Relevance statement
Collaborative care integrates physical and mental healthcare. It is more effective than usual care for managing depression over the long term in people with coexisting mental and physical health conditions (mental–physical multimorbidity). This is the first long-term evaluation of collaborative care for managing mental–physical multimorbidity in a UK setting. Additionally, collaborative care is good value for money over the long term and has the potential to deliver important health gains at levels generally considered to be cost-effective.
Introduction
Multimorbidity refers to the presence of two or more long-term conditions, which can include combinations of physical and mental symptoms. Multimorbidity is a critical burden on health systems, associated with increased mortality, reduced quality of life and increased use of unscheduled care.Reference Dickens, Katon and Blakemore1, Reference Nunes, Flores, Mielke, Thumé and Facchini2 The greatest reductions in quality of life are experienced by those with mental–physical multimorbidityReference Mujica-Mota, Roberts and Abel3 which is associated with 45–65% higher costs of care for long-term conditions.Reference Thorpe, Ogden and Galactionova4 Collaborative care is a model of care for people with co-occurring mental and physical health conditions that recognises the interplay between the two. The most widely accepted current definition of collaborative care includes four key criteria: a multi-professional approach to patient care, a structured management plan, scheduled patient follow-ups and enhanced inter-professional communication.Reference Archer, Bower and Gilbody5 A key element is the appointment of a care manager who acts as a conduit between patients and healthcare professionals, and works with the patient to promote better patient self-care.Reference Coventry, Lovell and Dickens6 There is some evidence that compared with usual care, collaborative care is more effective for treating depression and anxiety over the short to medium term, with or without multimorbid long-term conditions,Reference Panagioti, Bower and Kontopantelis7 but effectiveness beyond 12 months remains uncertain. The UK National Institute for Health and Care Excellence (NICE) concluded that there is currently an absence of clinical or cost-effectiveness evidence for collaborative care in multimorbidity.8 In the context of interventions for long-term health conditions it is especially important to evaluate long-term clinical effectiveness and cost-effectiveness. This article reports the long-term (24-month) clinical and cost-effectiveness of collaborative care for people with depression, in the context of high levels of multimorbidity, as part of the Collaborative Interventions for Circulation and Depression (COINCIDE) trial.
Method
Trial design and participants
The COINCIDE trial evaluated the clinical effectiveness of collaborative care over a short-term period (4 months); collaborative care was associated with a significantly greater improvement in depression symptoms compared with usual care (effect size: 0.30).Reference Coventry, Lovell and Dickens9 The study protocol (trial registration number: ISRCTN80309252) has been published previously.Reference Coventry, Lovell and Dickens6, Reference Coventry, Lovell and Dickens10
The evaluation was a two-arm, cluster randomised, controlled trial in the north-west of England. Participating general practices held electronic registers of patients with diabetes and/or coronary heart disease and were assigned a unique identifier. A total of 459 general practices across the north-west of England were invited to participate and 39 were allocated in a phased approach across the region (9 in Merseyside, 19 in Greater Manchester and 8 in East Lancashire); three practices withdrew before participants were recruited. Designated staff based remotely at the Clinical Trials Unit of the Christie National Health Service (NHS) Foundation Trust (Manchester, UK) provided a central randomisation service. A computerised random number generator was used. The first six practices recruited were allocated at a ratio of 1:1 at random, to either the collaborative care or usual care arm. Subsequent practices were then allocated by minimisation (with a probability weighting of 0.8),Reference Brown, Thorpe, Hawkins and Brown11 based on the Index of Multiple Deprivation and practice list size. An email confirmation of allocation details was sent to the trial manager. Research staff were blind to the allocation of enrolled participants. Details of sample size calculations are reported in full elsewhere;Reference Coventry, Lovell and Dickens9 the trial had 79% power to detect an effect size of 0.4.
Staff from the National Institute for Health's Mental Health Research Network searched clinical databases at participating practices for patients with a record of diabetes and/or coronary heart disease. Patients who met these eligibility criteria were sent a postal invitation followed by a reminder letter 3 weeks later; non-responders to the reminder postal invitation were telephoned. Patients were screened for depressive symptoms (≥10 on the nine-item Patient Health Questionnaire; PHQ-9)Reference Kroenke, Spitzer and Williams12 by the research team over the telephone twice over 2 weeks. Patients who met these criteria for at least 2 weeks were eligible to participate. We excluded patients with psychosis, patients with type I or type II bipolar disorder, those who were currently experiencing suicidal thoughts, those in receipt of services for substance misuse and those receiving psychological therapy for depression from a mental health service. Full details of the trial design are reported in detail in the published protocol.Reference Coventry, Lovell and Dickens6, Reference Coventry, Lovell and Dickens10
Ethical approval
The National Research Ethics Service Committee North West – Preston (approval number NRES/11/NW/0742) granted ethical approval, and research governance approvals were granted by participating primary care trusts. Informed consent was obtained in writing from all participants before data collection.
Interventions
Participants attending general practitioner (GP) practices allocated to the collaborative care arm received up to eight face-to-face sessions of brief psychological therapy delivered by a case manager over 3 months.Reference Coventry, Lovell and Dickens6, Reference Coventry, Lovell and Dickens10 Case managers were ‘psychological well-being practitioners’ (PWPs) employed by Improving Access to Psychological Therapy (IAPT) services, given specific training in delivering the COINCIDE collaborative care model. The first session was expected to last for 45 min, during which the PWP identified links between participants' mood and management of their long-term conditions with the aim of formulating a problem statement. Subsequent treatment sessions were scheduled to last for 30–40 min and participants could choose to engage with behavioural activation, graded exposure, cognitive restructuring and/or lifestyle changes. A 10 min collaborative meeting (by telephone or in person) between the participant, PWP and a practice nurse from the participant's general practice was scheduled to take place during treatment session two and eight, to facilitate the integration of care. These collaborative meetings focused on ensuring that psychological treatments did not complicate current management, reviewing patients' progress, reviewing relevant physical and mental health outcomes and planning future care.Reference Coventry, Lovell and Dickens9 The final session also included education about relapse prevention strategies. PWPs were expected to liaise with the practice nurse and participants' GPs about medication and update on participant progress. Participants attending general practices allocated to the control arm received treatment as usual based on the NICE stepped care model, provided by the participants' GPs.13 This could include treatment of depression with medication and/or onward referral to psychological therapy. If participants in the usual care arm were referred to psychological therapy provided for by Improving Access to Psychological Therapy services, they did not receive such care from COINCIDE-trained PWPs.
Outcomes
The primary outcome was self-reported depression severity on the 13 depression items of the Symptom Check List-90 scale (SCL-D13; range: 0–4) 24 months after randomisation.Reference Derogatis and Cleary14 Data on healthcare utilisation were collected with a bespoke patient questionnaire. Health status was measured with the EuroQol 5D-5L (EQ-5D-5L).Reference Herdman, Gudex, Lloyd, Janssen, Kind and Parkin15 All baseline measures were collected face to face by research staff blind to allocation; self-reported outcome measures at 24 months were collected by postal questionnaires. Participants who did not return the 24-month questionnaire were contacted by telephone and given the opportunity to complete the primary outcome over the telephone with a researcher blind to allocation.
For economic analyses, we used the UK NHS and personal social services perspective in line with NICE guidance for economic evaluations of healthcare interventions.16 The time horizon for the economic evaluation was 24 months. Data on resource utilisation were collected at 4 months (covering the period between baseline and 4 months) and 24 months (covering the period between 4 and 24 months). Therefore, it was not possible to distinguish which visits occurred in the period between 4 and 12 months and which occurred between 12 and 24 months. For this reason, costs associated with healthcare utilisation beyond 12 months were not discounted. For comparability, outcomes were also not discounted.
Use of healthcare services was collected by asking participants to report their total number of visits to different healthcare professionals in these categories: in-patient admission, out-patient, day patient (non-overnight hospital admission), accident and emergency, and primary/ community care (e.g. GP, nurse, social worker). Direct costs were also estimated for the intervention. Data on the resources used to provide the intervention only were collected from activity logs completed by PWPs and practice nurses who delivered care to participants in the collaborative care arm. The costs of training PWPs were also included in the primary economic analysis. We based PWP costs on NHS Agenda for Change salary band four (current salary range: £19 217–22 458; US$24 271–28 364; €21 793–25 469) and included employer National Insurance and pension contributions plus capital, administrative and managerial costs. We calculated cost per hour using standard working time assumptions,17 weighted to account for time spent on non-patient-facing activities. For each type of resource, the cost was estimated as the quantity of that resource or service used multiplied by nationally applicable unit costs.17, 18 All direct costs are reported in British pounds (£) and inflated to 2015–2016 prices by the Hospital and Community Health Services Index.17
The primary measure of cost-effectiveness was cost per quality-adjusted life-year (QALY).16 Utility values were derived at each time point from the EQ-5D-5L and associated utility tariffs for the UK, generated from a series of time trade-off and discrete choice experiments.Reference Devlin, Shah, Feng, Mulhern and Van Hout19 The EQ-5D-3L crosswalk methodReference van Hout, Janssen and Feng20 was used to estimate utility values as a sensitivity analysis. A mean utility value was calculated for each set of two time points (baseline to 4 months; 4–24 months). The mean utility value and length of the respective time period (e.g. a utility value of 1 is equivalent to 0.5 QALYs if accrued over 6 months, or 1 QALY if accrued over 12 months) was used to generate two QALY values (one for each time period), which were combined to estimate the total QALYs during the whole follow-up period.
Statistical analysis
We used an intention-to-treat approach for all clinical and cost-effectiveness analyses as per the original data analysis plan shared with the Data Monitoring and Ethics Committee. All analyses were conducted with Stata (Release 13). The trial originally had 80% power (two-sided α = 0.05) to detect a difference between groups on the primary outcome at follow-up, equivalent to a standardised effect size of 0.4, for which we required 15 practices per arm and 15 patients per practice (n = 450), allowing for 20% attrition and an intra-practice correlation of 0.06. Average recruitment in the first 11 practices was <15 patients per practice. We therefore increased the total number of clusters from 30 to 36, with a target of 10 patients per practice, giving 79% power to detect an effect of 0.4 under the same assumptions. The revised target sample was therefore 360 patients.Reference Coventry, Lovell and Dickens10
To compare the change in depression scores between participants randomised to collaborative care or usual care, multiple linear regression with robust standard errors was used; this accounted for the clustering of patients within practices. The following baseline characteristics were controlled for: depression score, age, gender, area deprivation (based on residential postcode), level of limitation of daily activities at baseline, use of antidepressants or antianxiety drugs (currently, previously or never), GP practice list size and GP practice area deprivation (the latter two as ‘design factors’). Multiple imputation was used to estimate missing scale scores and other data values at both baseline and follow-up. Thirty imputed data-sets were generated with chained equations, including covariates as listed above. As part of the imputation model, missing SCL-D13 values were restricted to between 0 and 4, in line with the possible questionnaire responses. To assess sensitivity of the results to multiple imputation, we conducted secondary analyses on complete cases, first with the same covariates as the main analysis and second with only baseline depression score included as a covariate. A further post hoc sensitivity analysis was conducted without restricting the range of imputed SCL-D13 values.
Economic analysis
The base–case (primary) economic analysis calculated incremental cost-effectiveness ratios (ICERs); accordingly, no parametric statistical tests of differences in mean costs or outcomes were conducted. Net QALYs (collaborative care versus usual care) were estimated by a linear regression model, and net costs were estimated by a generalised linear regression model with a log link and gamma family to allow for the non-normal distribution of costs. Regression models were adjusted for baseline variables identified through stepwise regression, using the following measures: World Health Organization Quality of Life Instrument,Reference Skevington, Lotfy and O'Connell21 Generalised Anxiety Disorder seven-item Scale,Reference Spitzer, Kroenke, Williams and Löwe22 PHQ-9, Self-Efficacy Questionnaire,Reference Lorig, Stewart, Ritter, Gonzalez, Laurent and Lynch23 Health Education Impact Questionnaire,Reference Osborne, Elsworth and Whitfeld24 Sheehan Disability Scale,Reference Sheehan, Harnett-Sheehan and Raj25 employment status and mobility (EQ-5D-5L). Robust standard errors were used to account for the clustering of patients within practices. The estimates of incremental costs and outcomes from the regression were bootstrapped to simulate either 2000 or 10 000 pairs of net cost and net outcomes for a cost-effectiveness acceptability analysis, as recommended by NICE for health technology appraisals.16
Missing data on costs and EQ-5D-5L utility scores were imputed five times with a chained-equation procedure. Costs were imputed by category and utility by individual EQ-5D-5L domain rather than as totals, so that all available data were used to inform the imputed values. The pattern of available cost and utility data across the different assessments are summarised in supplementary Table 1, available at https://doi.org/10.1192/bjp.2018.70. For the 24-month assessment, 69% of the original sample had a cost recorded for at least one category of healthcare and 69% had responded to at least one item on the EQ-5D-5L. All available (complete and partial) cost and outcome data for a particular participant was used to impute missing data. The number of imputed data-sets was chosen for pragmatic reasons: it was felt that this represented a balance between robustness and the computational burden of conducting the bootstrapping procedure on imputed data.
Sensitivity analyses assessed the effect of design and analysis choices on the cost-effectiveness of collaborative care. These were an alternative method for utility/QALY estimation (crosswalk approach),Reference van Hout, Janssen and Feng20 a complete case analysis, an alternative measure of health benefit (proportion of participants showing a ‘response’ – 40% improvement from baseline in SCL-D13 score),Reference Gwirtsman, Blehar, McCullough, Kocsis and Prien26 excluding the cost of training the PWPs and number of bootstrap simulations.
Results
19 practices were randomised to collaborative care and 20 to usual care. We identified 387 patients with depression and heart disease and/or diabetes and invited them to a baseline assessment. Follow-up data on 350 (90% of original sample) participants were collected at 4 months between 18 November 2012 and 4 October 2013, and follow-up data on 272 participants (71% of original sample) were collected at 24 months between 18 May 2014 and 4 June 2015 (see supplementary material for the Consolidated Standards of Reporting Trials (CONSORT) diagram). Characteristics of participating practices and participants are reported in Table 1. The majority (63%) of participants at baseline met criteria for moderately severe or severe depression, and 75% of participants met criteria for anxiety. Participants reported a mean of 6.2 (s.d. = 3.2) long-term conditions in addition to either diabetes or coronary heart disease; 15% of participants had a diagnosis of both diabetes and coronary heart disease. The mean age was 58.5 years and 38% of participants were female. Around half (54%; n = 211) of participants lived in areas ranked as highly deprived (index of multiple deprivation score ≥30). A total of 25% of participants were in paid employment. Half of participants were prescribed antidepressant or antianxiety medication at baseline. Full details about the delivery of the intervention have been previously reported.Reference Coventry, Lovell and Dickens9
Table 1 Baseline characteristics of COINCIDE trial practices and participants

COINCIDE, Collaborative Interventions for Circulation and Depression; QOF, quality outcomes framework; CHD, coronary heart disease; IMD, Index of Multiple Deprivation; PHQ-9, Patient Health Questionnaire 9; GAD-7, Generalised Anxiety Disorder seven-item Scale.
a. Mean (s.d.).
b. Excluding diabetes and CHD.
For the primary outcome measure, depression scores were available at 24 months for 62% of participants allocated to the collaborative care arm and 74% allocated to usual care. The primary measure of health benefit for the economic evaluation was completed by 61% of collaborative care participants and 68% of usual care participants at 24 months. Across the categories of healthcare utilisation, 70–83% of participants had complete data at 4 months and 64–68% at 24 months. However, when combined into a total cost, 34% of the collaborative care group and 40% of the usual care had data for all of the categories at both time points. An additional summary of available cost and utility data is reported in supplementary Table 2.
The unadjusted difference in mean SCL-D13 scores between baseline and first follow-up showed an improvement in both groups (4-month improvement: collaborative care 0.61; usual care 0.31) (Table 2 and Figure 1a). When compared with baseline values, 24-month depression scores were again lower in both groups, with the greater improvement maintained in the collaborative care arm (24-month improvement: collaborative care 0.84; usual care 0.55).
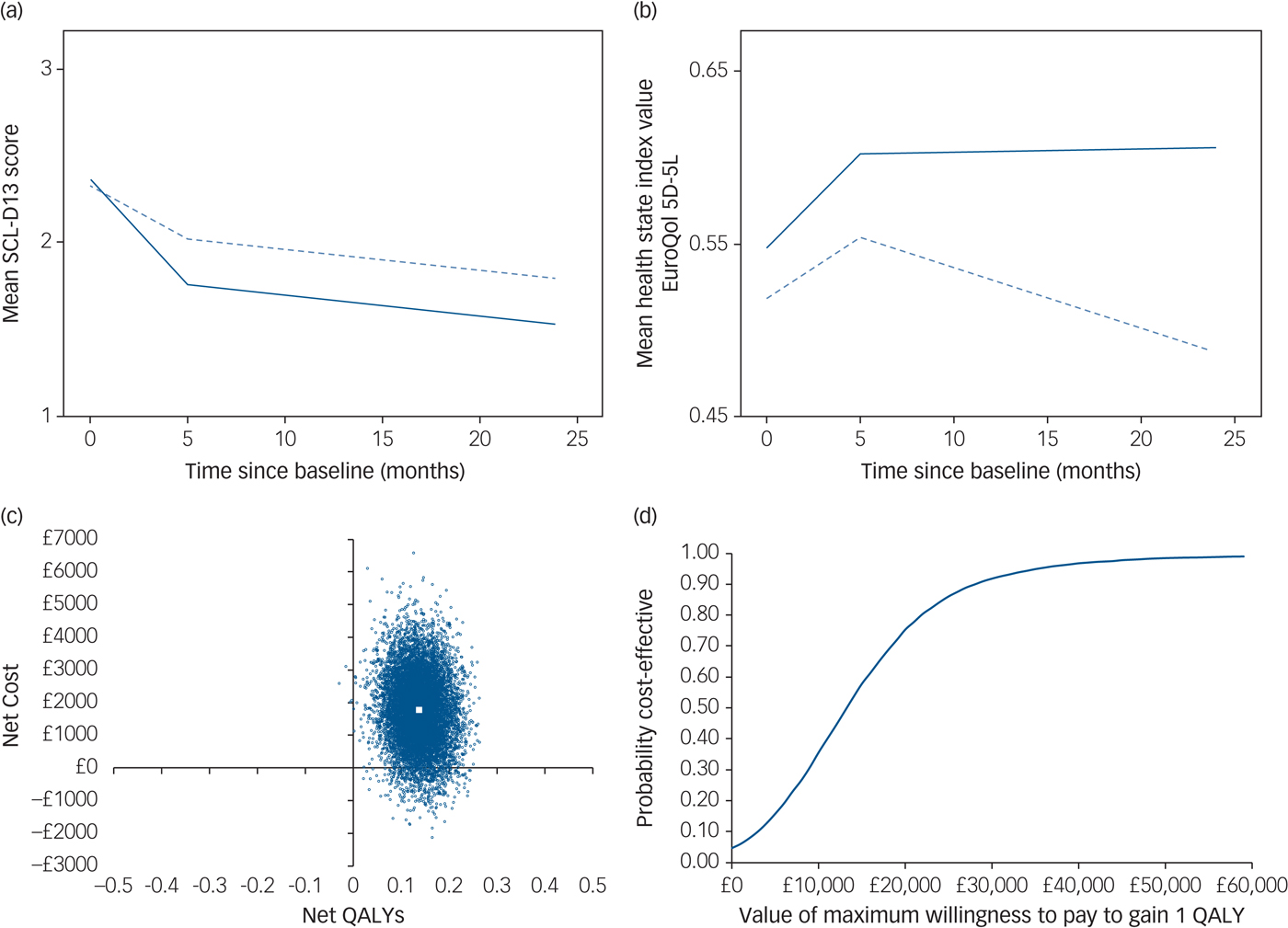
Fig. 1 Summary of clinical effectiveness and cost-effectiveness results.
(a) Mean Symptom Checklist-13 Depression Scale (SCL-D13) scores during follow-up by treatment group, unadjusted values (solid line represents collaborative care; dashed line represents usual care). (b) Mean health state index (EuroQol 5D-5L) scores during follow-up by treatment group, unadjusted values (solid line represents collaborative care; dashed line represents usual care). (c) Cost-effectiveness plane (primary analysis): distribution of 10 000 bootstrapped simulations of net cost and net quality-adjusted life-year (QALY) pairs (large white square indicates point estimate for incremental cost-effectiveness ratio). (d) Cost-effectiveness acceptability curve (primary analysis).
Table 2 Mean depression scores (SCL-D13) at all time points and change in depression scores between baseline and 24 months
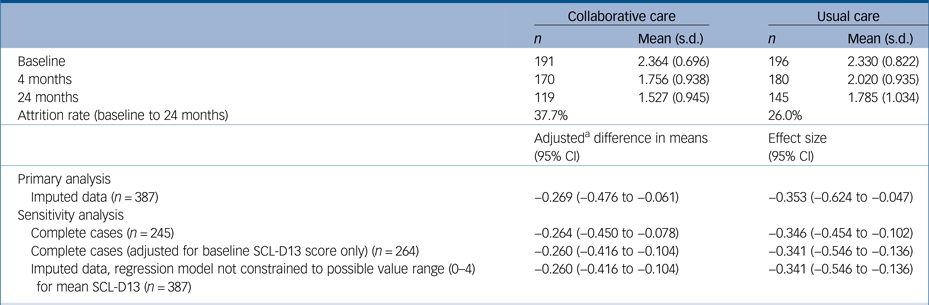
SCL-D13, Symptom Checklist-13 Depression Scale.
a. Adjusted for all following covariates unless otherwise indicated: age, gender, socioeconomic deprivation, limitation of daily activities owing to comorbidities, use of antidepressants or antianxiety drugs and general practitioner practice characteristics.
After adjustment for covariates, the mean SCL-D13 score at 24-month follow-up was 0.27 points lower (95% CI, −0.48 to −0.06; P = 0.014) in participants who received collaborative care compared with those who received usual care. This difference is equal to a standardised mean difference of −0.35 (95% CI, −0.62 to −0.05), using the baseline pooled s.d. for SCL-D13. Collaborative care was also found to be significantly more effective than usual care at 24 months in all sensitivity analyses, although there was very little difference in the estimated coefficients between models (Table 2).
Cost-effectiveness
The mean cost of the collaborative care intervention was £321, including a training cost of £130 per participant (total training costs divided by the total number of participants randomised to collaborative care irrespective of number of PWP sessions attended). For participants with complete cost data at all time points, the mean cost across all categories of healthcare was higher among those randomised to collaborative care; the cost of healthcare resources used was higher across all categories except day patient hospital visits and emergency department visits (see supplementary Table 3).
Unadjusted mean health state index scores show that the usual care group worsened overall between baseline and 24 months (Figure 1b; also see supplementary Table 3). The collaborative care group improved between baseline and first follow-up, which was sustained at the 24-month follow-up.
Regression analysis on multiple imputed data-sets also showed a higher net cost associated with collaborative care (compared with usual care); £1777 (95% CI, −£320 to £3875) over 24 months, although this difference is not statistically significant. Participants receiving collaborative care accrued significantly more QALYs over 24 months than those receiving usual care (0.136; 95% CI, 0.061 to 0.212). The bootstrapped estimates of net costs and QALYs are shown on a cost-effectiveness plane in Figure 1c. The simulations are predominantly located in the upper-right quadrant of the plane, indicating a net cost associated with collaborative care alongside a net health benefit (QALY gain). The points on the plane show more vertical than horizontal spread, illustrating that there is greater uncertainty around the estimated net cost than the estimated net benefit.
The cost per additional QALY gained from collaborative care (compared with usual care) is £13 069. The probability of collaborative care being cost-effective is 0.75 if decision makers are willing to pay £20 000 to gain one QALY. If decision makers are willing to pay £30 000 to gain one QALY, the probability that collaborative care is more cost-effective than usual care is 0.92. This is shown in the cost-effectiveness acceptability curve in Figure 1d.
There was little difference in the 95% confidence intervals generated from non-parametric bootstrapping 2000 or 10 000 pairs of net costs and benefits, and no effect on the cost-effectiveness recommendation (Table 3). When the crosswalk method was used for estimating utility values from the EQ-5D-5L, the net QALYs were slightly lower than for the time trade-off approach (0.118 v. 0.136), resulting in a slightly higher ICER (£15 063/QALY v. £13 069/QALY). When only participants with complete cost and QALY data were included in the analysis, net costs were higher and net QALYs lower than the primary (base–case) analysis. This resulted in an ICER of £38 032 per QALY at which collaborative care would be unlikely to be more cost-effective than usual care (probability 0.42 at a willingness-to-pay threshold of £30 000). When the measure of health benefit was the number of people showing a clinical response in terms of depression symptoms (40% reduction in total SCL-D13 scoreReference Gwirtsman, Blehar, McCullough, Kocsis and Prien26 between baseline and 24 months), there were 50 more ‘responders’ in the collaborative care group. Alongside the net cost of £1777, the cost per each additional responder was estimated to be £36. There is no guidance on how much decision makers are willing to pay for a treatment response, therefore it is not meaningful to calculate a probability of cost-effectiveness in terms of this outcome measure.
Table 3 Net costs and QALYs, ICER and probability collaborative care is cost-effective, using primary and sensitivity analyses, adjusted for baseline covariates, bootstrapped and imputed data (unless otherwise stated)
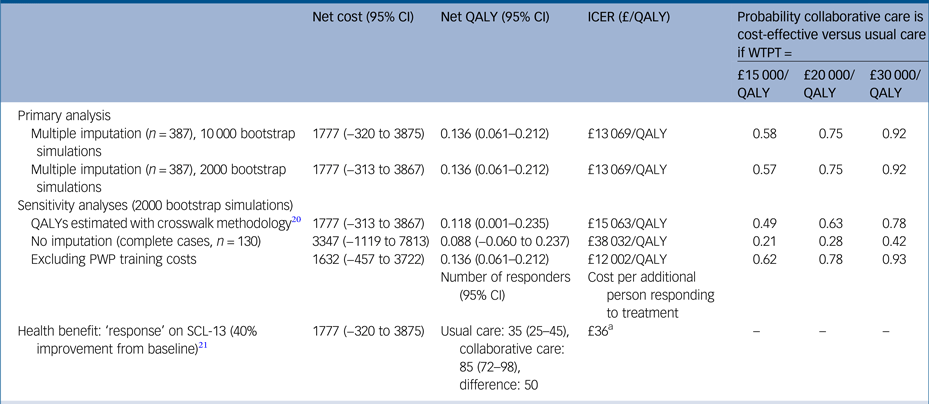
Covariates costs: baseline mobility (EuroQol-5D), general practitioner practice (cluster). Covariates QALYs: baseline scores for World Health Organization Quality of Life Instrument, Generalised Anxiety Disorder seven-item Scale, Patient Health Questionnaire 9, Self-Efficacy Questionnaire, Health Education Impact Questionnaire, Sheehan Disability Scale, employment status and general practitioner practice (cluster).
QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; WTPT, willingness-to-pay threshold; PWP, psychological well-being practitioner; SCL-D13, Symptom Checklist-13 Depression Scale.
a. Net cost (£1777) divided by the number of additional responders (50).
Discussion
Collaborative care for depression in the context of multimorbidity is clinically and cost-effective over the long term. Not only were the treatment effects of collaborative care maintained over 24 months, but they marginally exceeded those reported at 4 months (standardised mean difference 0.35 v. 0.30). Collaborative care was also associated with superior QALY gains compared with usual care (i.e. when both mental and physical domains of health are considered) in this context.
This is the first long-term evaluation of collaborative care for managing mental–physical multimorbidity in a UK primary care setting. The net QALY gain observed in the COINCIDE trial at 24 months was greater than that observed in a previous economic evaluation of collaborative care in UK primary care.Reference Green, Richards and Hill27 We previously reported results of an economic model estimating the long-term cost-effectiveness of collaborative care, based on data observed at 4 months in the COINCIDE trial.Reference Camacho, Ntais and Coventry28 The net QALY gain observed here from 24-month trial data (0.14) was similar to the modelled scenario, which assumed that the benefit of collaborative care accrued at 4 months would be maintained at the same level over 24 months (net QALY gain 0.15). Our findings here support this assumption; the mean utility values were similar at 4 and 24 months in the collaborative care group, although the ICER estimated from the model was somewhat lower than for the 24-month trial data (£3468/QALY (model) v. £13 069/QALY (trial)).
An important difference between the economic model and observed data is the estimated net cost. Net costs were notably higher using data observed within-trial, meaning that the additional healthcare resources used by participants randomised to collaborative care was greater than expected. Participants in the collaborative care arm used almost £1800 more of healthcare resources over 24 months (crudely, £900 over 12 months) than those receiving usual care. By comparison, in the Clinical and Cost Effectiveness of Collaborative Care for Depression in UK Primary Care Trial (CADET) the cost of healthcare resources over 12 months were almost identical between usual and collaborative care treatment groups.Reference Green, Richards and Hill27 The unexpectedly high resource use might partly be explained by participants in the collaborative care arm reporting improved navigation of health services and more engagement with health-directed behaviours, as evidenced by higher ratings on the Health Education Impact Questionnaire, compared with usual care.Reference Coventry, Lovell and Dickens9 This may have led to increased but more appropriate use of healthcare resources and thereby improved levels of both physical and mental functioning.
Strengths and limitations
The COINCIDE trial was a large, pragmatic trial conducted across a wide geographical area in the north-west of England that included a population with high levels of multimorbidity, disability and deprivation. This makes it particularly relevant to the management of mental–physical multimorbidity, which is more prevalent in younger adults from deprived regions.Reference Barnett, Mercer, Norbury, Watt, Wyke and Guthrie29 Cluster randomisation and analytic approaches for imputing data and adjusting for baseline characteristics offered greater opportunities to minimise bias and confounding. Additionally, the 24-month follow-up period is the longest time horizon used in an integrated economic and effectiveness evaluation of collaborative care outside the USA.
Our economic analysis conforms to the high standards of analysis and reporting expected of cost-effectiveness analyses of health interventions (see supplementary material for the Consolidated Health Economic Evaluation Reporting Standards checklist). However, data was not captured at baseline regarding use of health services before the intervention so it is not possible to determine or adjust for any underlying differences between the treatment groups.
Although multiple imputation of missing data reduced the potential for bias associated with missing data, the robustness of any imputation method declines as the level of missing data increases, reducing the validity and reliability of the analyses. Data from baseline and 24 months only were included in the clinical effectiveness analysis, and there was little difference in the change in depression score or effect size between the complete case analysis and the analysis after multiple imputation. The attrition rate was higher among participants in the collaborative care group (37.7%) compared with usual care (26.0%). We have explored key characteristics between participants with and without complete data (results not shown) and found only that in the usual care group, the mean age was higher (66 years) for those with incomplete data for the primary outcome measure than those with complete data (59 years). There were no other differences. Age was adjusted for in all analyses and included in the multiple imputation model, therefore this is unlikely to have unduly biased our results.
The economic evaluation included cost and utility data from baseline, 4 months and 24 months, and so there was greater scope for data to be missing. The proportion of participants with complete cost or utility data for all three time points (baseline, 4 months and 24 months) was lower than at the final time point (24 months) alone, suggesting that missing data items rather than loss to follow-up contributed to the level of missing data. Almost 70% of participants had complete cost or utility data; however, the proportion of participants with data for both was lower. As described in the Methods section, all available data were used to inform the imputed values and the role of multiple imputation can be thought of as filling in the blanks. A post hoc comparison of the characteristics of the participants with complete/incomplete economic data showed that only ethnicity was significantly different: there was a higher proportion of black and minority ethnic participants with complete data than incomplete data (see supplementary Table 2). This was surprising because for the participants who did not return the 24-month postal questionnaire, only English speakers were able to complete it over the telephone (because of limited resources). The cost-effectiveness results were somewhat sensitive to missing data. The direction of the effect on costs and QALYs were the same, but the cost to gain one QALY was higher in the complete case analysis.
Data on depression symptoms (SCL-D13), health status (EQ-5D-5L) and healthcare resource utilisation were self-reported by participants. Although pragmatic, self-reported methods are potentially prone to recall bias over a long-term follow-up. This is especially true in relation to capturing healthcare utilisation, particularly among those who use a large number of different healthcare services. However, use of costly services (e.g. an in-patient admission or major surgery) are notable events and likely to be recalled even over a long period.Reference Petrou, Murray, Cooper and Davidson30, Reference Bhandari and Wagner31 Furthermore, the intervention in this trial was not expected to affect recall of healthcare utilisation. Although total costs may be an underestimation, this is expected to be to the same extent in both groups and unlikely to influence the net cost. The second follow-up (at 24 months) had not been funded at the time of writing the original protocol for the COINCIDE trial, and so participants were only approached for second follow-up after the original study period had ended. If it had been possible to inform participants about the second follow-up earlier, the attrition rate may have been lower. However, a 68% retention rate over 24 months for the primary outcome measure is acceptable and, as described above, multiple imputation was used to minimise the effect of missing data. Additionally, we were only resourced to collect long-term follow-up data for the primary and economic outcomes, making it impossible to address questions about the long-term effect of the intervention on secondary outcomes evaluated in our short-term analysis.Reference Coventry, Lovell and Dickens9
Implications
A strong case for routinely using collaborative care as a framework of care for depression was made by the CADET trial.Reference Green, Richards and Hill27, Reference Richards, Hill and Gask32 We have shown that collaborative care is as effective in people with long-term conditions as it is in people with depression alone.Reference Panagioti, Bower and Kontopantelis7 However, current NICE guidance about the management of multimorbidity has excluded evidence from interventions that primarily target depression. The rationale for this was that any benefits for physical health may be an indirect effect of improvements in mood. Although the COINCIDE trial was designed as a depression intervention, it has been identified as an approach that can effectively integrate the mental and physical healthcare of people with multimorbidity in primary care.33 In the COINCIDE trial, collaborative care improved both depression and physical functioning in people with multimorbidity. Furthermore, collaborative care is also likely to be cost-effective over the long term. These findings address an evidence gap identified by NICE when developing their guideline/recommendations regarding the management of multimorbidity.8
In conclusion, the need for cost-effective interventions for multimorbidity is of paramount importance in high-, middle-, and low-income regions faced with the burden of managing ageing and/or deprived populations with complex health needs. Despite the limitations of this analysis, it can be concluded that collaborative care is clinically effective (with a moderate effect size) and cost-effective over the long term, as a treatment for people with depression in the context of multimorbidity. In addition to existing evidence, findings from the COINCIDE trial send a strong signal to clinicians and commissioners in primary care that collaborative care merits implementation to manage the effect of mental–physical multimorbidity over the long term.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.70.
Funding
This work was funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for Greater Manchester (NIHR CLAHRC-GM). The funder had no role in the identification, design, conduct, or reporting of this analysis. Views expressed in this article are those of the authors and not necessarily those of the NIHR, National Health Service, or the Department of Health.







eLetters
No eLetters have been published for this article.