas and Saarijarvi1998) similarly found no differences in negative symptoms according to smoking behaviour. Although these two naturalistic studies do not rule out the possibility of beneficial effects of nicotine on negative symptoms, they certainly suggest that the alleviation of other schizophrenic symptoms is more likely. Ziedonis et al (Reference Ziedonis, Kosten and Glazer1994) described lower levels of negative symptoms in heavy smokers (and higher levels of positive symptoms), in comparison with light smokers and non-smokers with schizophrenia. Goff et al (Reference Goff, Henderson and Amico1992) found higher levels of both negative and positive symptoms in smokers than in non-smokers, whereas Kelly & McCreadie (Reference Kelly and McCreadie1999) were not able to demonstrate significant differences between smokers and non-smokers.
The presence of disorganised symptoms was associated with a high dependence on nicotine in our sample and did not support the self-medication hypothesis.
Poor outcome and heavy smoking
Our results suggest that severe forms of schizophrenia with poor outcome, manifested by either residual disorganised symptoms or a greater number of hospital admissions without residual disorganised symptoms, were associated with heavy smoking. Certainly if nicotine has some beneficial influence in schizophrenia, it is not evident in those with a poor outcome. There are no clear-cut theories to explain the association between highly dependent smoking and poor long-term outcome in schizophrenia. The most likely underlying reason is that these individuals may have vulnerability to both high nicotine dependence and schizophrenia with poor outcome.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ In out-patients with schizophrenia, smoking behaviour may be related to symptoms, dose and type of medication and vulnerability to extrapyramidal side-effects.
-
▪ This study provided very limited support for a beneficial effect of nicotine (a beneficial effect is possible in total and positive symptoms in midly dependent smokers when compared with non-smokers). smokers when compared with non-smokers).
-
▪ High nicotine dependence is associated with poor-outcome schizophrenia.
LIMITATIONS
-
▪ The limitations of the cross-sectional design make it impossible to prove definitively or to deny a beneficial effect of smoking on schizophrenia.
-
▪ Although the interviewer made every effort to avoid bias, including the assessment of nicotine dependence at the end of the interview, she was not always blind regarding the smoking status of the patient.
-
▪ Nicotine dependence assessment was based on patient self-reporting.
Acknowledgements
The authors are grateful to patients and staff of the Granada-South and. Granada-North Community Mental Health Centres and their Out-patient. Rehabilitation Unit, for their collaboration, and to the Institutional Review. Boards of the San Cecilio and Virgen de las Nieves University Hospitals, who. approved this protocol. Juan Rivero helped with data collection. M.C.A.'s work in this study was supported by a grant from the Spanish. Agency for International Cooperation (AECI).








eLetters
No eLetters have been published for this article.