Dopamine D2 receptor blockade is thought to mediate antipsychotic action. In vivo studies in medicated patients have shown that treatments with traditional antipsychotic compounds such as haloperidol consistently induce 70-80% occupancy of the striatal D2 receptors (Reference Martinot, Paillère-Martinot and Loc'hMartinot et al, 1990; Reference Nordström, Farde and WieselNordströmet al, 1993). Furthermore, high levels of D2 receptor occupancy in the striatum are associated with higher risk of extrapyramidal side-effects (Reference Farde, Nordström and WieselFarde et al, 1992). At variance with traditional compounds, atypical antipsychotics such as clozapine have a low tendency to induce extrapyramidal side-effects (Reference MollerMoller, 2000). This may be consistent with the lower in vivo D2 striatal blockade reported with the usual doses of clozapine (Reference Farde, Nordström and WieselFarde et al, 1992). Whereas the nigro-striatal dopaminergic pathways appear to be involved in extrapyramidal side-effects, models of the antipsychotic mechanism of action involve extrastriatal cerebral structures such as the meso-cortico-limbic pathways (Reference Moore, West and GraceMoore et al, 1999). Therefore, the D2 receptor blockade by antipsychotic drugs in extrastriatal structures may be one of the mechanisms that account for the antipsychotic effect, as suggested by earlier single photon emission tomography studies (Reference Bigliani, Mulligan and ActonBigliani et al, 2000; Reference Stephenson, Bigliani and JonesStephenson et al, 2000). This hypothesis requires substantiation with the more sensitive and quantitative positron emission tomography (PET) technique. However, in the extrastriatal structures such as thalamus, limbic and temporal cortices, the D2 dopamine receptor density is 5— to 25-fold lower than in the striatal structures, where they exist in nanomolar concentrations (Reference Kessler, Whetsell and AnsariKessler et al, 1993). The development of [76Br]-FLB457, a bromine-labelled benzamide radioligand for PET with picomolar affinity and a high selectivity for D2 and D3 receptors (dissociation constant K i(D2)=0.018 nM) (Reference Loc'h, Halldin and BottlaenderLoc'h et al, 1996), has made possible the comparison of the binding of various antipsychotic compounds to extrastriatal and striatal structures. In order to compare four atypical antipsychotics with a traditional molecule at the recommended dose ranges for therapeutic antipsychotic effect, we studied the binding of the reference compound haloperidol (K i(D2)=1 nM) and that of four atypical antipsychotics — risperidone (K i(D2)=3 nM), clozapine (K i(D2)=125 nM), amisulpride (K i(D2)=3.4 nM andK i(D2)=3.5 nM) and olanzapine (K i(D2)=11 nM) (Reference Chivers, Gommeren and LeysenChivers et al, 1988) — in patients with schizophrenia treated with these compounds.
METHOD
Subjects
Nineteen patients with DSM-IV (American Psychiatric Association, 1994) schizophrenia (16 males, 3 females, mean age=32 years, s.d.=7) treated with haloperidol (n=4), risperidone (n=3), clozapine (n=3), amisulpride (n=5) or olanzapine (n=4) were included (Table 1). They were treated with daily dosages matching the recommended ranges for antipsychotic effect. For haloperidol this range was 3-20 mg/day (Editions du Vidal, 1993; Reference Zimbroff, Kane and TammingaZimbroff et al, 1997); for risperidone, 6-12 mg/day (Reference KasperKasper, 1998; Reference Nyberg, Erikson and OxenstiernaNyberg et al, 1999); for clozapine, 200-400 mg/day (Reference Fitton and HeelFitton & Heel, 1990); for amisulpride, 200-1200 mg/day (Reference Coukell, Spencer and BenfieldCoukell et al, 1996); and for olanzapine, 5-20 mg/day (Reference KasperKasper, 1998). Patients were scanned 18-20 h after the last evening dose of the antipsychotic, in order to avoid the peak plasma concentration following the drug dose intake. All patients were medicated for at least 15 days, which is much longer than the five half-lives necessary to reach the steady state for plasma antipsychotic concentration. Exclusion criteria included neurological or other medical conditions and substance misuse. Anticholinergic drugs or benzodiazepines that do not interfere with D2 dopamine receptors (Reference Burt, Creese and SnyderBurtet al, 1976; Reference Boyson, McGonicle and LuthinBoysonet al, 1988) were not withdrawn. The patient groups were compared with a group of six normal control men (mean age=24 years, s.d.=4) who underwent a medical examination and a psychiatric interview in order to rule out any medical condition. The Henri Mondor ethics committee approved the protocol. After a complete description of the study to the subjects, written informed consent was obtained.
Table 1 Characteristics of the patients
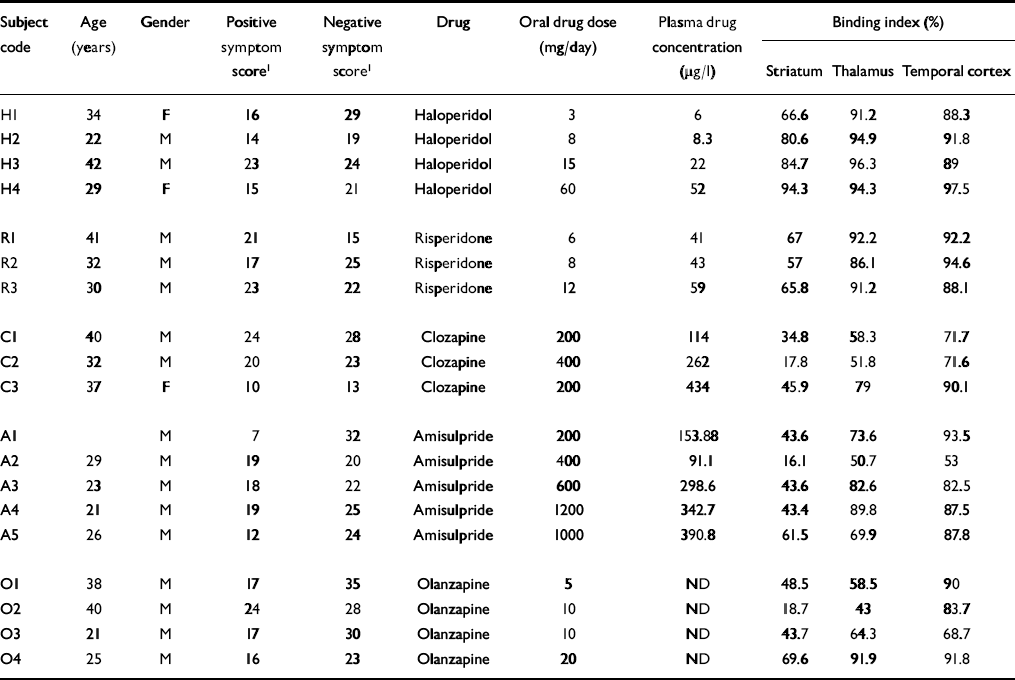
| Subject code | Age (years) | Gender | Positive symptom score1 | Negative symptom score1 | Drug | Oral drug dose (mg/day) | Plasma drug concentration (μg/I) | Binding index (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Striatum | Thalamus | Temporal cortex | ||||||||
| H1 | 34 | F | 16 | 29 | Haloperidol | 3 | 6 | 66.6 | 91.2 | 88.3 |
| H2 | 22 | M | 14 | 19 | Haloperidol | 8 | 8.3 | 80.6 | 94.9 | 91.8 |
| H3 | 42 | M | 23 | 24 | Haloperidol | 15 | 22 | 84.7 | 96.3 | 89 |
| H4 | 29 | F | 15 | 21 | Haloperidol | 60 | 52 | 94.3 | 94.3 | 97.5 |
| R1 | 41 | M | 21 | 15 | Risperidone | 6 | 41 | 67 | 92.2 | 92.2 |
| R2 | 32 | M | 17 | 25 | Risperidone | 8 | 43 | 57 | 86.1 | 94.6 |
| R3 | 30 | M | 23 | 22 | Risperidone | 12 | 59 | 65.8 | 91.2 | 88.1 |
| C1 | 40 | M | 24 | 28 | Clozapine | 200 | 114 | 34.8 | 58.3 | 71.7 |
| C2 | 32 | M | 20 | 23 | Clozapine | 400 | 262 | 17.8 | 51.8 | 71.6 |
| C3 | 37 | F | 10 | 13 | Clozapine | 200 | 434 | 45.9 | 79 | 90.1 |
| A1 | M | 7 | 32 | Amisulpride | 200 | 153.88 | 43.6 | 73.6 | 93.5 | |
| A2 | 29 | M | 19 | 20 | Amisulpride | 400 | 91.1 | 16.1 | 50.7 | 53 |
| A3 | 23 | M | 18 | 22 | Amisulpride | 600 | 298.6 | 43.6 | 82.6 | 82.5 |
| A4 | 21 | M | 19 | 25 | Amisulpride | 1200 | 342.7 | 43.4 | 89.8 | 87.5 |
| A5 | 26 | M | 12 | 24 | Amisulpride | 1000 | 390.8 | 61.5 | 69.9 | 87.8 |
| O1 | 38 | M | 17 | 35 | Olanzapine | 5 | ND | 48.5 | 58.5 | 90 |
| O2 | 40 | M | 24 | 28 | Olanzapine | 10 | ND | 18.7 | 43 | 83.7 |
| O3 | 21 | M | 17 | 30 | Olanzapine | 10 | ND | 43.7 | 64.3 | 68.7 |
| O4 | 25 | M | 16 | 23 | Olanzapine | 20 | ND | 69.6 | 91.9 | 91.8 |
Brain imaging
Brain imaging, image analysis and determination of the D2 dopamine blockade by the computation of a binding index were performed according to a methodology described previously (Reference Xiberas, Martinot and MalletXiberas et al, 2001). Briefly, for each subject a 1.5 T Signa Imager (General Electric, Milwaukee, WI) provided 128 anatomical slices parallel to the orbito-meatal line. Afterwards, 63 cerebral slices parallel to the orbito-meatal line were acquired with a Siemens HR+ positron tomograph (of spatial resolution 2.5 mm; Knoxville, TX). Just before injecting the [76Br]-FLB457, venous blood was sampled for plasma concentration of the antipsychotic drug. Approximately 1 mCi of [76Br]-FLB457 was injected into each subject (mean (s.d.)=0.98 (0.20) mCi for healthy subjects and 1.03 (0.23) mCi for patients, Mann—Whitney U=53.5,P=0.82), with a high specific activity of 264±164 μCi/nmol for healthy subjects and 481±299 μCi/nmol for patients (Mann—Whitney U=29, P=0.08). Afterwards, a first image series was acquired: two 5-min images, two 10-min images and two 15-min images. Then the subjects were removed from the tomograph for 60 min and afterwards placed in the same position using a thermoplastic-modelled mask as well as skin marks with respect to a laser beam system, in order to acquire a late image series: one 30-min and one 15-min image. Finally, a second transmission scan was performed for co-registration purposes.
Image analysis
For each subject, the first PET image series was co-registrated on the magnetic resonance image (MRI) using the first transmission scan (Reference Mangin, Frouin and BlochMangin et al, 1994). Because the radioactivity concentrated mainly in the striatum in the late image series, the cortical structures could not be used for registration. Therefore, the second transmission scan was used for PET to MRI co-registration. Regions of interest (ROIs) were drawn on the co-registrated MRI slices, following the visible borders of the structures in each hemisphere. Caudate and putamen were defined on five slices, thalamus on four slices and temporal cortices on four slices below the lowest striatum slice; finally; ROIs were defined in the cerebellar grey matter on four slices.
Regional radioactivity concentrations for each region (injected dose— and decay-corrected) were computed by pooling the corresponding ROI values and by summing the left and right regional values.
Determination of D2 dopamine receptor blockade: binding index computation
Regional radioactivity was measured for each sequential scan and plotted against time. Specific binding in the ROIs was defined as the difference between radioactivities in the ROIs and the cerebellum. The activity measured (A m) in the ROIs represents the sum of the free (and non-specific binding) and bound radioligand concentrations:
where A sb is the specifically bound radioactive concentration in the ROIs and C is the activity measured in the cerebellum, which represents (under the assumption that the concentration of D2 dopamine receptors in the cerebellum is negligible with respect to the concentration in the other regions) both free ligand and nonspecific binding. This method has been validated in vitro (Reference Altar, O'Neils and WalterAltar et al, 1985) and in vivo using PET with previous D2 dopamine receptor ligands (Reference Farde, Ehrin and ErikssonFarde et al, 1985), as well as for the [76Br]-FLB457 (Reference Xiberas, Martinot and MalletXiberas et al, 2001). Hence, for each patient a binding index (BI) of the antipsychotic compound was derived from the patient's measured regional radioactivity concentrations in the striatum, the extrastriatal regions (thalamus, temporal cortex) and the cerebellum at the time of 165 min, taking the measures of control subjects as a baseline:
Plasma drug concentration determination
Haloperidol
After extraction from alkalinised plasma by a mixture of hexane/isoamylic alcohol, haloperidol was purified from a Kromasil C8 column (5 μm, 250 × 4.6 mm) using a 73/23 mixture of phosphate buffer (pH 3) and acetonitrile as mobile phase. The wavelength was set at 280 nm. The detection limit was 1.0 μg/l.
Risperidone
After extraction from alkalinised plasma by a mixture of hexane/ethylacetate, the risperidone was purified from a Kromasil C8 column with the same characteristics as those described for haloperidol.
Clozapine
After extraction from alkalinised plasma by a mixture of hexane/isoamylic alcohol, the clozapine was purified from a Spherisorp C8 column (5 μm, 250 × 4.6 mm) using a 55/40/5 mixture of phosphate buffer (pH 3), acetonitrile and methanol as mobile phase. The wavelength was set at 230 nm. The detection limit was 2.5 μg/l.
Amisulpride
After extraction from alkalinised plasma by a mixture of diethyl ether and chloroform, the amisulpride was purified from a μBondapak C18 column (5 μ m, 150 × 4.6 mm) using a 90/10 mixture of phosphate buffer (pH 3) and acetonitrile as mobile phase. Detection was performed using a fluorimetric detector set at λex=280 nm and λem=370 nm. The detection limit was 0.5 μg/l.
Olanzapine
Owing to technical reasons, plasma drug concentration determinations were not available.
RESULTS
Individual characteristics of patients and treatments, as well as antipsychotic plasma concentrations and binding index values, are reported in Table 1. Figure 1 shows an example of time-radioactivity curves. Paired comparisons for the binding indices in striatal and extrastriatal regions across treatment groups (Table 2) showed that the striatal and thalamic binding indices graded the haloperidol and atypical antipsychotics (Fig. 2). This was not the case in the temporal cortex.
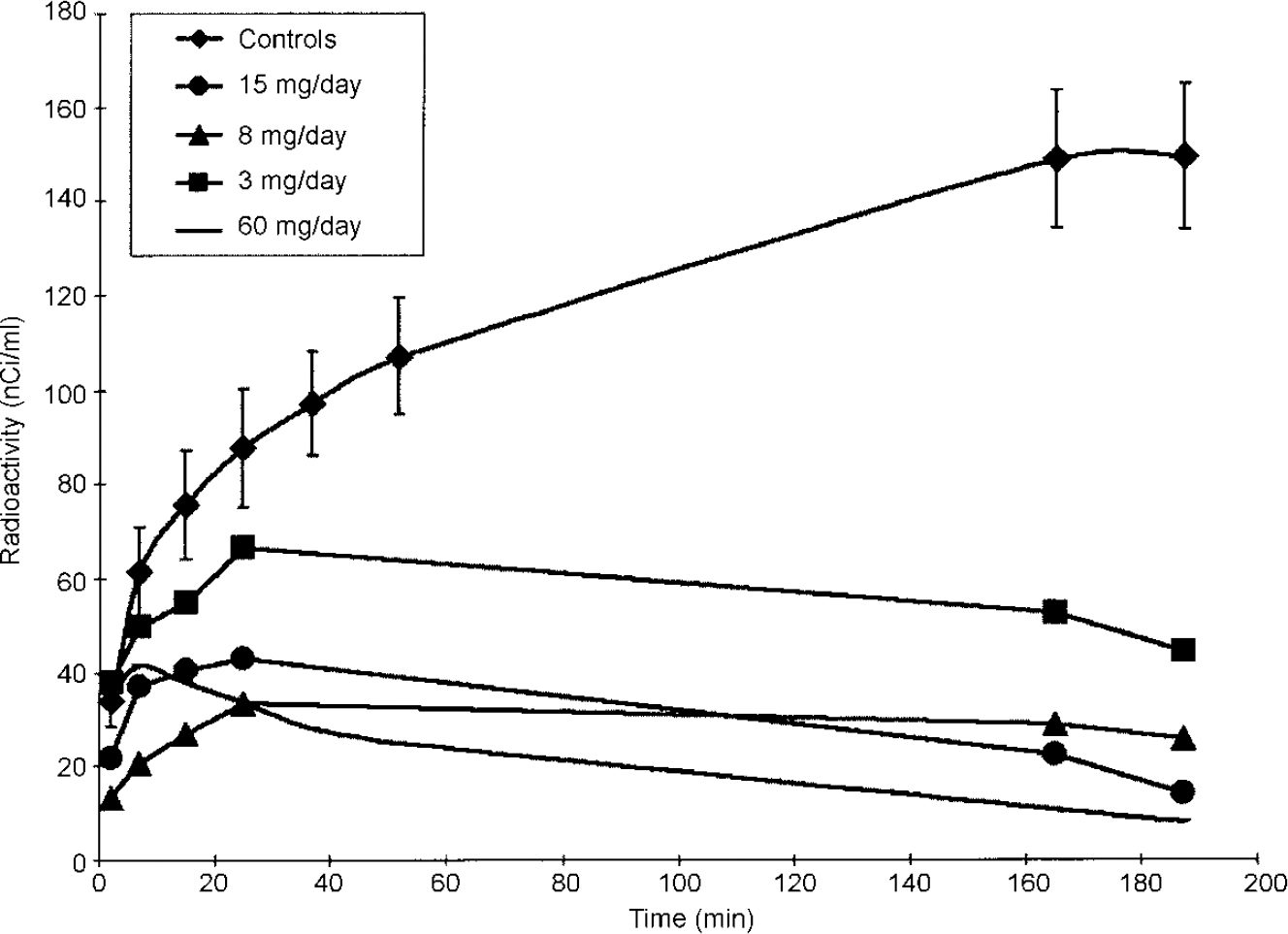
Fig. 1 Time—radioactivity curves in the striatum of control subject group and of patients treated with various dosages of haloperidol.
Table 2 Comparison of binding indices in striatum, thalamus and temporal cortex

| Intergroup comparisons by Kruskal—Wallis test | Paired-group comparisons (mean (s.d.)) by Mann—Whitney test | ||||
|---|---|---|---|---|---|
| Haloperidol v.risperidone | Haloperidol v.clozapine | Haloperidol v.amisulpride | Haloperidol v.olanzapine | ||
| Striatum | H=11.8; P=0.02 | 81.5 (11.5) v.63.3 (5.5) | 81.5 (11.5) v.32.8 (14.2) | 81.5 (11.5) v.41.6 (16.3) | 81.5 (11.5) v.45.1 (20.9) |
| U=1.0; P=0.11 | U=0.0; P=0.05 | U=0.0; P=0.01 | U=1; P=0.05 | ||
| Thalamus | H=11.3; P=0.02 | 94.2 (2.1) v.89.8 (3.3) | 94.2 (2.1) v.63.0 (14.2) | 94.2 (2.1) v.73.3 (14.8) | 94.2 (2.1) v.64.4 (20.4) |
| U=1.5; P=0.11 | U=0.0; P=0.05 | U=0.0; P=0.02 | U=1; P=0.05 | ||
| Temporal cortex | H=5.7; P=0.23 | 91.7 (4.2) v.91.6 (3.3) | 91.7 (4.2) v.77.8 (10.7) | 91.7 (4.2) v.80.9 (16.1) | 91.7 (4.2) v.83.6 (10.5) |
| U=6.0; P=1 | U=2.0; P=0.23 | U=3.0; P=0.11 | U=4.5; P=0.34 | ||
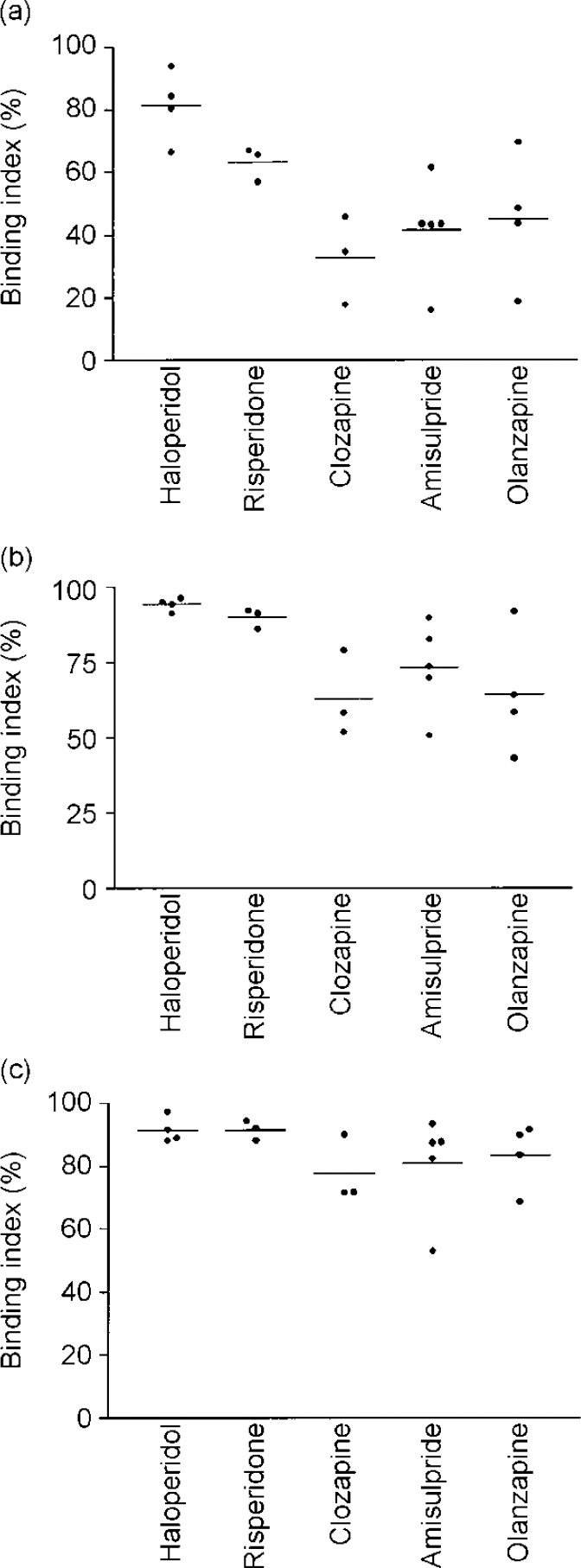
Fig. 2 Binding indices of haloperidol, risperidone, clozapine, amisulpride and olanzapine in the striatum (a), thalamus (b) and temporal cortex (c).
Striatal and thalamic binding indices
Striatal and thalamic binding indices clearly distinguished haloperidol-treated from atypical antipsychotic-treated patients: indices were lower with atypical antipsychotics. In the thalamus, the D2 binding indices induced by clozapine, amisulpride and olanzapine were lower than that induced by haloperidol. Analogous trends were detected in the thalamus and striatum of risperidone-treated patients.
Temporal binding indices
Binding indices in the temporal cortex were similarly high, whatever the antipsychotic compound.
DISCUSSION
Patients treated with the usual antipsychotic dose ranges of haloperidol, risperidone, clozapine, amisulpride and olanzapine had a similarly high D2 dopamine receptor blockade in the temporal cortex, as estimated by the binding index of [76Br]-FLB457. In contrast, the atypical antipsychotic drugs induced a significantly lower D2 binding index than haloperidol in basal ganglia (i.e. in the thalamus and particularly in the striatum).
Methodological considerations
Long-time-frame PET imaging
For each patient, a binding index of the antipsychotic drug was derived from the patient's measured regional radioactivity concentrations in the striatum, the extrastriatal regions (thalamus, temporal cortex) and the cerebellum 165 min after injection (Fig. 1), using the measures in control subjects as a baseline. The time was chosen from modelling studies in primates, because it tallies with the duration required for the radioligand's equilibrium in the extrastriatal regions (Reference Delforge, Bottlaender and Loc'hDelforge et al, 1999). However, according to the same model, equilibrium is reached only at t=300 min after injection in striatal regions. In one control subject, we measured the concentration of the radioactivity in the striata at 165 and 315 min after injection: an increase of 10% only (139v. 152 nCi/ml) was observed at 315 min. Thus, the binding in the striatum is probably slightly underestimated and values reported for striatal regions should not be considered as effective D2 receptor occupancy figures but as approximate values. They are, nevertheless, useful to compare the striatal D2 receptor blockade induced in vivo by various antipsychotic drugs.
Picomolar affinity radioligand
The picomolar affinity of [76Br]-FLB457 for D2 receptors accounts for the detection of a decrease of cerebellar radioactivity values in treated patients, which is likely to reflect some degree of occupancy of the small concentration of cerebellar D2 receptors. This was not detected in previous studies using lower-affinity D2 radioligands. Although the cerebellar radioactivity cannot be considered as solely reflecting non-specific binding of the [76Br]-FLB457, in the conditions of the present study the use of a subtractive operator in the binding index computation (i.e. regional A m —C) limits the incidence of the cerebellar specific binding for comparison of the binding index under various antipsychotic treatments. Indeed, by using the mean cerebellar value observed in the healthy subjects as a reference, underestimation of binding index values was below 5% in the striatum but ranged from 4% (haloperidol) to 12% (clozapine) in the temporal cortex.
This underestimation, more marked in cortical than in striatal structures, strengthens the observation of a high binding index in cortical regions with all antipsychotics and the differential binding index values between haloperidol and atypical compounds in the basal ganglia. This observation is in keeping with a modelling study, correlating the affinity of a ligand with the B max in the structure studied. According to that modelling, the apparent affinity of a radioligand is correlated with theB max in the structure: when the B max is low, the apparent affinity of the radioligand to its receptor increases (Reference Delforge, Bottlaender and Loc'hDelforge et al, 1999).
Consistency with previous ex vivo findings in animals
The high D2 blockade induced in the temporal cortex by each of the five antipsychotic drugs is consistent with reports using different methodologies. The topographical selectivity of atypical antipsychotics on dopamine blockade has been reported previously from ex vivo measurements in animals. For instance, studies have demonstrated a higher affinity of amisulpride for D2 dopamine receptors in the temporal regions than in striatum (Reference Schoemaker, Claustre and FageSchoemakeret al, 1997). Also, chronic treatment with clozapine has been shown specifically to upregulate cortical D2 mRNA turnover and cortical D2 receptor binding, at variance with haloperidol, which seems to upregulate both striatal and cortical D2 receptors (Reference Lidow and Goldman-RakicLidow & Goldman-Rakic, 1994). Atypical antipsychotics raise dopamine turnover more in limbic structures than in striatum, whereas traditional antipsychotics affect the dopamine turnover in both regions to the same degree (Reference Westerink, Lejeune and KorfWesterink et al, 1977). In addition, the c-fos-like immunoreactivity is increased by atypical antipsychotics in limbic areas and in cortices, whereas traditional antipsychotics also lead to an increased c-fos expression in the dorsolateral striatum (Reference Fink-Jensen and KristensenFink-Jensen & Kristensen, 1994).
Consistency with previous in vivo findings in humans
Our results obtained with a quantitative imaging technique are in agreement with previous in vivo reports using a radioligand with the same picomolar affinity for D2 receptors [123I]-epidepride) and single photon emission tomography to assess the ‘occupancy’ of D2 receptors by antipsychotic drugs in the striatum and temporal cortex. Indeed, the elevated occupancy figures observed with traditional compounds (haloperidol, fluphenazine, flupenthixol, pipotiazine, droperidol) in both temporal (mean 82%) and striatal (mean 73%) regions (Reference Bigliani, Mulligan and ActonBigliani et al, 1999), and the small difference between striatal and temporal cortex blockade were analogous to those determined in the haloperidol-treated patients of the present study. Thus, although a relative temporal cortex selectivity in D2 blockade (Reference Pilowsky, Mulligan and ActonPilowsky et al, 1997; Reference Bigliani, Mulligan and ActonBigliani et al, 2000) appears to characterise atypical antipsychotics, a similar high D2 blockade in both temporal and striatal regions is induced by usual antipsychotic doses of traditional antipsychotics.
On the whole, the convergence of ex vivo and in vivo data strongly suggests that cortical D2 dopamine receptors are a common target of both traditional and atypical antipsychotics for therapeutic action.
Finally, from a clinical point of view, our results are in keeping with the association of antipsychotic efficacy (attributed to an action on meso-cortico-limbic dopamine pathways) and lower extrapyramidal side-effects (attributed to an antidopaminergic activity in the dorsolateral striatum) with atypical antipsychotics than with traditional compounds. Searching for a highin vivo binding to the D2 receptors in the cortex co-occurring with a lower binding in the basal ganglia therefore could be suggested as an indicator of a favourable benefit/risk profile for a putative antipsychotic compound.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
• Cortical D2 dopamine receptors are a common target of both traditional and atypical antipsychotics for therapeutic action.
• In the basal ganglia, atypical antipsychotics antagonise the dopamine transmission mediated by the D2 receptor to a lower level than traditional antipsychotics.
• The co-occurrence of a high in vivo binding to the D2 receptors in the cortex and a lower binding in the basal ganglia could be an indicator of a favourable benefit/risk profile for a putative antipsychotic compound.
LIMITATIONS
• Owing to the small patient samples, inter-individual variability was not assessed extensively.
• The binding indices approximate the effective D2 receptor occupancy.
• The study was not designed to assess therapeutic effects longitudinally.
Acknowledgements
This study was possible through a BIOMED 2 grant of the European Union, coordinated by Professor Lars Farde. Dr Evelyne Chanut (Biology Laboratory, Paul Guiraud Hospital, Villejuif) is acknowledged for haloperidol, risperidone and clozapine plasma concentration determinations and Muriel Canal (Sanofi-Synthélabo) is acknowledged for amisulpride plasma concentration determinations.







eLetters
No eLetters have been published for this article.