Memory impairment in schizophrenia has been well documented since the early observations of Kraepelin and Bleuler in the 19th century. Recent reviews have documented significant stable and wide-ranging memory impairment in schizophrenia (Reference Aleman, Hijman and de HaanAleman et al, 1999). The importance of memory impairment stems from suggestions that it predicts functional outcome in schizophrenia (Reference GreenGreen, 1996). However, the extent of memory impairment is not clear, in part because of the shortage of epidemiological studies of this problem in schizophrenia: we found only one study that used a population-based approach (Reference Kelly, Sharkey and MorrisonKelly et al, 2000). In addition, the use of a variety of different memory batteries and terminology of memory subtypes might have contributed to the difficulty of finding the true prevalence of memory impairment in schizophrenia. Furthermore, the use of memory tests in a laboratory setting has been widely considered as having little relevance to everyday memory problems. In this study we evaluated the extent of impairment in a population-based sample using a standardised memory test that can be incorporated into clinical practice.
METHOD
Sample
We identified every patient with a possible diagnosis of schizophrenia, from psychiatric records, in one catchment area of approximately 100 000 people in south Leicestershire. This included examining old records of all psychiatric patients in the catchment area to make sure no potential patient was missed. The diagnoses were confirmed using ICD-10 criteria (World Health Organization, 1992). The area can be described as a suburban British residential area with a predominantly middle-class working population. The two consultants responsible for the area have a policy of not discharging patients with schizophrenia from their care even if the patients need minimal psychiatric input. The only exceptions were cases of severe and incapacitating schizophrenia that necessitated a referral to rehabilitation psychiatry. Such patients usually move out of the area into long-term care units or sheltered accommodation.
We excluded patients with organic brain disease, head injuries or comorbidity, and those whose first language was not English. None of the participants had had electroconvulsive therapy in the year prior to taking part in the study. Patients older than 60 years were also excluded, because Kelly et al (Reference Kelly, Sharkey and Morrison2000) suggested that people above this age with schizophrenia have a poorer cognitive performance than younger patients. The patients' performance on the memory test was compared with that of controls (n=71). Members of the control group live in the same city and were recruited by advertisements in the local hospital, university and supermarkets. They had no history of mental illness, and were subjected to the same exclusion criteria as the patient group.
Measures
Rivermead Behavioural Memory Test
Participants were assessed with the Rivermead Behavioural Memory Test (RBMT; Reference Wilson, Cockburn and BaddeleyWilson et al, 1985). This test of everyday memory has good ecological validity, and is made up of 12 measures, each aimed at testing one aspect of everyday memory:
-
(a) remembering a name;
-
(b) remembering a hidden belonging;
-
(c) remembering an appointment;
-
(d) picture recognition;
-
(e) immediate recall of a newspaper article;
-
(f) delayed recall of a newspaper article;
-
(g) face recognition;
-
(h) remembering a new route (immediate);
-
(i) remembering a new route (delayed);
-
(j) delivering a message;
-
(k) orientation questions;
-
(l) knowing the date.
The RBMT has a screening score (0-12), and is not very demanding in terms of effort or time (it takes 25-30 min to administer). It has been used before in schizophrenia studies, for example by McKenna et al (Reference McKenna, Tamlyn and Lund1990) and Kelly et al (Reference Kelly, Sharkey and Morrison2000).
National Adult Reading Test
The National Adult Reading Test (NART; Reference NelsonNelson, 1982) is an estimate measure of premorbid intelligence. It has been widely used in psychiatric research and in particular in studies of schizophrenia (Reference Gilvarry, Russell and JonesGilvarry et al, 2001).
Positive and Negative Syndrome Scale
The Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) was given to patients only. It is a widely used scale for symptom ratings in schizophrenia.
Health of the Nation Outcome Scales
The Health of the Nation Outcome Scales (HoNOS; Reference Wing, Beevor and CurtisWing et al, 1998) were used for assessment of the psychiatric patients' current community functioning.
Demographic factors
Demographic data for all participants were documented, including occupation group using the Office for National Statistics classification (see Appendix). In addition, duration of illness and age at onset for the patients were documented through information provided by the patient and verified from medical records, including first documentation by general practitioners. Duration of illness was defined as the period between the time that first psychotic symptoms were reported and the time of current assessment. Age at onset was defined as the age when first psychotic symptoms were reported.
Assessment procedure
A psychologist administered the cognitive assessments independently from the clinical assessment, which was made by a clinician (M.A.-U.) masked to the cognitive assessment. The responsible consultants (J.B. and S.F.) assessed the patients' level of community functioning using the HoNOS, and were also unaware of the cognitive assessment. Illness duration and age at onset were calculated independently by D.M.
Statistical analysis
Sample size was calculated to achieve statistical power of an 80% possibility of obtaining significant results at 5%, when submitted for ethical committee approval. Differences between patients and controls were examined by t-test or χ2 test, as appropriate, for demographic variables; RBMT scores were examined by logistic regression; within-group differences were examined using analysis of variance (ANOVA); correlations were examined using Pearson's r or Spearman's rho as appropriate. The Statistical Package for the Social Sciences version 12 for Windows was used to analyse the data.
RESULTS
We identified 190 patients, of whom 133 were potentially eligible for the study. Of those not eligible, 2 did not fulfil strict diagnostic criteria for schizophrenia, 1 died before testing, 5 had a diagnosis of substance misuse, 8 had a history of organic brain disease or head injury, 30 were over 60 years old, and for 11 English was not their first language. Of the 133 eligible patients, 60 declined to take part, leaving a total of 73 (55%) patients who were eligible and volunteered to take part in the study (Fig. 1). All patients who took part in the study were in a stable state and living in the community, except one who was an in-patient at the time of the study.
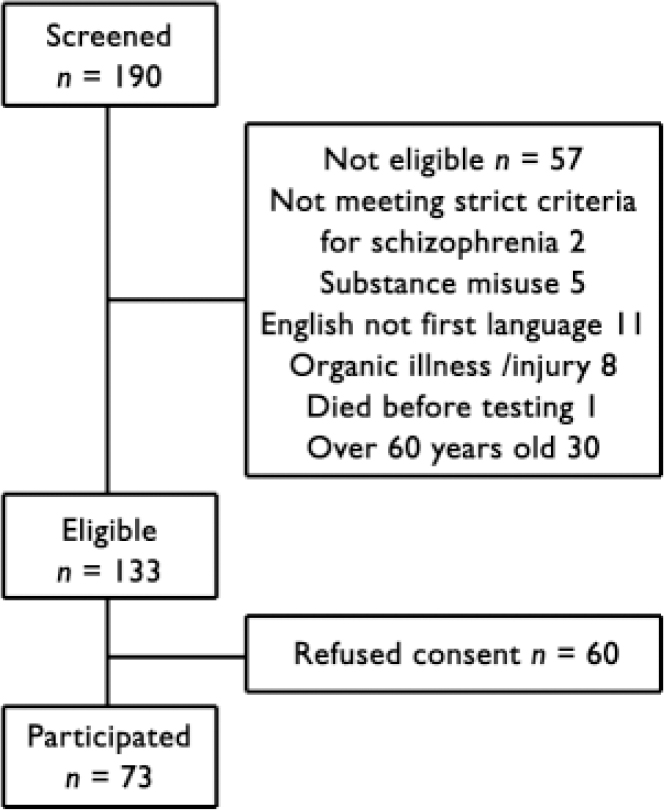
Fig. 1 Flow chart of patients' selection for the study.
We compared the known socio-demographic and clinical characteristics of patients who participated in the study and those who did not (Table 1). These characteristics included age, gender, years in education, accommodation, employment status, medication prescribed, age at onset, length of in-patient stay and illness duration. There was no significant difference between the two groups except that the participant group had more years in education. The participants were slightly older, on average, at the time of disease onset, but this difference did not reach significance.
Table 1 Comparison between patients who participated or refused to take part in the study
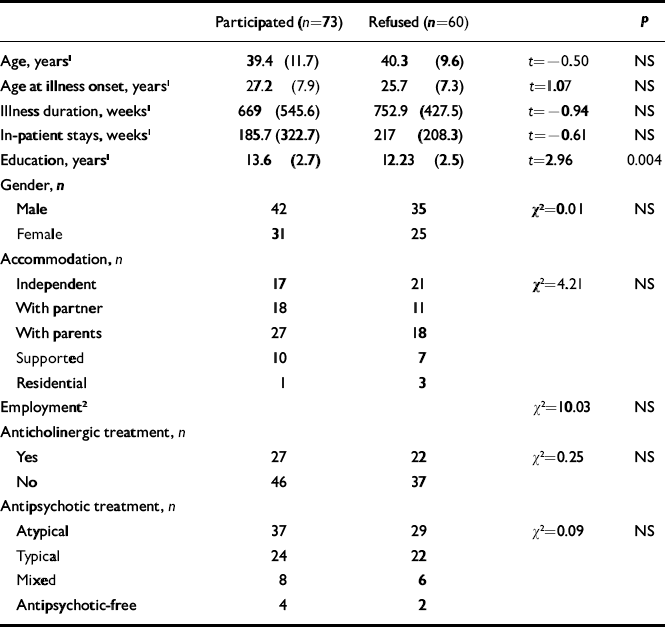
| Participated (n=73) | Refused (n=60) | P | ||
|---|---|---|---|---|
| Age, years 1 | 39.4 (11.7) | 40.3 (9.6) | t=–0.50 | NS |
| Age at illness onset, years 1 | 27.2 (7.9) | 25.7 (7.3) | t=1.07 | NS |
| Illness duration, weeks 1 | 669 (545.6) | 752.9 (427.5) | t=–0.94 | NS |
| In-patient stays, weeks 1 | 185.7 (322.7) | 217 (208.3) | t=–0.61 | NS |
| Education, years 1 | 13.6 (2.7) | 12.23 (2.5) | t=2.96 | 0.004 |
| Gender, n | ||||
| Male | 42 | 35 | χ2=0.01 | NS |
| Female | 31 | 25 | ||
| Accommodation, n | ||||
| Independent | 17 | 21 | χ2=4.21 | NS |
| With partner | 18 | 11 | ||
| With parents | 27 | 18 | ||
| Supported | 10 | 7 | ||
| Residential | 1 | 3 | ||
| Employment 2 | χ2=10.03 | NS | ||
| Anticholinergic treatment, n | ||||
| Yes | 27 | 22 | χ2=0.25 | NS |
| No | 46 | 37 | ||
| Antipsychotic treatment, n | ||||
| Atypical | 37 | 29 | χ2=0.09 | NS |
| Typical | 24 | 22 | ||
| Mixed | 8 | 6 | ||
| Antipsychotic-free | 4 | 2 |
1. Mean (s.d.)
2. Standard Occupational Classification 2000 (see Appendix)
The participating group comprised 31 women and 42 men, and the control group 33 women and 38 men. There was a small but statistically significant difference in age (t=2.48, d.f.=142, P=0.014) and NART score (t =-2.49, d.f.=132, P=0.014) between patients and controls (Table 2).
Table 2 Comparison between patients and control group demographics
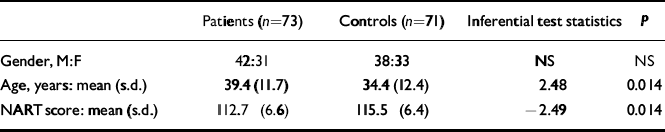
| Patients (n=73) | Controls (n=71) | Inferential test statistics | P | |
|---|---|---|---|---|
| Gender, M:F | 42:31 | 38:33 | NS | NS |
| Age, years: mean (s.d.) | 39.4 (11.7) | 34.4 (12.4) | 2.48 | 0.014 |
| NART score: mean (s.d.) | 112.7 (6.6) | 115.5 (6.4) | –2.49 | 0.014 |
F, female; M, male; NART, National Adult Reading Test
RBMT scores analysis
Binary logistic regression showed that patients as a group performed significantly worse than controls on the RBMT, even after correcting for NART score and age (B=0.665, P<0.001). Table 3 shows the distribution of both patients and controls across different scores categories of the RBMT scores. Reducing this into a 2 × 2 table (Table 4) showed that 81% of patients had impaired memory compared with 28% of controls. Thus, using RBMT scores of impaired v. normal gives a 76% chance of correctly predicting group membership (patients or controls respectively).
Table 3 Rivermead Behavioural Memory Test screening score
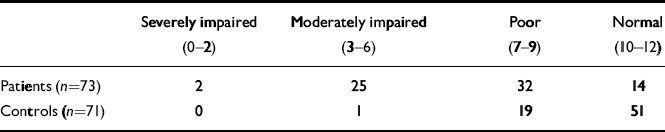
| Severely impaired (0–2) | Moderately impaired (3–6) | Poor (7–9) | Normal (10–12) | |
|---|---|---|---|---|
| Patients (n=73) | 2 | 25 | 32 | 14 |
| Controls (n=71) | 0 | 1 | 19 | 51 |
Table 4 Rivermead Behavioural Memory Test screening scores reduced to ‘impaired’ or ‘normal’

| Impaired memory (0–9) | Normal memory (10–12) | Total | |||
|---|---|---|---|---|---|
| n (%) | |||||
| Patients | 59 (81) | 14 (19) | 73 (100) | ||
| Controls | 20 (28) | 51 (72) | 71 (100) | ||
χ2=39.569, d.f.=1, P<0.001 (two-tailed)
Age
There was a significant inverse correlation between age and RBMT score for the whole sample (patients and controls): r=-0.375, two-tailed, P<0.001. That was also the case when correlations for both groups were examined separately, although the correlation was stronger between age and RBMT score in patients (r=-0.369, P=0.001) than in controls (r=-0.277, P=0.020). To further examine the age effect on both groups separately, we divided the two samples (patients and controls) into three age-groups: 18-30 years, 31-45 years and 46-60 years (see Fig. 2).
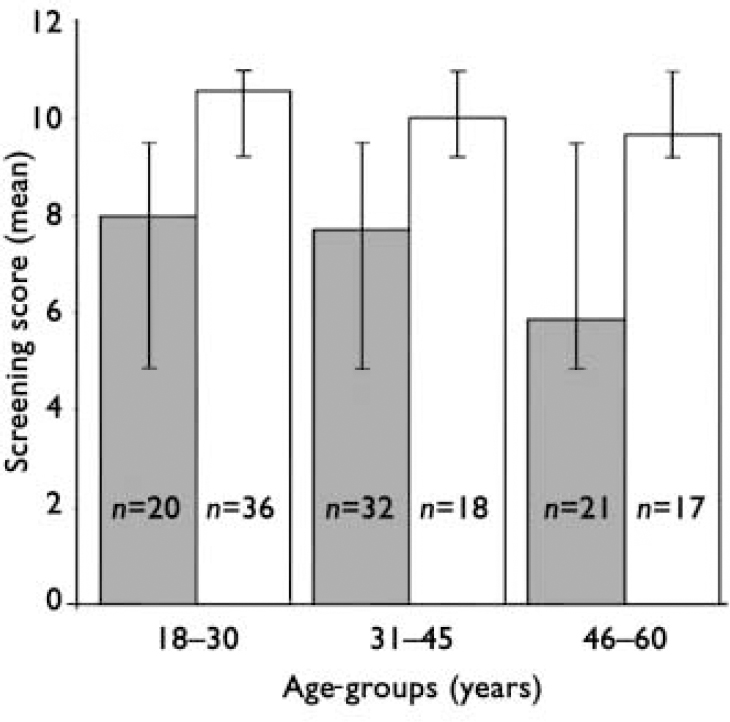
Fig. 2 Performance of different age-groups on the Rivermead Behavioural Memory Test (P<0.001 for patients v. controls in all age-groups). ▪, patients (n=73); □, controls (n=71).
Using ANOVA, we found a significant difference in RBMT scores between diagnostic groups (patients v. controls) (F=72.4, d.f.=1, P<0.0001) and the three age-groups (F=6.5, d.f.=2, P=0.002), but no significant interaction between diagnosis and age (F=1.7, d.f.=2, P=0.18). The reason for the lack of significant interaction is that the age effect was not linear, but was particularly pronounced in the oldest age-group (46-60 years). There was no drop in the RBMT score with age in the control group, but there was a drop in the oldest age-group in participants with schizophrenia.
Using one-way ANOVA, there was no significant difference in RBMT scores among the different age-groups of controls. However, there were significant differences among different age-groups in patients (F=4.686, d.f.=2, P=0.007). The post hoc Tukey honestly significant difference test showed significant differences between the youngest (18-30 years) and oldest (46-60 years) groups (P=0.012) and between the middle (31-45 years) and oldest age-groups (P=0.018), but no significant difference between the youngest and middle age-groups.
Illness duration and age at onset
For 67 patients we were able to obtain accurate information on the illness duration (mean 669 weeks, median 504, s.d.=546), length of stay (mean 186 days, median 75, s.d.=323) and age at onset (mean 27.2 years, median 26, s.d.=7.9). Using Pearson correlation, we found a significant inverse correlation between illness duration and RBMT screening score (r=-0.335, P=0.006). In contrast, there was no significant correlation between length of stay or age at onset and RBMT screening score.
Medication
Patients were on different antipsychotic medication regimens: 29 were taking atypical antipsychotic agents, 33 typical agents, 7 were taking both, and 4 were taking no antipsychotic at the time of the study. Twenty-seven were taking anticholinergic medication. Analysis of variance showed no significant effect for type of antipsychotic on the RBMT score; it also showed no significant difference on RBMT score between patients taking or not taking anticholinergic medication.
Symptom ratings
The total PANSS rating showed mild psychopathological disorder in 73 patients (mean 50.77, mode 42, range 30-84). Using Pearson correlation there was a significant inverse correlation between RBMT score and the negative sub-scale of the PANSS (r=-0.262, two-tailed, P=0.027). However, the correlation was not significant with the total score, general or positive sub-scales.
Occupation and HoNOS score
There was a significant inverse correlation between RBMT score and occupational groups (Spearman's rho=-0.332, two-tailed, P<0.001), i.e. the lower the score on the RBMT the higher the category of occupational group (1, managers and senior officials; 10, unemployed). This is to say that the lower a person scores on RBMT, the more likely that person would be to be unemployed.
We were able to obtain HoNOS scores for 58 patients. There was no significant correlation between RBMT score and the total HoNOS score. However, there was a significant correlation between the RBMT score and the functional impairment subscale (items 4 and 5) of the HoNOS (Pearson's r=-0.297, P=0.02).
DISCUSSION
We report a high prevalence of memory impairment (over 80%) in a population-based study of patients with schizophrenia. This is based on the screening score of the RBMT, where a score of less than 10 is considered to represent impaired memory. This is, to the best of our knowledge, the second population-based study of cognitive impairment in schizophrenia after that by Kelly et al (Reference Kelly, Sharkey and Morrison2000). Significantly, we were able to replicate their findings regarding memory impairment using the same test, but in a demographically different population.
The patients who took part in our study were relatively young and free from psychotic symptoms, living in the community and with no documented comorbidity. The exclusion criteria were also designed to avoid the participation of any patients disadvantaged in terms of age and language. Except for years in education, there was no significant demographic or clinical difference between the patients who took part in the study and those who declined. This suggests that participants might have better memory functioning than those who declined to take part in the study. Therefore, the prevalence of memory impairment reported would be a conservative estimate of its overall prevalence in schizophrenia when taking other confounding factors (clinical or demographic) into consideration. This is supported by the findings of Tamlyn et al (Reference Tamlyn, McKenna and Mortimer1992) who used the same test (RBMT) to examine their cohort; they reported a much higher prevalence of memory impairment in their subgroup of chronically ill and hospitalised patients, 27 out of 28 of whom scored in the impaired range.
The prevalence of schizophrenia in our study population (1.9 per 1000) is at the lower end of that expected (1.4-4.6 per 1000 population; Reference JablenskyJablensky, 2000). This could be explained by the demographic characteristics of the catchment area. As a suburban district, it is more likely to have a lower prevalence of psychotic disorders compared with city centres, which are associated with higher morbidity in general (Reference Mortensen, Pedersen and WestergaardMortensen et al, 1999). In addition, patients who develop schizophrenia might well migrate towards the city centre, especially when they need supported or hostel accommodation, which is most likely to be available in urban areas. This was particularly true for our study because patients who needed rehabilitation services and supported accommodation were moved outside the catchment area.
RBMTand schizophrenia
Our study suggests that the RBMT is a good clinical marker for memory impairment in schizophrenia. This is supported by previous use of the RBMT in studies of schizophrenia, which consistently showed that people with this disorder underperform on this test (Reference McKenna, Tamlyn and LundMcKenna et al, 1990; Reference Kelly, Sharkey and MorrisonKelly et al, 2000). Our study had the advantage, compared with previous studies, of the inclusion of a control group. This made it possible to examine the ability of the RBMT in discriminating between patients and controls. It is not common in psychiatric research to have an instrument with such a good ability (76%) to predict patient or control status. A similar ability (76%) was reported in previous work (Reference Palmer, Heaton and PaulsonPalmer et al, 1997); however, this involved a more demanding neuropsychological battery which is difficult to incorporate into everyday clinical practice, and furthermore lacked the specificity of everyday memory. Therefore, the RBMT has the potential to become an important tool in our clinical practice for the identification of memory impairment in schizophrenia, which may help predict functional outcome.
Specificity of memory impairment
The premorbid IQ reported for the patients in this study was much higher than that reported in previous studies. This is another indication that our sample can be considered among the less ill of patients with schizophrenia, making the memory impairment reported even more significant. The difference in premorbid IQ between patients and controls was small in clinical terms, but statistically significant. However, even after correcting for this difference in premorbid IQ, patients' performance on the RBMT was worse than that of controls. Therefore, the underperformance of patients on the RBMT, as a measure of working memory, cannot be explained as a symptom of generalised reduction of intellectual ability, but is rather a specific cognitive deficit. Furthermore, this deficit was not related to symptom rating, except for negative symptoms, or medication in clinically stable patients. This supports the view that memory impairment is a core element of the clinical presentation of schizophrenia.
The association between memory impairment and the negative symptoms sub-scale of the PANSS is an important replication of previous findings (Reference Berman, Veigner and MersonBerman et al, 1997). Conceptually, both denote the lack of a normally existing function. More importantly, this is further evidence that they may have a common underlying substrate (Reference Rossi, Mancini and StrattaRossi et al, 1997). This is an important contribution of neuropsychology towards better understanding of the underlying pathophysiology of schizophrenia.
Memory impairment and level of functioning
The association of memory impairment with occupational group provides further evidence for the importance of such impairment in schizophrenia. This echoes previous findings (Reference GreenGreen, 1996), which suggested an association between memory impairment and functional outcome. This would have important implications for the development of any intervention that involves the use of memory. First, it suggests that patients with such impairment might not benefit from interventions that require intact memory. Second, it might be necessary to include memory remediation programmes in rehabilitation services to improve level of functioning. Further validity for the RBMT comes from the significant correlation with the functional impairment sub-scale of the HoNOS. This finding echoes that previously reported by Kelly et al (Reference Kelly, Sharkey and Morrison2000), which reinforces the importance of memory impairment in influencing level of functioning in patients with mental illness.
Age and memory impairment
An interesting finding emerged when we divided the patient and control groups, separately, into three different age categories. The average RBMT scores for the controls were not significantly different across age-groups and remained within the normal memory category. In contrast, the patients' average RBMT scores remained within the impaired memory range across age-groups. In addition, there was a significant reduction in the average score for the oldest group of patients, which suggests that memory impairment as a subset of cognitive performance is compromised before the age of 60 years (cf. Reference Kelly, Sharkey and MorrisonKelly et al, 2000). We can conclude that memory decline might have a different course in schizophrenia compared with that in the general population and that older people with schizophrenia (aged 46-60 years) are significantly disadvantaged compared with younger people with this disorder.
The significance of the association between illness duration and memory impairment reported in this study raises important issues. Ostensibly, one can conclude that memory function in schizophrenia has a deteriorating course. However, it is important to examine the impact of potential mediating factors, such as the course of the illness, before such a conclusion can be drawn definitively. This is particularly important in the absence of clear neuropathological evidence to support a degenerative nature of the illness (Reference WoodsWoods, 1998). Therefore, what can be concluded from the result of this study is that longer illness duration might carry a higher risk of worsening memory impairment.
Finally, it is not known whether memory impairment which was identified by the RBMT is exclusive to schizophrenia or extends to other psychotic disorders. Bipolar affective disorders have also been associated with cognitive impairment, including memory impairment, during the acute phase of the illness as well as during euthymic periods (Reference Thompson, Gallagher and HughesThompson et al, 2005). However, a review by Reference Martinez-Aran, Vieta and ColomMartinez-Aran et al, 2000 suggested that during symptom remission cognitive dysfunction in patients with bipolar disorder is more likely to improve. In addition, relatives of patients with schizophrenia show cognitive deficits such as memory impairment, whereas relatives of patients with affective bipolar disorders do not show such impairment (Reference Keri, Kelemen and BenedekKeri et al, 2001). These findings suggest that cognitive dysfunction in general, and memory impairment in particular, may be a possible trait marker for schizophrenia to a greater degree than for bipolar affective disorders. Further research is needed to clarify this issue.
APPENDIX
Standard Occupational Classification 2000 (from the Office for National Statistics)
-
1 Managers and senior officials
-
2 Professional occupations
-
3 Associate professional and technical occupations
-
4 Administrative and secretarial occupations
-
5 Skilled trades occupations
-
6 Personal service occupations
-
7 Sales and customer service occupations
-
8 Process, plant and machine operatives
-
9 Elementary occupations
-
10 Unemployed
Acknowledgements
We thank Mr Nick Taub, from the Trent Institute for Health Service Research, for his statistical advice, and Ms Kate Martin for her assistance in data collection. An earlier version of this paper was presented at the British Psychopharmacology Association meeting in Harrogate in July 2002.









eLetters
No eLetters have been published for this article.