The Global Burden of Disease (GBD) study launched by the World Health Organization (WHO) in the 1990s aimed to provide a set of summary measures that would be comprehensive and provide information on disease and injury, including non-fatal health outcomes, to inform global priority-setting for health research and to inform international health policy and planning (Reference Murray and LopezMurray & Lopez, 1996). The study showed that unipolar depressive disorders place an enormous burden on society, and ranked as the fourth leading cause of burden among all diseases, accounting for 3.7% of total disability-adjusted life-years (DALYs), and were one of the leading causes of years lived with disability (YLD), accounting for 10.7% of total YLDs. In 2001, the WHO embarked on a new assessment of the Global Burden of Disease for the year 2000 (the GBD 2000 study) using new epidemiological estimates. The present paper reports the data, methods and results for the new estimates of depression burden for the year 2000.
METHOD
The three goals articulated for the GBD 1990 study remain central in the GBD 2000 study. These are: to decouple epidemiological assessment of the magnitude of health problems from advocacy by interest groups favouring particular health policies or interventions; to include, in international health policy debates, information on non-fatal health outcomes along with information on mortality; and to undertake the quantification of health problems into time-based units that can also be used in economic appraisal.
A major focus of the GBD 2000 project was to improve the comparability, validity and reliability of the descriptive epidemiology for mortality and non-fatal health outcomes attributed to various diseases, injuries and risk factors. The GBD 2000 working group identified the need to review the epidemiological estimates and disease models developed for neuropsychiatric disorders that had been used in the original GBD project's assessment of disability. As a consequence of this review process, major changes have been made in the GBD 2000 study in the conceptualisation of depression, and new epidemiological estimates have been used for estimating DALYs.
Case definition
The definitions of depressive episodes established by the ICD–10 were used (codes F 32, F 33) (World Health Organization, 1992). Major depressive episodes as defined in DSM–IV (American Psychiatric Association, 1994) and DSM–III–R (American Psychiatric Association, 1987) were considered alternative definitions, so that we would be able to use epidemiological data from survey studies employing either of these two standard psychiatric classification systems.
Regions
For geographic disaggregation of the GBD 2000, the six WHO regions of the world (i.e. Africa, Americas, Eastern Mediterranean, Europe, South-East Asia, Western Pacific) were further divided into 17 epidemiological subregions, based on levels of child (under 5 years) and adult (15–59 years) mortality for WHO member states. Five mortality strata were defined in terms of quintiles of the distribution of child and adult mortality (both genders combined). When these mortality strata are applied to the six WHO regions, they produce 14 mortality subregions. For the purposes of burden of disease epidemiological analyses, two of these regions were further subdivided: EurB into EurB1 and EurB2, the latter including the central Asian states; and WprB into WprB1 (mainly China), WprB2 (South-East Asian countries) and WprB3 (Pacific Islands). Additionally, some member states have been reclassified into subregions with similar epidemiological/geographical/ethnic patterns in order to maximise the epidemiological homogeneity of the subregions for the purposes of epidemiological analysis. A detailed table of these epidemiological subregions can be downloaded from the WHO website at http://www.who.int/whr/2003/en/member_states_182-184_en.pdf.
Years of life lost to mortality due to depression
The first analytical step in the GBD 2000 study was to estimate the age-specific death rates, by gender, for the GBD subregions for the year 2000. The number of deaths, by age and gender, provides an essential ‘envelope’ which constrains individual disease and injury estimates of deaths. Competing claims for the magnitude of deaths from various causes must be reconciled within this envelope. From the estimated age-specific mortality rates, life tables for the populations of the subregions can be derived using standard methods. The sources of mortality data for GBD 2000 estimates are described elsewhere (Reference Mathers, Stein and Ma FatMathers et al, 2002). Table 1 presents world deaths related to depression and other neuropsychiatric conditions by gender and cause for the year 2000 that were used for the estimation of years of life lost due to premature mortality (YLLs) as a result of depression in the GBD 2000 analysis.
Table 1 Global Burden of Disease 2000 study: world deaths related to neuropsychiatric conditions by gender and cause for the year 2000
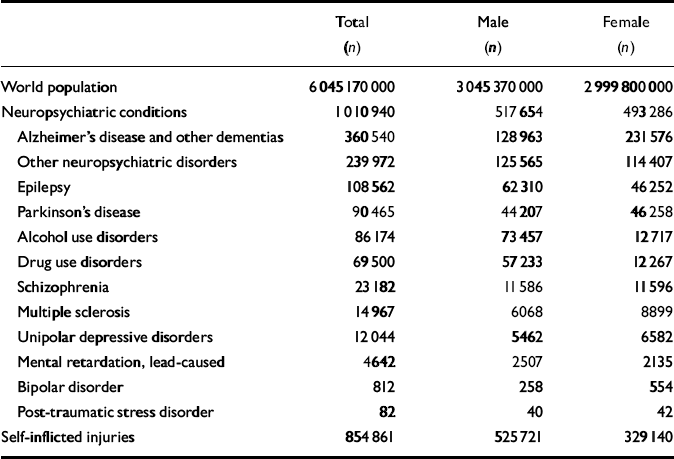
| Total (n) | Male (n) | Female (n) | |
|---|---|---|---|
| World population | 6 045 170 000 | 3 045 370 000 | 2 999 800 000 |
| Neuropsychiatric conditions | 1 0 10 940 | 517 654 | 493 286 |
| Alzheimer's disease and other dementias | 360 540 | 128 963 | 231 576 |
| Other neuropsychiatric disorders | 239 972 | 125 565 | 114 407 |
| Epilepsy | 108 562 | 62 310 | 46 252 |
| Parkinson's disease | 90 465 | 44 207 | 46 258 |
| Alcohol use disorders | 86 174 | 73 457 | 12 717 |
| Drug use disorders | 69 500 | 57 233 | 12 267 |
| Schizophrenia | 23 182 | 11 586 | 11 596 |
| Multiple sclerosis | 14 967 | 6068 | 8899 |
| Unipolar depressive disorders | 12 044 | 5462 | 6582 |
| Mental retardation, lead-caused | 4642 | 2507 | 2135 |
| Bipolar disorder | 812 | 258 | 554 |
| Post-traumatic stress disorder | 82 | 40 | 42 |
| Self-inflicted injuries | 854 861 | 525 721 | 329 140 |
Episode duration
The GBD 2000 study assumes a 6-month duration for episodes of depression. This figure is based on recent studies and is consistent with classical descriptions from the pre-antidepressant era. The same duration was used in GBD 1990 and the US Burden of Disease Study. The length of depressive episodes has been the subject of many investigations, most of which were carried out with inadequate methodologies. The duration of episodes had a log-normal distribution; therefore, the best way to report this variable is in terms of logarithmic values or non-parametric parameters, such as medians and quartiles. However, in the GBD project, the arithmetic mean has been used for the estimation of the YLD across all the conditions. Therefore, an effort was required to obtain information on duration of depressive episodes reported as an arithmetic mean, to be consistent with the methods in the GBD project.
Data gathered as part of more recent epidemiological studies were re-analysed to establish the mean duration of episodes in subjects with depression in the community. The first study was the Baltimore Epidemiologic Catchment Area Follow-up (Reference Eaton, Kramer and AnthonyEaton et al, 1989). In 1981, 3481 residents of Baltimore, from a probabilistic sample of 4238 residents, completed a psychiatric evaluation with the National Institute of Mental Health Diagnostic Interview Schedule (DIS). In 1993, these 3481 people were targeted for follow-up and interviewed again with the DIS. A specific analysis of this database was conducted to obtain estimates for the different parameters of the epidemiologic queuing formula: P=I×D, where P is the point prevalence, I is the incidence density and D is the mean duration of episodes. A total of 1725 respondents were interviewed and 536 episodes of depression were evaluated. The mean duration of episodes in weeks was 26.57 for both genders (male=25.7; female=26.85).
The second psychiatric database that was analysed to obtain estimates of the duration of episodes was the National Comorbidity Survey (Reference Kessler, McGonagle and ZhaoKessler et al, 1994). This survey did not include a longitudinal follow-up of cases identified in the community, but the diagnostic interview included questions related to the duration of lifetime episodes of DSM–III–R depression. For the purpose of this analysis, 3% of the subjects were excluded for having especially long durations. For the remaining 97% of the sample with episodes of depression, the overall mean duration was 22.6 weeks (Reference Üstün and KesslerÜstün & Kessler, 2002).
Finally, the NEMESIS study, a major epidemiological survey carried out in a national representative sample from The Netherlands, was also used to address the question of episode duration. This was a prospective study on the prevalence of psychiatric disorders in the Dutch population aged 18–64. A total of 7076 people were interviewed personally in 1996 with the Composite International Diagnostic Interview (CIDI), and re-interviewed later as part of an incidence study. The survival analysis of the 250 persons with newly originated episodes during the follow-up period found a mean duration of 8.4 months (Reference Spijker, de Graaf and BijlSpijker et al, 2002).
Prevalence estimates
We completed a systematic review of all available published and non-published papers of meaningful population studies on depressive disorders in order to create the most up-to-date data-set on the epidemiology of depressive disorders. Criteria for studies to be included in this review were as follows:
-
(a) population-based studies (with sample sizes of n > 1000);
-
(b) studies that reported prevalence (wherever possible, with a specification of the period covered: 2 weeks; 1 month; 6 months; 12 months; and lifetime); whenever possible, these data were converted into point prevalence;
-
(c) studies that reported incidence, preferably with specific age and gender distribution; from these studies data were regrouped into the eight age groups, as follows: 0–4; 5–14; 15–29; 30–44; 45–59; 60–69; 70–79; 80+ years (this was expanded from the original five age groups used in the GBD 1990);
-
(d) studies using a clearly specified method for sampling (a design that would yield a probabilistic national/regional representative sample) and implementation.
For depression, prevalence estimates were made by experts on the basis of published and unpublished studies. In addition, prevalence figures for ICD depressive episodes were derived from the CIDI interviews (World Health Organization, 1997) carried out as part of the WHO multicountry survey 2000–2001. This was a household survey carried out with the objective of assessing health states, disability and provision of health services in nationally representative samples (n=5000–10 000) of adults living in rural and urban areas of different WHO regions: China, Colombia, Egypt, Georgia, India, Indonesia, Mexico, Nigeria, the Slovak Republic and Turkey. Where no data for a region were available, experts on psychiatric epidemiology were encouraged to make informed estimates. Frequently, age patterns of incidence and prevalence were based on the assumption that some regions have similar epidemiological patterns but might differ in the level of incidence or prevalence. In the worst cases, where no information whatsoever was available, estimates were based exclusively on data or information from other regions. Table 2 presents a summary of the data sources and assumptions regarding the estimates for depressive disorders for all the WHO regions used in the burden calculations. Table 3 features age-standardised prevalence rate estimates for depressive episodes in the WHO epidemiological subregions. Comorbidity is common with psychiatric conditions, especially between anxiety and depressive disorders. For comorbidity between depression and anxiety disorders, comorbid states were attributed to the depressive illness – more severe of the two conditions in terms of the disability weight assigned to the condition. The GBD estimates of anxiety disorder prevalence, incidence and severity, therefore, reflect no comorbidity with depression. This approach translates to lower burden estimates for anxiety disorders.
Table 2 Summary of data sources and assumptions by World Health Organization epidemiological subregion within Africa (AFRO), the Americas (AMRO), the Eastern Mediterranean (EMRO), Europe (EURO), South-East Asia (SEARO) and the Western Pacific (WPRO)
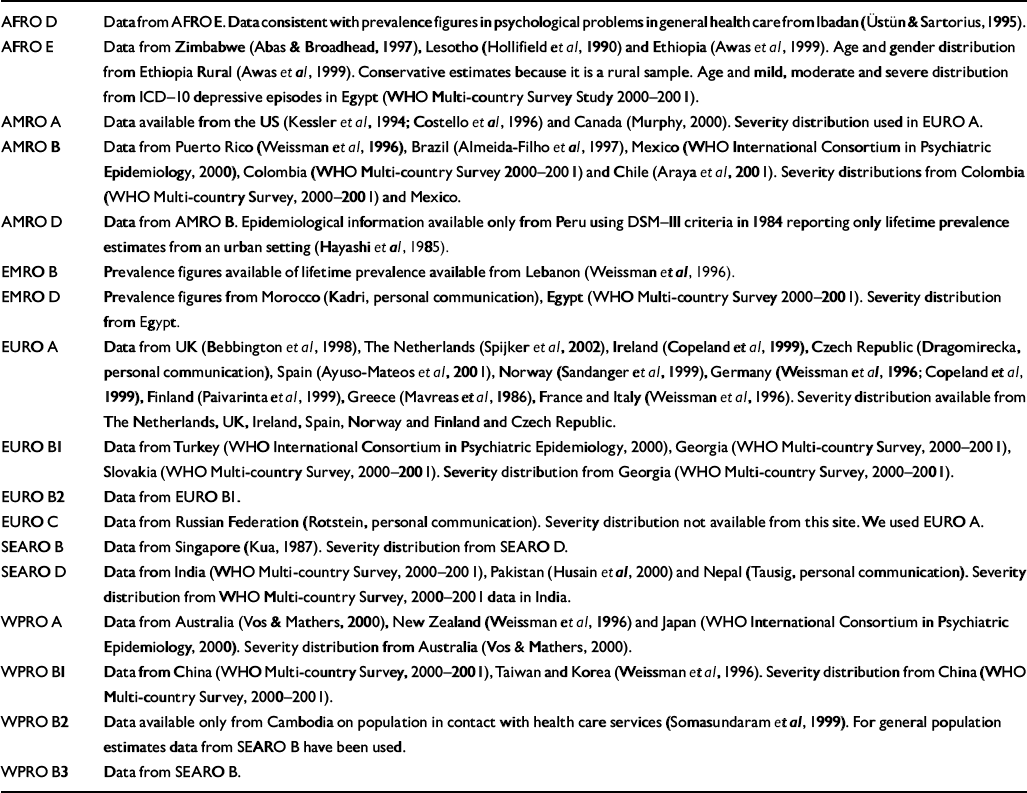
| AFRO D | Data from AFRO E. Data consistent with prevalence figures in psychological problems in general health care from Ibadan (Reference Üstün and SartoriusÜstün & Sartorius, 1995). |
| AFRO E | Data from Zimbabwe (Reference Abas and BroadheadAbas & Broadhead, 1997), Lesotho (Reference Hollifield, Katon and SpainHollifield et al, 1990) and Ethiopia (Reference Awas, Kebede and AlemAwas et al, 1999). Age and gender distribution from Ethiopia Rural (Reference Awas, Kebede and AlemAwas et al, 1999). Conservative estimates because it is a rural sample. Age and mild, moderate and severe distribution from ICD—10 depressive episodes in Egypt (WHO Multi-country Survey Study 2000—2001). |
| AMRO A | Data available from the US (Reference Kessler, McGonagle and ZhaoKessler et al, 1994; Reference Costello, Angold and BurnsCostello et al, 1996) and Canada (Reference MurphyMurphy, 2000). Severity distribution used in EURO A. |
| AMRO B | Data from Puerto Rico (Reference Weissman, Bland and CaninoWeissman et al, 1996), Brazil (Reference Almeida-Filho, de Mari and CoutinhoAlmeida-Filho et al, 1997), Mexico (WHO International Consortium in Psychiatric Epidemiology, 2000), Colombia (WHO Multi-country Survey 2000—2001) and Chile (Reference Araya, Rojas and FritschAraya et al, 2001). Severity distributions from Colombia (WHO Multi-country Survey, 2000—2001) and Mexico. |
| AMRO D | Data from AMRO B. Epidemiological information available only from Peru using DSM—III criteria in 1984 reporting only lifetime prevalence estimates from an urban setting (Reference Hayashi, Perales and SogiHayashi et al, 1985). |
| EMRO B | Prevalence figures available of lifetime prevalence available from Lebanon (Reference Weissman, Bland and CaninoWeissman et al, 1996). |
| EMRO D | Prevalence figures from Morocco (Kadri, personal communication), Egypt (WHO Multi-country Survey 2000—2001). Severity distribution from Egypt. |
| EURO A | Data from UK (Reference Bebbington, Dunn and JenkinsBebbington et al, 1998), The Netherlands (Reference Spijker, de Graaf and BijlSpijker et al, 2002), Ireland (Reference Copeland, Beekman and DeweyCopeland et al, 1999), Czech Republic (Dragomirecka, personal communication), Spain (Reference Ayuso-Mateos, Vázquez-Barquero and DowrickAyuso-Mateos et al, 2001), Norway (Reference Sandanger, Nygard and IngebrigtsenSandanger et al, 1999), Germany (Reference Weissman, Bland and CaninoWeissman et al, 1996; Reference Copeland, Beekman and DeweyCopeland et al, 1999), Finland (Reference Paivarinta, Verkkoniemi and NiinistoPaivarinta et al, 1999), Greece (Reference Mavreas, Beis and MouyiasMavreas et al, 1986), France and Italy (Reference Weissman, Bland and CaninoWeissman et al, 1996). Severity distribution available from The Netherlands, UK, Ireland, Spain, Norway and Finland and Czech Republic. |
| EURO B1 | Data from Turkey (WHO International Consortium in Psychiatric Epidemiology, 2000), Georgia (WHO Multi-country Survey, 2000—2001), Slovakia (WHO Multi-country Survey, 2000—2001). Severity distribution from Georgia (WHO Multi-country Survey, 2000—2001). |
| EURO B2 | Data from EURO B1. |
| EURO C | Data from Russian Federation (Rotstein, personal communication). Severity distribution not available from this site. We used EURO A. |
| SEARO B | Data from Singapore (Reference KuaKua, 1987). Severity distribution from SEARO D. |
| SEARO D | Data from India (WHO Multi-country Survey, 2000—2001), Pakistan (Reference Husain, Creed and TomensonHusain et al, 2000) and Nepal (Tausig, personal communication). Severity distribution from WHO Multi-country Survey, 2000—2001 data in India. |
| WPRO A | Data from Australia (Reference Vos and MathersVos & Mathers, 2000), New Zealand (Reference Weissman, Bland and CaninoWeissman et al, 1996) and Japan (WHO International Consortium in Psychiatric Epidemiology, 2000). Severity distribution from Australia (Reference Vos and MathersVos & Mathers, 2000). |
| WPRO B1 | Data from China (WHO Multi-country Survey, 2000—2001), Taiwan and Korea (Reference Weissman, Bland and CaninoWeissman et al, 1996). Severity distribution from China (WHO Multi-country Survey, 2000—2001). |
| WPRO B2 | Data available only from Cambodia on population in contact with health care services (Reference Somasundaram and van de PutSomasundaram et al, 1999). For general population estimates data from SEARO B have been used. |
| WPRO B3 | Data from SEARO B. |
Table 3 Age-standardised incidence and prevalence rate estimates for unipolar depressive disorders in World Health Organization epidemiological subregions, 2000
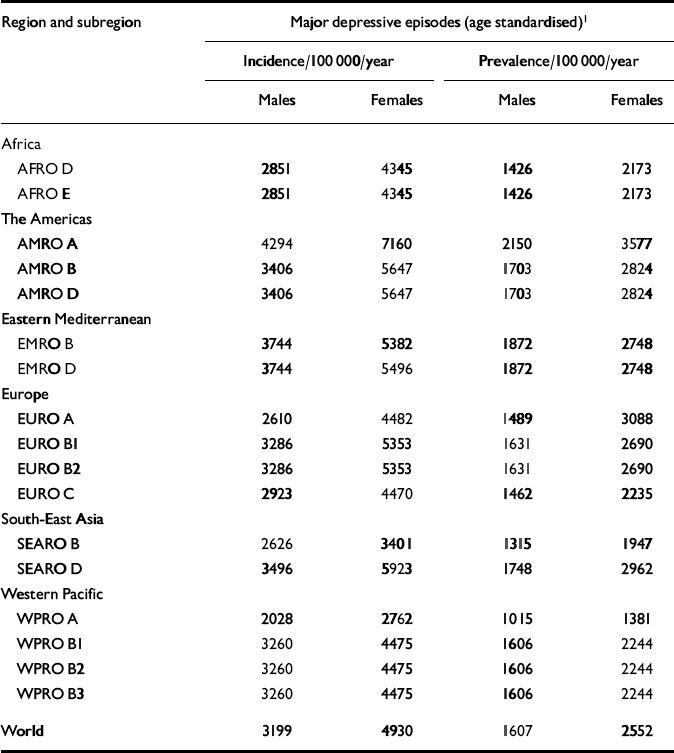
| Region and subregion | Major depressive episodes (age standardised)1 | |||
|---|---|---|---|---|
| Incidence/100 000/year | Prevalence/100 000/year | |||
| Males | Females | Males | Females | |
| Africa | ||||
| AFRO D | 2851 | 4345 | 1426 | 2173 |
| AFRO E | 2851 | 4345 | 1426 | 2173 |
| The Americas | ||||
| AMRO A | 4294 | 7160 | 2150 | 3577 |
| AMRO B | 3406 | 5647 | 1703 | 2824 |
| AMRO D | 3406 | 5647 | 1703 | 2824 |
| Eastern Mediterranean | ||||
| EMRO B | 3744 | 5382 | 1872 | 2748 |
| EMRO D | 3744 | 5496 | 1872 | 2748 |
| Europe | ||||
| EURO A | 2610 | 4482 | 1489 | 3088 |
| EURO B1 | 3286 | 5353 | 1631 | 2690 |
| EURO B2 | 3286 | 5353 | 1631 | 2690 |
| EURO C | 2923 | 4470 | 1462 | 2235 |
| South-East Asia | ||||
| SEARO B | 2626 | 3401 | 1315 | 1947 |
| SEARO D | 3496 | 5923 | 1748 | 2962 |
| Western Pacific | ||||
| WPRO A | 2028 | 2762 | 1015 | 1381 |
| WPRO B1 | 3260 | 4475 | 1606 | 2244 |
| WPRO B2 | 3260 | 4475 | 1606 | 2244 |
| WPRO B3 | 3260 | 4475 | 1606 | 2244 |
| World | 3199 | 4930 | 1607 | 2552 |
Incidence estimates
For the epidemiology of depression, the primary sources of information are cross-sectional population prevalence surveys. Incidence rates need to be estimated based on these observed prevalence rates. Other sources of information, such as small-scale follow-up studies, could be useful in suggesting a credible range of incidence.
In GBD 2000, incidence estimates for depressive episodes were derived from prevalence and duration with the epidemiologic queuing formula P=I×D. The incidence estimates of depressive episodes used are presented in Table 3, broken down by gender. We compared these theoretical incidence estimates with the published results of the few studies that provide data on the incidence of depressive disorders in community samples. Murphy (Reference Murphy2000) reviewed this issue and found an interval effect which has a major impact on the final incidence figures found in follow-up studies. Short-interval studies, such as the Epidemiologic Catchment Area follow-up study (Reference Eaton, Kramer and AnthonyEaton et al, 1989), found lower annual incidence rates than the long-interval studies, such as the Stirling County study (Reference MurphyMurphy, 2000). We considered it more appropriate to use short-interval studies in order to check the consistency of our epidemiological estimates. The incidence figure of depressive episodes will be closer to the incidence of depressive disorder obtained in short-interval studies than that obtained in long-interval studies.
Disability weights and severity breakdown
The YLD estimates of the GBD 2000 are based largely on the GBD 1990 disability weights or on the Dutch disability weights (Reference Stouthard, Essink-Bot and BonselStouthard et al, 1997). Three different severity levels of depressive episodes have been considered in the disease model used for the estimation of the burden of depression: mild, moderate and severe, with a disability weight of 0.14, 0.35 and 0.76, respectively.
RESULTS
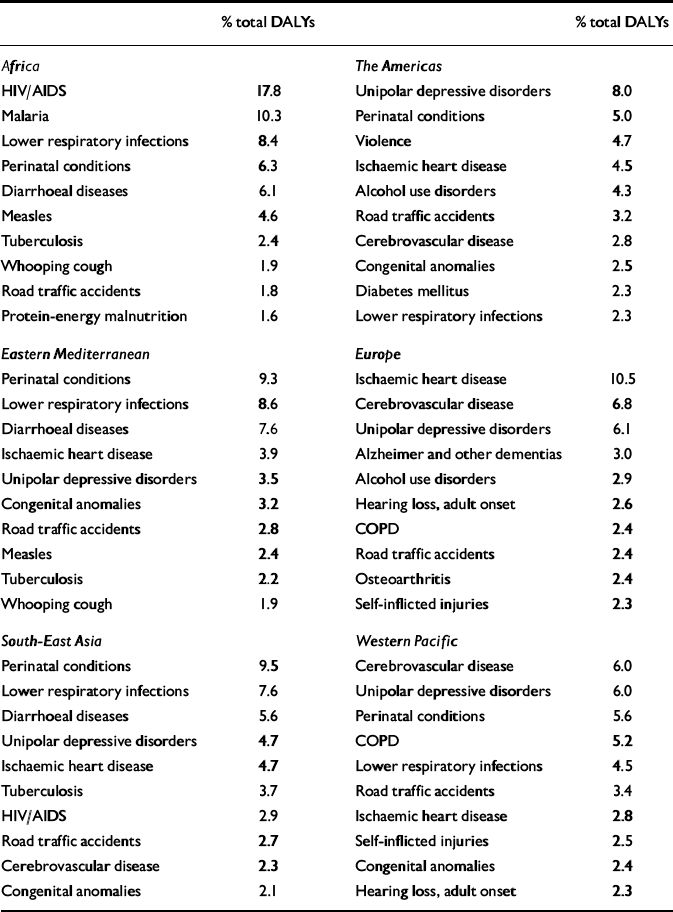
Depressive disorders were estimated to be the leading cause of disability (i.e. non-fatal burden) in the world in 1990, accounting for 10.7% of total YLD. Correspondingly, these disorders were the fourth leading cause of total global burden of disease, accounting for 3.7% of total DALYs (Reference Murray and LopezMurray & Lopez, 1996). A decade later, according to these revised estimates for the GBD 2000 study (World Health Organization, 2002), unipolar depressive disorders remain one of the leading causes of total DALYs worldwide (Table 4). Globally, they account for 4.46% of total DALYs and for 12.1% of total YLDs. Perinatal conditions, lower respiratory infections, HIV/AIDS and unipolar depressive disorders are the four leading causes of DALYs for men and women combined. The total DALYs for perinatal conditions, lower respiratory infections and HIV/AIDS are similar in magnitude for men and women. A more important gender difference is for depression, which is the fourth leading cause of disease burden in women but ranks seventh for men (5.6% v. 3.4% of total DALYs, respectively). Detailed tables for deaths, YLLs, YLDs and DALYs by subregion, cause, gender and age group can also be downloaded from the WHO website at http://www.who.int/evidence/bod.
Table 4 Leading causes of disability-adjusted life-years (DALYs) in World Health Organization (WHO) regions, estimates for 2000
| % total DALYs | % total DALYs | ||
|---|---|---|---|
| Africa | The Americas | ||
| HIV/AIDS | 17.8 | Unipolar depressive disorders | 8.0 |
| Malaria | 10.3 | Perinatal conditions | 5.0 |
| Lower respiratory infections | 8.4 | Violence | 4.7 |
| Perinatal conditions | 6.3 | Ischaemic heart disease | 4.5 |
| Diarrhoeal diseases | 6.1 | Alcohol use disorders | 4.3 |
| Measles | 4.6 | Road traffic accidents | 3.2 |
| Tuberculosis | 2.4 | Cerebrovascular disease | 2.8 |
| Whooping cough | 1.9 | Congenital anomalies | 2.5 |
| Road traffic accidents | 1.8 | Diabetes mellitus | 2.3 |
| Protein-energy malnutrition | 1.6 | Lower respiratory infections | 2.3 |
| Eastern Mediterranean | Europe | ||
| Perinatal conditions | 9.3 | Ischaemic heart disease | 10.5 |
| Lower respiratory infections | 8.6 | Cerebrovascular disease | 6.8 |
| Diarrhoeal diseases | 7.6 | Unipolar depressive disorders | 6.1 |
| Ischaemic heart disease | 3.9 | Alzheimer and other dementias | 3.0 |
| Unipolar depressive disorders | 3.5 | Alcohol use disorders | 2.9 |
| Congenital anomalies | 3.2 | Hearing loss, adult onset | 2.6 |
| Road traffic accidents | 2.8 | COPD | 2.4 |
| Measles | 2.4 | Road traffic accidents | 2.4 |
| Tuberculosis | 2.2 | Osteoarthritis | 2.4 |
| Whooping cough | 1.9 | Self-inflicted injuries | 2.3 |
| South-East Asia | Western Pacific | ||
| Perinatal conditions | 9.5 | Cerebrovascular disease | 6.0 |
| Lower respiratory infections | 7.6 | Unipolar depressive disorders | 6.0 |
| Diarrhoeal diseases | 5.6 | Perinatal conditions | 5.6 |
| Unipolar depressive disorders | 4.7 | COPD | 5.2 |
| Ischaemic heart disease | 4.7 | Lower respiratory infections | 4.5 |
| Tuberculosis | 3.7 | Road traffic accidents | 3.4 |
| HIV/AIDS | 2.9 | Ischaemic heart disease | 2.8 |
| Road traffic accidents | 2.7 | Self-inflicted injuries | 2.5 |
| Cerebrovascular disease | 2.3 | Congenital anomalies | 2.4 |
| Congenital anomalies | 2.1 | Hearing loss, adult onset | 2.3 |
There is a marked contrast in the epidemiological patterns between rich and poor regions of the world. Thus, in the more developed countries, the share of disease burden from communicable, maternal, perinatal and nutritional conditions is typically around 5%, compared with 70–75% in Africa. The contribution of depression to the total disease burden in Africa in 2000 was 1.2%, ranking in 13th position; in the Americas it was the leading cause, representing 8% of the total burden. Overall, in high-income countries the burden of depressive disorders was 8.9%, whereas in middle- and low-income countries the burden was 4.1% of the total DALYs.
DISCUSSION
The present paper has summarised the methods and data sources used for the estimation of the burden of depressive episodes within the Global Burden of Disease 2000 project. It documents the bases for the burden estimates published in the World Health Report 2002, and forms the basis of the cost-effectiveness analysis of key health interventions carried out as part of the WHO-CHOICE project (Reference Chisholm, Sanderson and Ayuso-MateosChisholm et al, 2004).
Comparison of GBD 1990 and GBD 2000
The original Global Burden of Disease study, GBD 1990, highlighted the public health significance of depressive disorders, providing the tool for comparative assessment in a general health context. However, in assessing the burden of depressive disorders, the GBD 1990 study had certain shortfalls that GBD 2000 tried to overcome. The first was that the epidemiological data used as input for the original GBD study to calculate the burden due to depressive disorders remain debatable: episode incidence was modelled as 29 per 100 000 per year for women, and 16 per 100 000 per year for men. Average age at onset was taken as 37.1 years and the average episode duration was considered to be 6 months (Reference Murray and LopezMurray & Lopez, 1996). These incidence estimates are very low in comparison with the recent findings from epidemiological surveys. In addition, depression is now known to occur in younger age groups, often between 20 and 25 years, as compared with the estimate of 37.1 used in the GBD analysis. This, in fact, means that the degrees of burden estimated from the GBD results were actually underestimates for depressive disorders. Moreover, the GBD study considered depression as only an adult disease. There is overwhelming evidence at present that depression occurs with considerable frequency in childhood and adolescence (Reference Costello, Angold and BurnsCostello et al, 1996). In the GBD 2000 the incidence estimates used were higher (49 per 100 000 per year for women and 31 per 100 000 per year for men) and with incident cases of depressive episodes appearing at younger ages, than in the GBD 1990. Finally, in the GBD 1990 study, the disability weight for depressive disorder was taken as 0.6 for untreated cases, irrespective of severity of depression (i.e. mild, moderate or severe). However, it is evident that various levels of illness severity are associated with different degrees of disability. Different disability weights were assigned for the different levels of severity in the GBD 2000.
Limitations
There are varying degrees of uncertainty in GBD 2000 estimates for depressive disorders, reflecting uncertainty in the prevalence of depression in different regions of the world and uncertainty in the variation of their severity distribution. Despite intense efforts to obtain information on prevalence estimates of this frequent condition in all the WHO regions, there are still extensive and highly populated areas of the world where the epidemiology of depression is largely unknown because of a lack of data. There is a tendency in descriptive epidemiology to refuse to make estimates where data are sparse, uncertain or based on studies that do not reach certain methodological standards. To the contrary, disciplines such as demography and economics often aim to make the best possible estimates using any available data, employing a range of techniques, depending on the type and quality of evidence. Thus, the GBD 1990 has been criticised by some epidemiologists for using ‘estimates’ rather than ‘actual data’. This is not a relevant discussion for comparative burden estimates because all epidemiological data relating to population are ‘estimates’ of varying degrees of precision or uncertainty. The GBD 2000 seeks to use all available relevant data, to maximise the use of high-quality population-based data, and, even for regions and conditions where data are sparse, to use the available evidence and the best available methods to make inferences. Otherwise, limitations on the evidence base for the epidemiology of diseases, including depression, in some areas of the world translate to ‘no burden’ rather than the best achievable (even if uncertain) estimates of burden, thus presenting health decision makers with a picture that is highly misleading.
The WHO has initiated the World Mental Health Surveys, which will implement and analyse general population epidemiological surveys of mental, substance use and behavioural disorders in at least 18 countries throughout all WHO regions. This initiative will yield needed epidemiological parameters for many regions of the world and will improve the accuracy of the prevalence and incidence figures used for the estimation of the burden of depressive disorders in the future.
Depression as a public health priority
The fact that depressive disorders rank fourth as a source of DALYs, even though they cause few deaths, underscores how assessment of both fatal and non-fatal health outcomes affects the ranking of disease burden. Until recently, counting deaths was the only way to determine the priorities for public health actions and to control whether public health programmes were succeeding. Mental disorders have never been ranked on the top 10 priority list of public health significance when mortality indicators alone were used. Once the mortality and disability effects of disease were combined into a single metric, such as the DALYs, the magnitude of the burden of mental disorders in general, and of depression in particular, became apparent. With this new approach, depressive disorders are properly classified as being priority health problems. This high burden is a result of a combination of a high prevalence of depression, high impact on functioning and early age of onset.
The importance of depression worldwide was one of the key findings of the original Global Burden of Disease study, which has been confirmed for the analysis of the year 2000. The results of the GBD 2000 study have shown variations by regions, but patterns and trends are remarkably similar worldwide. Depressive disorders constitute a large proportion in the global burden of disease, both in the developed and developing countries.
The Global Burden of Disease results have attracted the attention of policy makers and public health experts alike, because they provide a common metric for evaluating and priority-setting across a wide range of health problems. DALYs, being based on a universal measure of time, life-years, provide a trans-professional currency to determine priorities for health and human services and to evaluate their effectiveness.
There is a strong interest among policy makers to monitor the impact of health care reforms and other interventions, using a common cost-effectiveness measure. The appeal of the DALY measure is that it provides a potentially useful tool for health policy purposes: the transformation of epidemiological data into informed decisions about resource allocation for health care. DALYs can be used in different ways. They can be used to set priorities for service provision, as an outcome measure to monitor/evaluate performance of services in terms of consumer outcomes and to compare cost-effectiveness of different interventions.
These results of the Global Burden of Disease study have provided the most powerful scientific and advocacy support for mental health to date. It is now time to see how these findings and these tools can be applied to policy-making, planning and programme implementation.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ There is a need to develop and adapt interventions for reducing the disability associated with depression.
-
▪ To reduce the burden, effective strategies need to be found to shorten episode duration and prevent recurrence in at-risk populations.
-
▪ Because of the high burden of depression worldwide, there is a need to integrate its treatment into primary care services and treat mental disorders with parity in health systems and in coverage in insurance schemes.
LIMITATIONS
-
▪ Underreporting in official statistics of deaths related to mental disorders, in general, and depression in particular.
-
▪ Uncertainty of prevalence and incidence estimates.
-
▪ Limitations in the generalisability of surveys in sub-populations to broader population groups.
Acknowledgements
The work was supported by the WHO/NIH Joint Project on Assessment and. Classification of Disability (principal investigator, T. B. Üstün, National Institutes of Health Grant UOIMH35883) and WHO's Global. Programme on Evidence for Health Policy. J.L.A.-M. was supported by. Spain's national Fondo de Investigación Sanitaria (contract. no. G03/061).
The views expressed are those of the authors and not necessarily those of. the organisations they represent.







eLetters
No eLetters have been published for this article.