Cannabis is the world's most popular illicit substance. Prevalence statistics suggest that, despite its illegal status, a greater number of 16- to 18-year-olds in the UK have smoked cannabis than have not. 1 Whereas cannabis dependence was a rare phenomenon even a decade ago, figures from the European Monitoring Centre for Drugs and Drug Abuse estimate that the numbers of people seeking treatment for dependence has increased markedly since 1999. 2 In 2004, cannabis was the main reason for referral to drug services in 15% of all cases, making it second only to heroin. Further evidence suggests that cannabis use is now associated with a greater incidence of psychosis, with earlier onset. Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones and Burke3 There is robust evidence of an acute cognitive impairment following cannabis use but findings of longer-term effects remain equivocal. Reference Gonzalez4 Although most cannabis users experience at least transient cognitive impairments, only a small minority develop psychosis or become dependent on the drug. This begs the question of what determines vulnerability to the harmful effects of cannabis. One critical factor may be the type of cannabis actually consumed.
Cannabis contains a myriad of different chemicals, around 70 of which are unique to the plant and called cannabinoids. The main psychoactive ingredient is Δ9-tetrahydrocannabinol (THC) and this produces the effects that users seek. Reference Curran, Brignell, Fletcher, Middleton and Henry5 When given intravenously to healthy humans, THC produces psychotic-like and anxiogenic effects. Reference D'Souza, Perry, MacDougall, Ammerman, Cooper and Wu6,Reference D'Souza, Ranganathan, Braley, Gueorguieva, Zimolo and Cooper7 In contrast, cannabidiol, another major constituent of cannabis, appears to have antipsychotic properties, Reference Zuardi, Crippa, Hallak, Moreira and Guimaraes8 is anxiolytic Reference Guimares, Chiaretti and Graeff9 and may be neuroprotective in humans. Reference Hermann, Sartorius, Welzel, Walter, Skopp and Ende10 The relative THC/cannabidiol ratio of cannabis varies greatly. Levels of cannabidiol can range from virtually none to up to 40%. Reference Hardwick and King11 Higher levels of THC are found in hydroponically grown varieties like skunk and in cross-bred strains that are increasingly common throughout Europe and beyond.
We have recently found evidence to suggest that use of strains richer in cannabidiol may protect cannabis users from the chronic psychotic-like effects of THC. Reference Morgan and Curran12 Given the opposing neuropharmacological actions of THC and cannabidiol – the former is a partial agonist whereas the latter is an antagonist at CB1 and CB2 receptors Reference Pertwee13 – we hypothesised that cannabidiol may also protect users against other harmful effects of the drug such as cognitive impairment and psychosis-like effects. The current study set out to test these hypotheses by employing a novel methodology that enabled analysis of cannabinoids in the cannabis actually smoked by each individual user.
Method
Design and participants
A repeated measures design compared a convenience sample of 134 cannabis users aged between 16 and 23 years on two test occasions approximately 7 days (and at least 5 days) apart. Inclusion criteria required that participants had English as a native language, were not dyslexic, had no history of psychotic illnesses and had normal or corrected-to-normal vision. Participants needed to have used the drug at least once a month for at least 1 year. They were recruited by word of mouth and ‘snowball sampling’. Reference Solowij, Hall and Lee14
All participants provided written, witnessed, informed consent on both occasions. This study was approved by the University College London Graduate School Ethics Committee and its aims were supported by the UK Home Office.
Procedure
All participants were tested on two separate occasions. One testing session occurred when the participant was under the influence of the drug (intoxicated day) and the other when they were drug free (not intoxicated day), with session order being counterbalanced. Participants were required to abstain from recreational drugs and alcohol for 24 h before testing commenced. They were also asked to abstain from cannabis for 24 h prior to testing and were told that this would be verified with the saliva samples. Participants were tested in their own or friends' homes on two occasions, separated by a minimum of 5 days. Participants informed the experimenter when they would next be using cannabis and when they would not be; two testing appointments were arranged accordingly. On the intoxicated day each participant smoked cannabis in front of the experimenter. They were asked to smoke at their usual inhalation rate and to smoke as much they would normally do to feel stoned. At this point, the testing began. A sample of the cannabis each participant smoked was taken on the intoxicated day and analysed for levels of THC and cannabidiol (Forensic Science Service, UK). Saliva samples were also collected 40 min after participants had smoked cannabis, again to assess levels of cannabinoids. Urine tests were administered on the drug-free day to confirm abstinence from other drugs (opiates, cocaine, amphetamine, benzodiazepines and other related compounds; THC remains detectable in the body for up to 4 weeks so the 24-h abstinence from cannabis use was not verifiable). Participants then completed the assessments described below, with test versions being balanced across the two testing days.
On the not intoxicated day, participants also completed the Severity of Dependence Scale, Reference Gossop, Darke, Griffiths, Hando, Powis and Hall15 a brief five-item questionnaire regarding their drug use; the Wechsler Adult Reading Test (WTAR) Reference Wechsler16 to estimate reading ability as an analogue of premorbid IQ; and they self-reported their drug use in a drug history questionnaire. The Schizotypal Personality Questionnaire (SPQ) Reference Raine17 was used to assess trait schizotypy or psychosis-proneness. The assessments reported here formed part of a wider test battery on which data collection is still underway. Following testing on the second occasion, participants were fully debriefed and compensated for their time.
Assessments on each testing day
Cognitive measures
Verbal memory was tested using Prose Recall. Reference Wilson, Cockburn and Baddeley18 Participants recalled a short prose passage immediately after hearing it and again after a delay filled with other assessments. To test verbal and category fluency participants were required to generate in 60 s as many words as possible: beginning with particular letters of the alphabet; and exemplars of particular categories. To test episodic memory, i.e. awareness of when and where a stimulus was encoded, a source memory Reference Wilding and Rugg19 test was used. Stimuli consisted of 80 low-frequency words that were divided randomly into two study lists of 40 words. In each study list half the words were spoken by a female voice and half by a male voice (allocation was randomly determined). All study words were presented to participants aurally; participants listened to each word, repeated it aloud and then, depending on the gender of the voice it is presented in, rated the word as either ‘pleasant/unpleasant’ or ‘abstract/concrete’. After a filled delay of 6 min, participants were given a recognition test list that combined the study list with the unpresented list. Participants said aloud whether each word was one that they had heard before and if so, whether it had been presented by a male or female voice.
Mood
A 100 mm visual analogue scale anchored ‘not at all anxious,’, ‘extremely anxious’ and ‘not at all stoned,’ ‘extremely stoned’ was administered.
Psychotic symptoms
A 48-item questionnaire, the Psychotomimetic States Inventory (PSI), Reference Mason, Morgan, Stefanovic and Curran20 was used to assess current schizotypal symptoms. It has been shown previously to be sensitive to acute cannabis-induced psychotomimetic effects. Reference Wilson, Cockburn and Baddeley18 Participants rate statements describing their current experience from 0 (not at all) to 3 (strongly). An abridged version of the Brief Psychiatric Rating Scale (BPRS), Reference Overall and Gorham21 in line with Krystal et al, Reference Krystal, Karper, Bennett, D'Souza, Abi-Dargham and Morrissey22 was used with selected items rated by the experimenters: four key Positive Symptoms, three key Negative Symptoms, three key Activation and six key Anxious Depression.
Type of cannabis
Participants were asked to label the cannabis they had smoked as being either skunk, herb or resin.
Statistical analyses
Because of the markedly uneven distribution of cannabinoids measured in the samples of cannabis actually smoked, in order to determine the impact of THC and cannabidiol the sample was divided into two groups according to the percentage of cannabidiol in the cannabis. The low-cannabidiol group (n = 22) comprised individuals whose samples had less than 0.14% cannabidiol and the high-cannabidiol group (n = 22) those whose samples had more than 0.75% cannabidiol (see Fig. 1). Using these two groups, memory and psychotic symptom data were subjected to a 2×2 repeated measures ANOVA with group (highest, lowest) as a between-individual factor and day (intoxicated, not intoxicated) as a within-individual factor. An additional factor of ‘delay’ (immediate, delayed) was added for Prose Recall data. Post hoc comparisons were Bonferroni corrected one-way ANOVAs to explore interactions, or Bonferroni comparisons to explore main effects. The WTAR scores were covaried in the analysis of memory measures. Mann–Whitney tests were used where data were not normally distributed. Signal detection analysis was used for the source memory data and d-prime, the index of discriminability between stimuli, was calculated as:
The criterion (C), an index of bias, was calculated as:
where Ht = hit and Fa = false alarms.
Results
Demographics and drug use
The whole sample comprised 98 males and 36 females, aged 20.64 years (s.d. = 2.02) with a mean of 14.60 years (s.d. = 2.15) in education and WTAR score of 42.63 (s.d. = 6.33). They used cannabis 13.8 days (s.d. = 11.18) per month.

Fig. 1 A scatterplot of the levels of Δ9-tetrahydrocannabinol (THC) and cannabidiol in each of the 134 cannabis samples collected in the study.
Cannabinoids
Over the whole sample, cannabidiol in the cannabis smoked positively correlated with salivary cannabidiol (r = 0.323, P = 0.002) and salivary THC-COOH (r = 0372, P<0.001). The THC in the cannabis smoked was not correlated with salivary THC, THC-OH, THC-COOH or cannabidiol.
Levels of cannabidiol and THC in the cannabis smoked by the 134 users are depicted in Fig. 1. As can be seen, cannabidiol was relatively scarce with only 22 samples showing levels above 0.75%. To determine the impact of cannabidiol on THC, these 22 individuals were compared with 22 whose samples showed the lowest levels of cannabidiol.
Highest v. lowest cannabidiol content
There were no differences in the THC content of the cannabis smoked by these two groups (Table 1). As expected, there was a highly significant difference in cannabidiol content (F(1,43) = 118.11, P<0.001) and in cannabidiol/THC ratios (F(1,43) = 111.98, P<0.001). Salivary levels on the intoxicated day showed a trend towards a group difference in cannabidiol (U = 248.5, P = 0.099) but no differences in levels of THC. No cannabinoids were found in any saliva sample from either group on the not intoxicated day. The high-cannabidiol group had higher WTAR scores than the low-cannabidiol group (F(1,42) = 5.00, P = 0.031) and as premorbid IQ can be associated with verbal memory, WTAR scores were covaried from all memory measures throughout the analysis. The low-cannabidiol group reported consuming more units of alcohol in a typical drinking session than the high-cannabidiol group (F(1,40) = 10.351, P = 0.003); no other group differences emerged.
Table 1 Means (s.d.) for demographic, cannabidiol and THC data across the low- and high-cannabidiol user groups
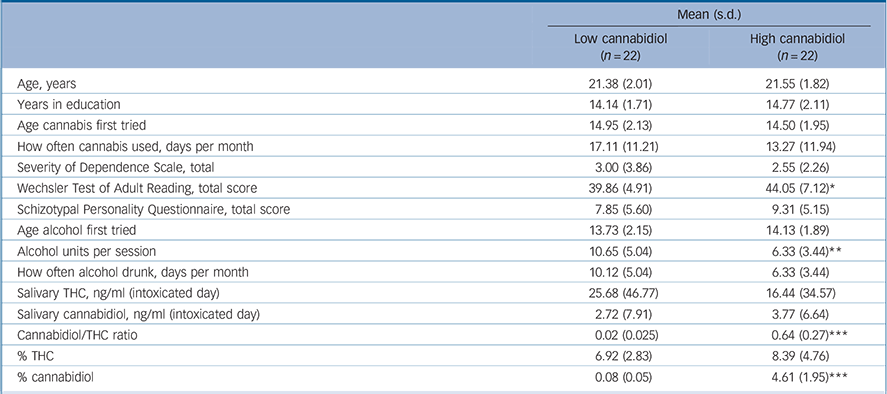
| Mean (s.d.) | ||
|---|---|---|
| Low cannabidiol (n = 22) | High cannabidiol (n = 22) | |
| Age, years | 21.38 (2.01) | 21.55 (1.82) |
| Years in education | 14.14 (1.71) | 14.77 (2.11) |
| Age cannabis first tried | 14.95 (2.13) | 14.50 (1.95) |
| How often cannabis used, days per month | 17.11 (11.21) | 13.27 (11.94) |
| Severity of Dependence Scale, total | 3.00 (3.86) | 2.55 (2.26) |
| Wechsler Test of Adult Reading, total score | 39.86 (4.91) | 44.05 (7.12)* |
| Schizotypal Personality Questionnaire, total score | 7.85 (5.60) | 9.31 (5.15) |
| Age alcohol first tried | 13.73 (2.15) | 14.13 (1.89) |
| Alcohol units per session | 10.65 (5.04) | 6.33 (3.44)** |
| How often alcohol drunk, days per month | 10.12 (5.04) | 6.33 (3.44) |
| Salivary THC, ng/ml (intoxicated day) | 25.68 (46.77) | 16.44 (34.57) |
| Salivary cannabidiol, ng/ml (intoxicated day) | 2.72 (7.91) | 3.77 (6.64) |
| Cannabidiol/THC ratio | 0.02 (0.025) | 0.64 (0.27)*** |
| % THC | 6.92 (2.83) | 8.39 (4.76) |
| % cannabidiol | 0.08 (0.05) | 4.61 (1.95)*** |
Cognitive measures
Prose Recall
A2×2×2 repeated measures ANOVA found a significant day× group interaction (F(1,40) = 4.19, P = 0.047) and a significant main effect of group (F(1,40) = 5.46, P = 0.025). Post hoc comparisons (Bonferroni corrected) revealed no differences on the not intoxicated day but significantly poorer performance by the low-cannabidiol group (compared with the high-cannabidiol group) when intoxicated on both immediate (P = 0.002) and delayed recall (P<0.001) (Fig. 2).
Source Memory
There were no main effects of group or interactions for the recognition or source memory data.
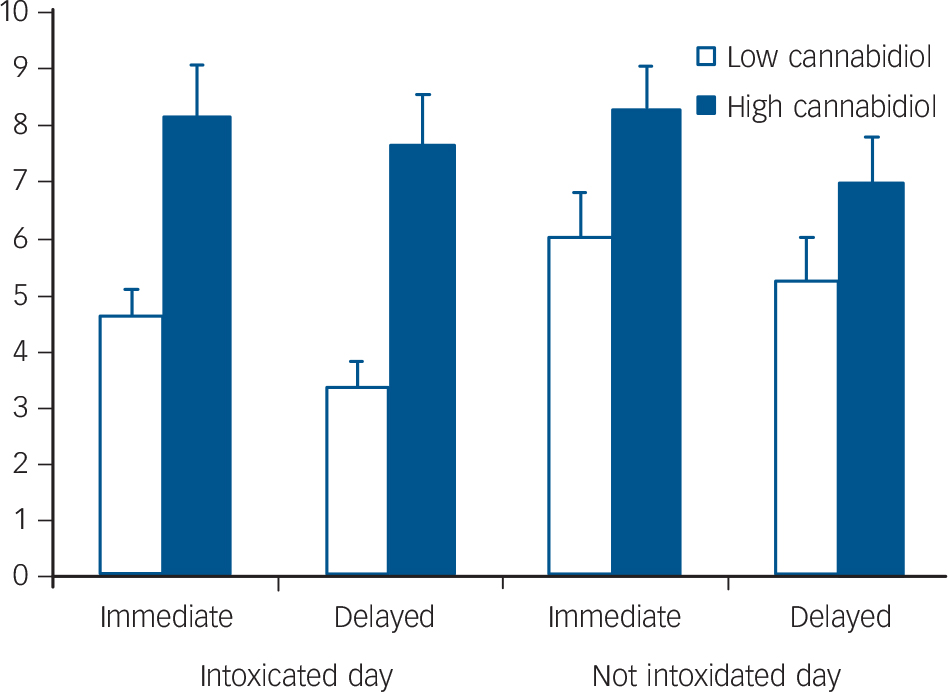
Fig. 2 Mean scores for immediate and delayed prose recall when intoxicated and not intoxicated in low- and high-cannabidiol groups.
Verbal and category fluency
A2×2 repeated measures ANOVA of verbal fluency data found a significant day×cannabidiol group interaction [F(1,40) = 7.46, P = 0.009]. Post hoc analyses demonstrated no significant differences. For category fluency data, the same analysis demonstrated no significant interactions or main effects (Table 2).
Table 2 Means (s.d.) for cognitive and self-rating data in the low- and high-cannabidiol user groups when intoxicated and not intoxicated with their own cannabisa

| Mean (s.d.) | ||||
|---|---|---|---|---|
| Low cannabidiol (n = 22) | High cannabidiol (n = 22) | |||
| Intoxicated | Not intoxicated | Intoxicated | Not intoxicated | |
| Verbal fluency | 16.05(4.92) | 14.05 (5.78) | 15.05 (4.34) | 16.91 (4.73) |
| Category fluency | 16.55 (4.08) | 16.10 (5.97) | 19.10 (7.30) | 17.68 (4.65) |
| Psychotomimetic States Inventory, total | 34.38 (19.99) | 18.57 (11.18) | 25.90 (15.16) | 18.19 (15.35) |
| Brief Psychiatric Rating Scale | 18.05 (3.40) | 17.19 (3.40) | 17.29 (4.65) | 16.00 (1.38) |
| ‘Stoned’ | 6.82 (2.30) | 1.62 (1.43) | 5.81 (1.87) | 1.46 (1.22) |
| Anxiety | 4.27 (2.78)* | 1.81 (1.25) | 2.76 (1.87)* | 1.29 (0.64) |
| Source Memory | ||||
| d' | 2.06 (0.84) | 2.22 (0.83) | 2.18 (0.85) | 2.32 (0.66) |
| C | 0.30 (0.39) | 0.52 (0.35) | 0.30 (0.31) | 0.68 (0.43) |
| Proportion correct | 0.58 (0.23) | 0.64 (0.23) | 0.67 (0.22) | 0.62 (0.24) |
Psychotic-like symptoms
Psychotomimetic States Inventory
A2×2 repeated measures ANOVA of PSI data demonstrated a significant main effect of day (F(1,40) = 13.82, P = 0.001), with greater scores on the intoxicated day in both groups but no other main effects or interactions (Table 2).
Brief Psychiatric Rating Scale
A2×2 repeated measures ANOVA of BPRS data showed no main effects or interactions.
Ratings of anxiety and ‘stoned’
Anxiety ratings showed a significant group difference across days (F(1,39) = 4.42, P = 0.042) and a significant main effect of day (F(1,39) = 31.19, P<0.001) but no interaction. The low-cannabidiol group had higher ratings of anxiety than the high-cannabidiol group; both groups rated higher anxiety on the intoxicated day. There was a main effect of day on the ratings of ‘stoned’ (F(1,40) = 173.1, P<0.001), reflecting intoxication but no significant group differences.
Correlations
There were no significant correlations between measures of cannabidiol/THC and prose recall.
Type of cannabis smoked
In the high-cannabidiol group, 18 participants classified their cannabis as resin and 4 as herbal. In the low-cannabidiol group, 1 classified it as herbal and 21 as skunk.
Discussion
The main findings of this study were acute deficits in recall of prose in individuals who had smoked cannabis containing a low percentage of cannabidiol. Higher levels of cannabidiol in cannabis appeared to protect against any memory impairment, as the high-cannabidiol group performed at the same level when they were acutely intoxicated as when they were sober.
THC, cannabidiol and cognition
Only participants who smoked cannabis low in cannabidiol content showed impairment in immediate and delayed prose recall when acutely intoxicated. The high- and low-cannabidiol groups did not differ in performance when drug free and thus this finding cannot be attributable to any pre-existing group differences. Episodic memory deficits following acute doses of THC administered in laboratory studies are very robust and most pronounced in delayed recall tasks, Reference D'Souza, Ranganathan, Braley, Gueorguieva, Zimolo and Cooper7,Reference D'Souza, Perry, MacDougall, Ammerman, Cooper and Wu23 as we found here in the low-cannabidiol group. Importantly, however, people in our study who smoked higher cannabidiol strains of cannabis did not show any acute deficit. Indeed their performance when intoxicated was virtually indistinguishable from that when drug free. Only one previous study in humans has assessed effects of cannabidiol on THC-induced memory impairments Reference Ilan, Gevins, Coleman, Elsohly and de Wit24 and it reported no interaction although it administered very much lower doses of THC and cannabidiol than were self-administered by our participants. Our findings are, however, consistent with recent research demonstrating the reversal of THC-induced memory deficits in rats by the CB1 receptor antagonist, rimonabant. Reference Wise, Thorpe and Lichtman25 They are also redolent of recent findings that oral THC and cannabidiol have opposite effects on activation in the striatum during verbal recall. Reference Bhattacharyya, Morrison, Fusar-Poli, Martin-Santos, Borgwardt and Winton-Brown26
The memory impairing effects of THC are thought to be attributable to the involvement of the CB1 receptor: Reference Ranganathan and D'Souza27 CB1 knockout mice have been shown to exhibit reduced forgetting on memory tasks Reference Marsicano, Wotjak, Azad, Bisogno, Rammes and Cascio28 and endogenous cannabinoids have been shown to enhance hippocampal long-term potentiation. Reference de Oliveira, de Oliveira, Camboim, Diehl, Genro and Lanziotti29 It has been suggested that similar to endocannabinoids, exogenous THC prevents or reduces consolidation of newly learned memories. Reference Ranganathan and D'Souza27 Cannabidiol, through its antagonism of THC activity, may reverse this anti-consolidation effect. To our knowledge, this is the first study in humans to demonstrate this effect and the findings are exciting as they suggest that cannabidiol may not only provide mechanistic insights into memory, but also possible therapeutic treatments for disorders involving memory deficits.
Prose recall has been found to be the best predictor of everyday memory performance Reference Watts and Cooper30 and so our findings are relevant to users’ daily functioning. No group differences emerged on the source memory task, which has less ecological validity and is more ‘artificial’ in requiring recognition of single words in lists. However, the memory impairment observed here in the low-cannabidiol group was not generalised across both tasks. Other cognitive effects observed in this study were indications of poorer fluency when the low-cannabidiol/THC group were not intoxicated. This may reflect the tendency towards increased anxiety in this group while not intoxicated, as anxiety can influence performance on fluency tasks. Reference Horwitz and McCaffrey31 The other possibilities are pre-existing group differences or, highly speculatively, chronic effects related to smoking different strains.
THC, cannabidiol and psychotic symptoms
In line with our previous findings, Reference Mason, Morgan, Dhiman, Patel, Parti and Patel32 smoking cannabis in a naturalistic setting reliably increased psychotic-like symptoms as indexed by the PSI. However, there were no group differences. One of our hypotheses – based on our previous findings from hair analysis in cannabis users Reference Hermann, Sartorius, Welzel, Walter, Skopp and Ende10 as well as laboratory research with cannabis-naive individuals and intravenous THC and cannabidiol Reference D'Souza, Perry, MacDougall, Ammerman, Cooper and Wu6 – was that acutely, cannabis containing higher levels of cannabidiol would produce less psychotomimetic effects than lower-cannabidiol cannabis. This was not the case. As this is a null finding, we will not dwell long on interpretation, but given the excitement generated by the potential antipsychotic properties of cannabidiol we feel it merits some consideration. One key reason for the absence of group differences may be that individuals in this study were all regular cannabis smokers, having started using at around 14–15 years of age and currently smoking every other day. They would be defined as ‘heavy’ smokers by criteria in other studies and heavy smokers have been shown to experience blunted psychotomimetic effects following THC. Reference D'Souza, Ranganathan, Braley, Gueorguieva, Zimolo and Cooper7 The ratio of cannabidiol to THC was also much lower than in the laboratory studies, where double the concentration of cannabidiol to THC has been given. Reference Zuardi, Crippa, Hallak, Moreira and Guimaraes8 In relation to our previous findings, it is possible that the potential protective effects of cannabidiol, at the lower doses in which it occurs in street cannabis, are cumulative over time rather than acute effects. Indeed, chronic neuroprotective-like effects have been observed in long-term cannabis users. Reference Hermann, Sartorius, Welzel, Walter, Skopp and Ende33 Di Forti et al Reference Di Forti, Morgan, Dazzan, Pariante, Mondelli and Marques34 recently reported that individuals with first-episode psychosis show a preference for using higher potency (skunk) types of cannabis. In the present study, there was no difference in psychosis-proneness (SPQ score) between users of skunk (low cannabidiol) and resin/herb (higher cannabidiol), suggesting that the preference seen in individuals in first episode is not seen in a non-patient group of recreational cannabis users.
Methodological issues
Cannabidiol in the sample correlated with metabolites of THC (THC-COOH). This may support suggestions that cannabidiol speeds the metabolism of THC. Salivary THC did not correlate with the percentage THC and cannabidiol in the cannabis over the whole sample. The absence of correlation between THC levels in cannabis samples and salivary levels of THC and its metabolites may also be tentatively interpreted as suggesting that users may titrate the dose of THC in order to achieve an ‘optimal’ subjective state, as has been suggested previously. Reference Cappell, Kucher and Webster35 However, the excretion of THC and cannabidiol into saliva is not well understood, therefore we are cautious of conclusions based on levels of cannabinoids in oral fluid.
This was a naturalistic study and, without giving out cannabis of different varieties – in effect, ‘supplying’ a drug – to be smoked, it could not employ double-blind procedures. On the other hand, a key strength of this study was that it objectively assessed cannabinoid content of whatever cannabis each participant actually chose to smoke in real life.
Implications
In summary, unlike the clear memory impairment of individuals who smoked cannabis low in cannabidiol, participants smoking cannabis high in cannabidiol showed no acute memory impairment in immediate or delayed prose recall. Cannabidiol content did not affect psychotomimetic symptoms, which were elevated in both groups when acutely intoxicated. The antagonistic (or perhaps inverse agonist) effects of cannabidiol at the CB1 receptor are likely to be responsible for its profile in smoked cannabis, attenuating the acute memory-impairing effects of THC.
The constituents of street cannabis have changed over the past 20 years with high THC, low-cannabidiol strains Reference Hardwick and King11 now dominating the market. Our findings suggest that this increases the cognitive harms to cannabis users. The research reported here also contributes to the growing body that suggests a range of potential therapeutic uses of cannabidiol, including the ability to modulate the acute amnestic effects of THC. Given the widespread use of cannabis across the globe, there are clear public health implications of this study. In terms of harm reduction, users should be made aware of the higher risk of memory impairment associated with smoking low-cannabidiol strains of cannabis like skunk and encouraged to use strains containing higher levels of cannabidiol.
Acknowledgements
The authors are grateful to the Home Office and the Forensic Science Service for their support of the study. We would also like to thank all the participants who donated their time and cannabis.







eLetters
No eLetters have been published for this article.