Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder of childhood onset characterised by severe, developmentally inappropriate motor hyperactivity, inattention and impulsiveness that results in impairment (for example, school failure) and must be present in more than one setting – usually home and school. There is increasing recognition that ADHD symptoms and clinically defined disorder can persist into adult life and are associated with later drug and alcohol misuse and social and work difficulties. In some cases the disorder is associated with antisocial behaviour and criminality. When ADHD co-exists with antisocial behaviour, both problems are clinically more severe and persistent, have a worse prognosis and show stronger association with neurocognitive deficits than when they occur alone (Reference Thapar, Langley and O'DonovanThapar et al, 2006). However the mechanisms by which ADHD leads to antisocial behaviour are not clear-cut. Thus ADHD is not only the most common reason for follow-up in child and adolescent mental health services, it is also likely to be seen in adult psychiatry, learning disability, forensic and substance misuse services.
There is consistent evidence that genetic factors contribute to the aetiology of ADHD and that susceptibility genes interact with environmental risk factors in complex ways. The same risk factors that influence the origins of ADHD may have a role in the developmental course of the disorder, although it is also possible that a different set of risk and protective factors influence the course and outcome of ADHD (Fig. 1). It is crucial that research studies consider the developmental nature of ADHD and the variation in phenotypic manifestation over time, notably the association with antisocial behaviour (Reference Thapar, Langley and O'DonovanThapar et al, 2006), and take into consideration the role of environmental factors.
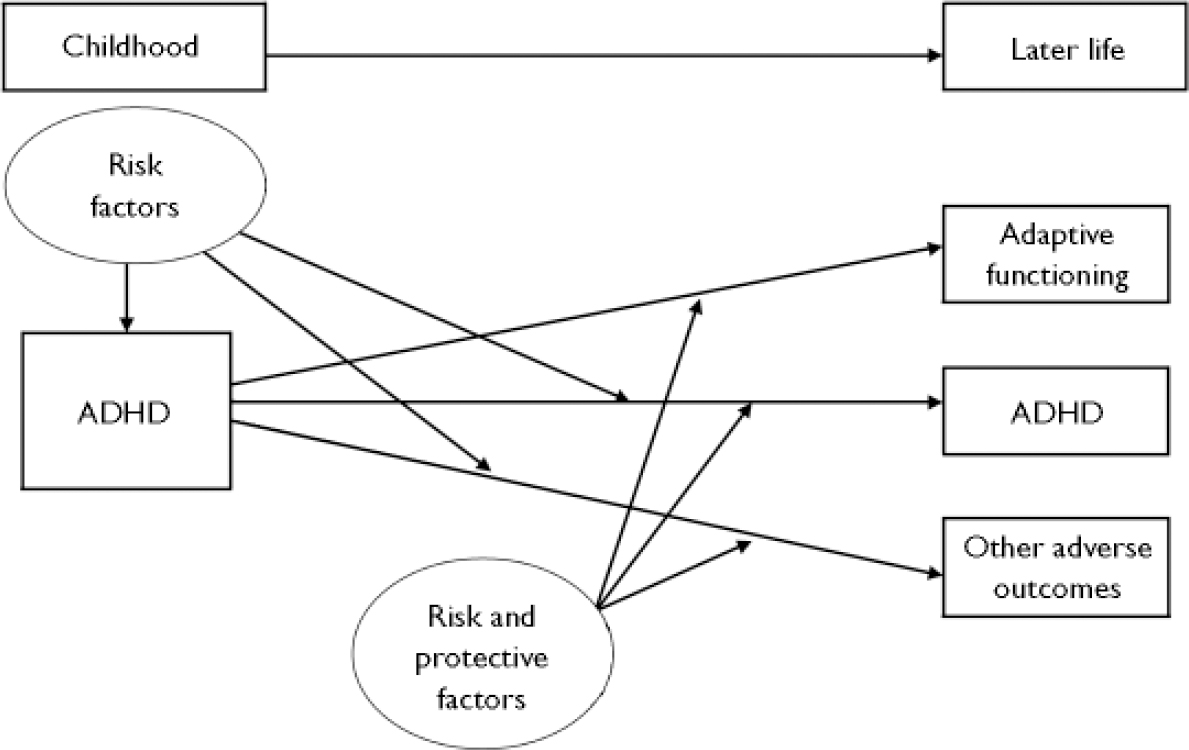
Fig. 1 The course of attention-deficit hyperactivity disorder (ADHD).
EVIDENCE THAT GENES CONTRIBUTE TO ADHD AND ITS DEVELOPMENTAL COURSE
Attention-deficit hyperactivity disorder runs in families, with first-degree relatives of affected individuals showing higher rates of the disorder (relative risk 4–5). The familial risk appears to be higher among relatives of those with both ADHD and conduct disorder. Twin and adoption studies have provided consistent evidence that genetic factors contribute to the aetiology of ADHD, with estimates of heritability of 60–91%; (Reference Thapar, O'Donovan and OwenThapar et al, 2005b ). Twin studies also show that genetic factors are the main contributor to continuity of ADHD symptoms over time and to the link between ADHD and antisocial behaviour (Reference Thapar, Langley and O'DonovanThapar et al, 2006). Overall, most studies suggest that there are genetic risk factors that influence both ADHD and its developmental course. However, it appears that there are additional risk factors (both genetic and environmental) that do not influence the origins of ADHD but do contribute to its clinical course and outcome.
PROGRESS IN IDENTIFYING SUSCEPTIBILITY GENES
There are a number of susceptibility gene variants for ADHD for which findings have been independently replicated and pooled or for which meta-analyses of data have yielded significant evidence of association. So far, these gene variants have been identified from functional candidate gene association studies. This type of design has been criticised because candidate genes are selected on the a priori assumption of involvement in the disorder, whereas for neuropsychiatric disorders the pathophysiology is usually unknown. However, for ADHD a reasonable prior hypothesis supporting monoaminergic system genes existed, and so far this approach has worked well.
Association between a variant in the dopamine D4 receptor gene (the 7-repeat allele of a 48 base pair repeat sequence) and ADHD has been widely replicated, with estimated odds ratios of 1.16–1.45 from meta-analysis and pooled analysis of data (Reference Faraone, Perlis and DoyleFaraone et al, 2005). Pooled analysis of data on a variant (microsatellite cytosine–adenine repeat) in the dopamine receptor DRD5 gene has also yielded significant evidence of association, with an estimated odds ratio of 1.24 (Reference Lowe, Kirley and HawiLowe et al, 2004). Finally, the most recent pooled analysis found small but significant association between a dopamine transporter gene variant – a variable number of tandem repeats (VNTR) 10-repeat allele – and ADHD, in which the odds ratio was 1.1 (Reference Faraone, Perlis and DoyleFaraone et al, 2005). More recently there have been several reports of association between variants in the SNAP-25 gene and ADHD (pooled OR=1.19), although the associated variants have differed between studies. This gene became of interest following reports that a SNAP-25-deficient mouse mutant shows hyperactivity. Other gene variants have been examined but these need further study (Reference Thapar, O'Donovan and OwenThapar et al, 2005b ). Thus, replicated genetic findings are emerging, but it is necessary to know more about how variants result in disorder, at a biological and phenotypic level.
DO THE SAME OR DIFFERENT SUSCEPTIBILITY GENES INFLUENCE THE DEVELOPMENTAL COURSE OF ADHD?
So far few studies have examined this question. One study has shown that the DRD4 7-repeat risk allele influenced persistence of ADHD over time (Reference El-Faddagh, Laucht and MarasEl-Faddagh et al, 2004). There has been interest in investigating what gene variants influence antisocial behaviour in ADHD, and here there have been several sets of interesting findings. First, the DRD4 7-repeat allele was found to be associated with antisocial behaviour in ADHD in a joint analysis of data from Cardiff, London and Dublin. These findings suggest that this allele might be important in influencing the course as well as the origins of ADHD. More recently the Cardiff group found that a functional variant in the gene encoding the enzyme COMT (previously found to be associated with measures of prefrontal cognitive functioning) was associated with antisocial behaviour in ADHD but not with ADHD itself (Reference Thapar, Langley and FowlerThapar et al, 2005a ). Finally, a variant in MAOA, a gene encoding another enzyme involved in neurotransmitter breakdown, was found to be associated with antisocial behaviour in ADHD but not with ADHD itself (Reference Thapar, Langley and O'DonovanThapar et al, 2006).
GENE–ENVIRONMENT INTERACTION
Attention-deficit hyperactivity disorder and its subsequent developmental course are not entirely explained by genes. There are a number of environmental factors that also appear to be associated with ADHD, two of which have withstood meta-analysis or pooled analyses: exposure to maternal smoking in pregnancy (estimated odds ratio 2.39; Reference Langley, Rice and van den BreeLangley et al, 2005) and low birth weight/prematurity (odds ratio 2.64; Reference Bhutta, Cleves and CaseyBhutta et al, 2002). It is well recognised that not all of those who are exposed to environmental adversity go on to develop ADHD. Gene–environment interaction (G×E), whereby genes operate by influencing sensitivity or response to environmental adversity, is becomingly increasingly recognised as important. To date there have been few published studies examining the contribution of G×E to ADHD and its course. For example, a recent study found that the association between a DAT1 haplotype (combination of risk alleles) and ADHD was stronger when the mother had drunk alcohol during pregnancy (Reference Brookes, Mill and GuindaliniBrookes et al, 2006). Another group suggested that the DAT1 risk allele previously found to be associated with ADHD was only associated with hyperactive–impulsive symptoms in those who had been exposed to maternal smoking during pregnancy (Reference Kahn, Khoury and NicholsKahn et al, 2003). In a study that focused on childhood-onset conduct disorder symptoms in ADHD, those who carried the COMT gene risk variant appeared to be more susceptible to the adverse effects of lower birth weight (Reference Thapar, Langley and FowlerThapar et al, 2005a ). All these findings now require replication but the evidence so far suggests that some genes may influence the origins and developmental course of ADHD by affecting individual sensitivity to environmental adversity.
In conclusion, genetic factors contribute to ADHD and replicated molecular genetic findings are now emerging. It is, however, important to recognise the phenotypic complexity of ADHD and acknowledge that it is a developmental disorder showing continuity and change in clinical presentation over time that is influenced by prenatal, biological and psychosocial environmental risk factors (see Fig. 1). Genes also appear to contribute to ADHD continuity and the development of antisocial behaviour in this disorder, and some of these genetic factors interact with environmental risk factors. However, risk factors for ADHD as a clinically defined disorder are not necessarily the same as those that influence its developmental course.
CLINICAL IMPLICATIONS
Understanding the aetiology and origins of ADHD, as with all psychiatric disorders, is important for paving the way to developing new and effective treatments (biological and non-biological) and for providing information and understanding to families and clinicians that in turn provides a framework for clinical management. Identifying genetic and environmental risk factors and examining how they co-act and interact to increase susceptibility to ADHD also provide a method of unpacking the heterogeneity of a clinically defined disorder in a meaningful way. This may lead to different ways of conceptualising the disorder and its diagnostic boundaries, and influence current methods of diagnostic classification.
In clinical practice some of the key goals are reducing symptoms, impairment and associated problems – notably antisocial behaviour in those already affected. Medication improves symptoms, but the long-term benefits for wider outcomes, including antisocial behaviour, are uncertain. Thus, additional risk reduction strategies aimed at reducing adverse outcomes are important (for example, this could involve reducing family conflict in those at highest genetic risk). Identifying both genetic and environmental risk factors that contribute to the course of the disorder is an important area of research activity so that the risk and protective pathways that lead to adverse outcomes and impairment can be elucidated. These types of research findings then provide an evidence base to inform the development of effective risk reduction strategies in the long-term management of ADHD. Intensive interventions for all children with ADHD is not pragmatic or necessarily desirable. Thus, identifying genetic and environmental risk factors as well as clinical characteristics that predict outcome can also be helpful in targeting resources and more carefully monitoring those who are at greatest risk of adverse consequences.
Acknowledgements
We acknowledge funding from the Wellcome Trust (A.T., M.G.) and the Medical Research Council (P.A.). K.L. is supported by a Wellcome Trust Value in People Award. We thank Professors Mike Owen and Michael O'Donovan for their contributions.




eLetters
No eLetters have been published for this article.