Although several studies have been performed to assess the neural correlates of memory encoding and retrieval in schizophrenia, there is still no consensus on the specific brain regions that seem most affected. A new quantitative meta-analytic method is available for combining the results from different functional brain imaging studies (Reference Chein, Fissell and JacobsChein et al, 2002; Reference Turkeltaub, Eden and JonesTurkeltaub et al, 2002). This type of meta-analysis is theoretically and methodo-logically different from standard meta-analyses based on effect size (Reference Fox, Parsons and LancasterFox et al, 1998). In contrast to the typical pooling of effect sizes from different studies, this new technique assesses whether the peaks of activation reported in different studies are significantly clustered (agglomerated) in certain brain regions. We used this technique to review published reports of functional brain imaging studies of episodic memory in schizophrenia; our aim was to identify the brain regions that are consistently affected during the performance of episodic memory tasks.
METHOD
Literature search and criteria for inclusion
A literature search was performed to retrieve studies that compared the brain activation elicited by an episodic memory task between a group of people with schizophrenia and a control group of healthy people. Our inclusion criteria were:
-
(a) participation of a group of people with an established diagnosis of schizophrenia (or schizophrenia-spectrum disorders) and a healthy control group;
-
(b) administration of a target episodic memory task (encoding or retrieval) and a reference (baseline) task concomitant to the acquisition of functional neuroimaging data;
-
(c) direct statistical comparison between the pattern of activation elicited by this memory task (relative to the reference task) in the schizophrenia group v. the control group;
-
(d) reporting of the focus of the peaks of differential activation between the two groups according to the Talairach/Montreal Neurological Institute (MNI) coordinate system (Reference Talairach and TournouxTalairach & Tournoux, 1988; Reference Evans, Collins and MillsEvans et al, 1993).
Data extraction and processing
Eighteen studies published between January 1996 and October 2004 met the criteria for inclusion in our meta-analysis (Table 1). Other studies meeting our inclusion criteria except for the absence of Talairach/MNI coordinates (Reference Ragland, Gur and GlahnRagland et al, 1998; Reference Shihabuddin, Buchsbaum and HazlettShihabuddin et al, 1998; Hazlett et al, Reference Hazlett, Buchsbaum and Byne1999, Reference Hazlett, Buchsbaum and Jeu2000; Reference Nohara, Suzuki and KurachiNohara et al, 2000) were reviewed but were not included in the meta-analysis. Altogether, 228 foci reported in these studies corresponded to a between-group difference in brain activation during the performance of a memory task. Studies often used different methods of analysis and different thresholds and so we included all foci reported to be significant using the criteria designated in the individual studies. Following a technique similar to that used by other groups (Reference Chein, Fissell and JacobsChein et al, 2002; Reference Turkeltaub, Eden and JonesTurkeltaub et al, 2002; Wager et al, Reference Wager, Phan and Liberzon2003, Reference Wager, Jonides and Reading2004), we created a three-dimensional file with a value of 1 at each of these 228 foci and smoothed it to model each focus as a Gaussian sphere with a full width at half-maximum of 14 mm. After smoothing, the overlap of neighbouring Gaussian spheres led to greater values, termed activation likelihood estimate (ALE) values by Turkeltaub et al (Reference Turkeltaub, Eden and Jones2002), in certain brain regions where multiple foci agglomerated.
Table 1 Studies included in the meta-analysis
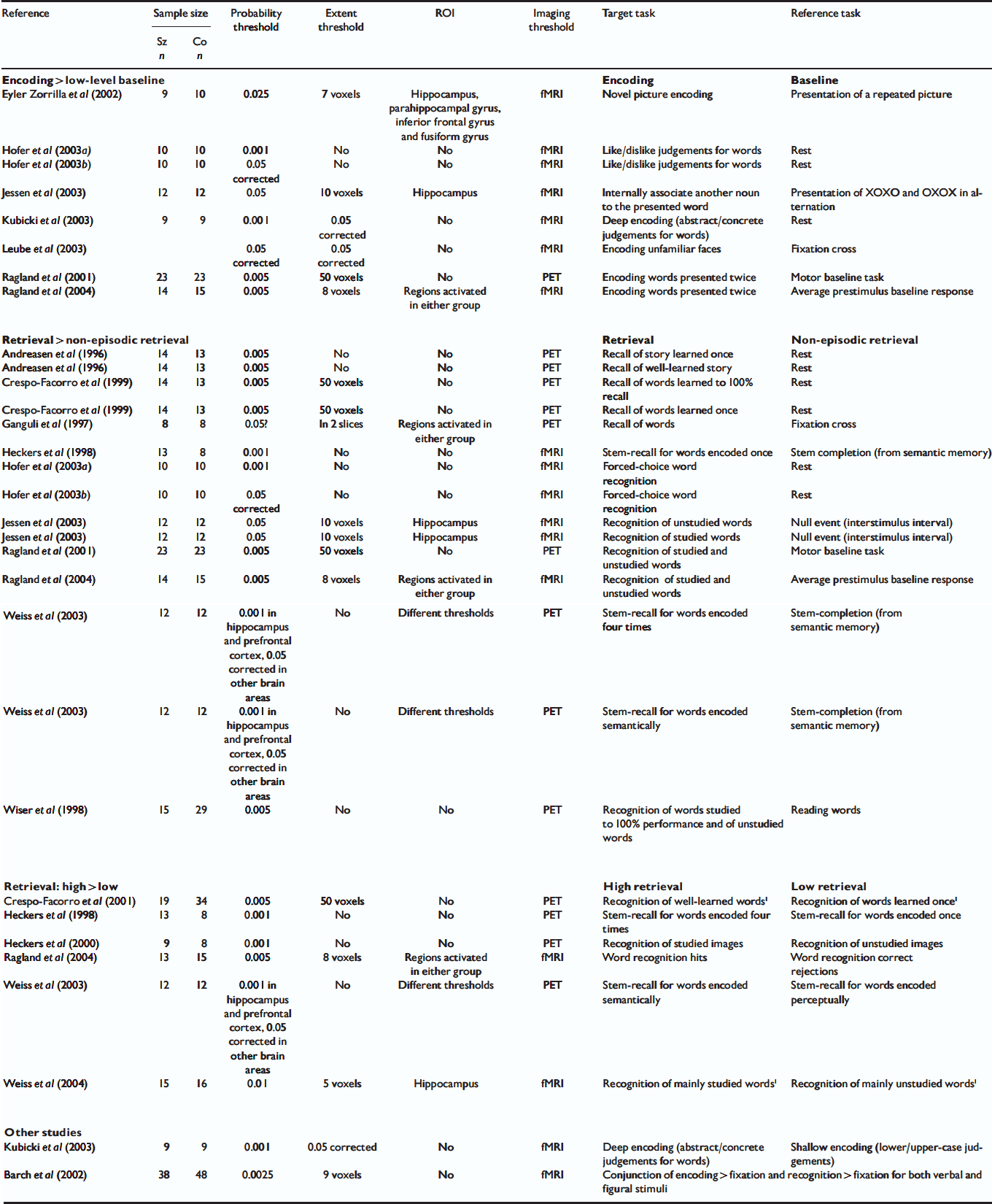
| Reference | Sample size | Probability threshold | Extent threshold | ROI | Imaging threshold | Target task | Reference task | |
|---|---|---|---|---|---|---|---|---|
| Sz n | Co n | |||||||
| Encoding > low-level baseline | Encoding | Baseline | ||||||
| Eyler Zorrilla et al (Reference Eyler Zorrilla, Jeste and Paulus2002) | 9 | 10 | 0.025 | 7 voxels | Hippocampus, parahippocampal gyrus, inferior frontal gyrus and fusiform gyrus | fMRI | Novel picture encoding | Presentation of a repeated picture |
| Hofer et al (Reference Hofer, Weiss and Golaszewski2003a ) | 10 | 10 | 0.001 | No | No | fMRI | Like/dislike judgements for words | Rest |
| Hofer et al (Reference Hofer, Weiss and Golaszewski2003b ) | 10 | 10 | 0.05 corrected | No | No | fMRI | Like/dislike judgements for words | Rest |
| Jessen et al (Reference Jessen, Scheef and Germeshausen2003) | 12 | 12 | 0.05 | 10 voxels | Hippocampus | fMRI | Internally associate another noun to the presented word | Presentation of XOXO and OXOX in alternation |
| Kubicki et al (Reference Kubicki, McCarley and Nestor2003) | 9 | 9 | 0.001 | 0.05 corrected | No | fMRI | Deep encoding (abstract/concrete judgements for words) | Rest |
| Leube et al (Reference Leube, Rapp and Buchkremer2003) | 0.05 corrected | 0.05 corrected | No | fMRI | Encoding unfamiliar faces | Fixation cross | ||
| Ragland et al (Reference Ragland, Gur and Raz2001) | 23 | 23 | 0.005 | 50 voxels | No | PET | Encoding words presented twice | Motor baseline task |
| Ragland et al (Reference Ragland, Gur and Valdez2004) | 14 | 15 | 0.005 | 8 voxels | Regions activated in either group | fMRI | Encoding words presented twice | Average prestimulus baseline response |
| Retrieval > non-episodic retrieval | Retrieval | Non-episodic retrieval | ||||||
| Andreasen et al (Reference Andreasen, O'Leary and Cizadlo1996) | 14 | 13 | 0.005 | No | No | PET | Recall of story learned once | Rest |
| Andreasen et al (Reference Andreasen, O'Leary and Cizadlo1996) | 14 | 13 | 0.005 | No | No | PET | Recall of well-learned story | Rest |
| Crespo-Facorro et al (Reference Crespo-Facorro, Paradiso and Andreasen1999) | 14 | 13 | 0.005 | 50 voxels | No | PET | Recall of words learned to 100% recall | Rest |
| Crespo-Facorro et al (Reference Crespo-Facorro, Paradiso and Andreasen1999) | 14 | 13 | 0.005 | 50 voxels | No | PET | Recall of words learned once | Rest |
| Ganguli et al (Reference Ganguli, Carter and Mintun1997) | 8 | 8 | 0.05? | In 2 slices | Regions activated in either group | PET | Recall of words | Fixation cross |
| Heckers et al (Reference Heckers, Rauch and Goff1998) | 13 | 8 | 0.001 | No | No | fMRI | Stem-recall for words encoded once | Stem completion (from semantic memory) |
| Hofer et al (Reference Hofer, Weiss and Golaszewski2003a ) | 10 | 10 | 0.001 | No | No | fMRI | Forced-choice word recognition | Rest |
| Hofer et al (Reference Hofer, Weiss and Golaszewski2003b ) | 10 | 10 | 0.05 corrected | No | No | fMRI | Forced-choice word recognition | Rest |
| Jessen et al (Reference Jessen, Scheef and Germeshausen2003) | 12 | 12 | 0.05 | 10 voxels | Hippocampus | fMRI | Recognition of unstudied words | Null event (interstimulus interval) |
| Jessen et al (Reference Jessen, Scheef and Germeshausen2003) | 12 | 12 | 0.05 | 10 voxels | Hippocampus | fMRI | Recognition of studied words | Null event (interstimulus interval) |
| Ragland et al (Reference Ragland, Gur and Raz2001) | 23 | 23 | 0.005 | 50 voxels | No | PET | Recognition of studied and unstudied words | Motor baseline task |
| Ragland et al (Reference Ragland, Gur and Valdez2004) | 14 | 15 | 0.005 | 8 voxels | Regions activated in either group | fMRI | Recognition of studied and unstudied words | Average prestimulus baseline response |
| Weiss et al (Reference Weiss, Schacter and Goff2003) | 12 | 12 | 0.001 in hippocampus and prefrontal cortex, 0.05 corrected in other brain areas | No | Different thresholds | PET | Stem-recall for words encoded four times | Stem-completion (from semantic memory) |
| Weiss et al (Reference Weiss, Schacter and Goff2003) | 12 | 12 | 0.001 in hippocampus and prefrontal cortex, 0.05 corrected in other brain areas | No | Different thresholds | PET | Stem-recall for words encoded semantically | Stem-completion (from semantic memory) |
| Wiser et al (Reference Wiser, Andreasen and O'Leary1998) | 15 | 29 | 0.005 | No | No | PET | Recognition of words studied to 100% performance and of unstudied words | Reading words |
| Retrieval: high > low | High retrieval | Low retrieval | ||||||
| Crespo-Facorro et al (Reference Crespo-Facorro, Wiser and Andreasen2001) | 19 | 34 | 0.005 | 50 voxels | No | PET | Recognition of well-learned words1 | Recognition of words learned once1 |
| Heckers et al (Reference Heckers, Rauch and Goff1998) | 13 | 8 | 0.001 | No | No | PET | Stem-recall for words encoded four times | Stem-recall for words encoded once |
| Heckers et al (Reference Heckers, Curran and Goff2000) | 9 | 8 | 0.001 | No | No | PET | Recognition of studied images | Recognition of unstudied images |
| Ragland et al (Reference Ragland, Gur and Valdez2004) | 13 | 15 | 0.005 | 8 voxels | Regions activated in either group | fMRI | Word recognition hits | Word recognition correct rejections |
| Weiss et al (Reference Weiss, Schacter and Goff2003) | 12 | 12 | 0.001 in hippocampus and prefrontal cortex, 0.05 corrected in other brain areas | No | Different thresholds | PET | Stem-recall for words encoded semantically | Stem-recall for words encoded perceptually |
| Weiss et al (Reference Weiss, Zalesak and DeWitt2004) | 15 | 16 | 0.01 | 5 voxels | Hippocampus | fMRI | Recognition of mainly studied words1 | Recognition of mainly unstudied words1 |
| Other studies | ||||||||
| Kubicki et al (Reference Kubicki, McCarley and Nestor2003) | 9 | 9 | 0.001 | 0.05 corrected | No | fMRI | Deep encoding (abstract/concrete judgements for words) | Shallow encoding (lower/upper-case judgements) |
| Barch et al (Reference Barch, Csernansky and Conturo2002) | 38 | 48 | 0.0025 | 9 voxels | No | fMRI | Conjunction of encoding > fixation and recognition > fixation for both verbal and figural stimuli | |
Table 2 Thresholds associated with each category of contrasts to which the meta-analysis procedure was applied
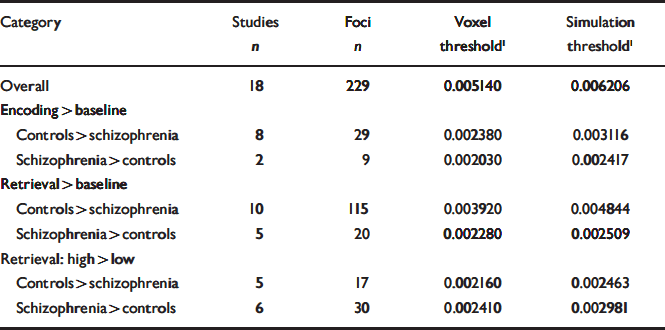
| Category | Studies n | Foci n | Voxel threshold1 | Simulation threshold1 |
|---|---|---|---|---|
| Overall | 18 | 229 | 0.005140 | 0.006206 |
| Encoding > baseline | ||||
| Controls > schizophrenia | 8 | 29 | 0.002380 | 0.003116 |
| Schizophrenia > controls | 2 | 9 | 0.002030 | 0.002417 |
| Retrieval > baseline | ||||
| Controls > schizophrenia | 10 | 115 | 0.003920 | 0.004844 |
| Schizophrenia > controls | 5 | 20 | 0.002280 | 0.002509 |
| Retrieval: high > low | ||||
| Controls > schizophrenia | 5 | 17 | 0.002160 | 0.002463 |
| Schizophrenia > controls | 6 | 30 | 0.002410 | 0.002981 |
Statistical significance of these ALE values was then determined using 1000 simulated sets of 228 randomly distributed foci throughout the brain. Based on these simulations, two methods were used to determine a significance threshold. First, following the method described by Turkeltaub et al (Reference Turkeltaub, Eden and Jones2002), we used as a threshold the ALE value that was observed with a voxel probability of 0.001 (i.e. obtained by chance only once in every 1000 voxels in the simulations). Second, we also extracted the value corresponding to 0.05 simulations (i.e. obtained by chance in at least 1 voxel in only 5% of the simulations). This threshold was found to be more restrictive than the voxel threshold and the regions that met this threshold are highlighted in bold characters in Tables 3 and 4.
Table 3 Overall regions of significant agglomeration of foci of differential activation between participants with schizophrenia and controls during the performance of memory tasks. The foci reported in bold also met the simulation threshold
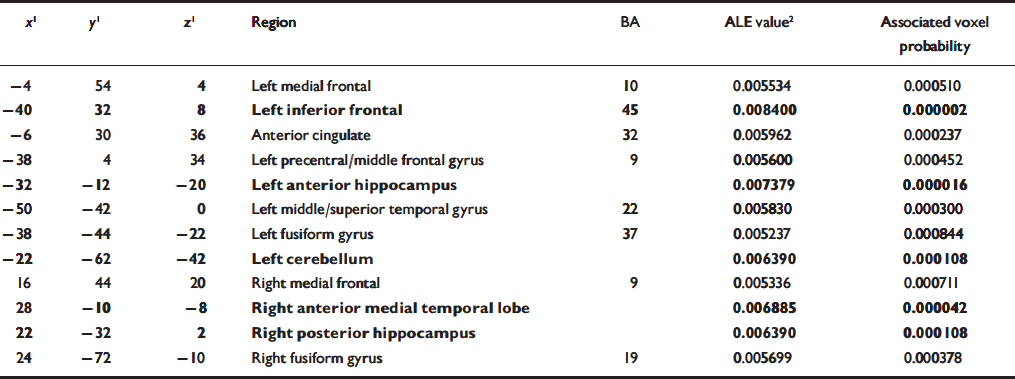
| x 1 | y 1 | z 1 | Region | BA | ALE value2 | Associated voxel probability |
|---|---|---|---|---|---|---|
| –4 | 54 | 4 | Left medial frontal | 10 | 0.005534 | 0.000510 |
| –40 | 32 | 8 | Left inferior frontal | 45 | 0.008400 | 0.000002 |
| –6 | 30 | 36 | Anterior cingulate | 32 | 0.005962 | 0.000237 |
| –38 | 4 | 34 | Left precentral/middle frontal gyrus | 9 | 0.005600 | 0.000452 |
| –32 | –12 | –20 | Left anterior hippocampus | 0.007379 | 0.000016 | |
| –50 | –42 | 0 | Left middle/superior temporal gyrus | 22 | 0.005830 | 0.000300 |
| –38 | –44 | –22 | Left fusiform gyrus | 37 | 0.005237 | 0.000844 |
| –22 | –62 | –42 | Left cerebellum | 0.006390 | 0.000108 | |
| 16 | 44 | 20 | Right medial frontal | 9 | 0.005336 | 0.000711 |
| 28 | –10 | –8 | Right anterior medial temporal lobe | 0.006885 | 0.000042 | |
| 22 | –32 | 2 | Right posterior hippocampus | 0.006390 | 0.000108 | |
| 24 | –72 | –10 | Right fusiform gyrus | 19 | 0.005699 | 0.000378 |
Table 4 Regions of significant agglomeration of foci of differential activation between participants with schizophrenia and controls for three categories of contrasts. The foci reported in bold also met the simulation threshold
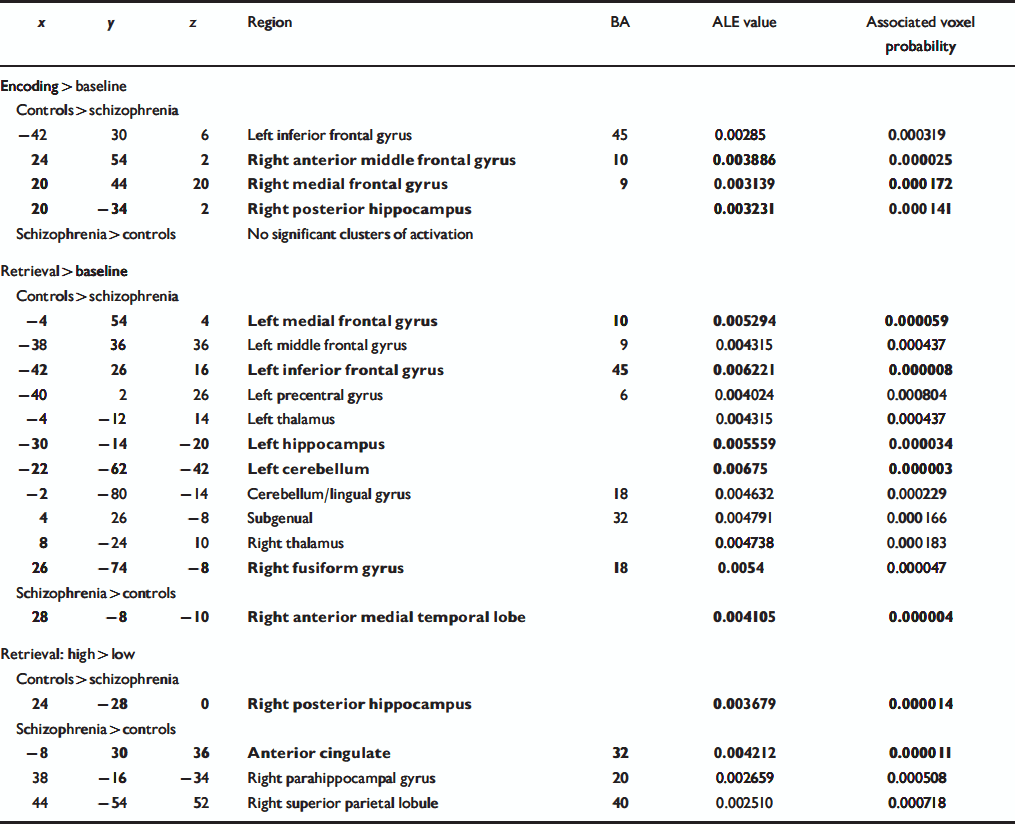
| x | y | z | Region | BA | ALE value | Associated voxel probability |
|---|---|---|---|---|---|---|
| Encoding > baseline | ||||||
| Controls > schizophrenia | ||||||
| –42 | 30 | 6 | Left inferior frontal gyrus | 45 | 0.00285 | 0.000319 |
| 24 | 54 | 2 | Right anterior middle frontal gyrus | 10 | 0.003886 | 0.000025 |
| 20 | 44 | 20 | Right medial frontal gyrus | 9 | 0.003139 | 0.000172 |
| 20 | –34 | 2 | Right posterior hippocampus | 0.003231 | 0.000141 | |
| Schizophrenia > controls | No significant clusters of activation | |||||
| Retrieval > baseline | ||||||
| Controls > schizophrenia | ||||||
| –4 | 54 | 4 | Left medial frontal gyrus | 10 | 0.005294 | 0.000059 |
| –38 | 36 | 36 | Left middle frontal gyrus | 9 | 0.004315 | 0.000437 |
| –42 | 26 | 16 | Left inferior frontal gyrus | 45 | 0.006221 | 0.000008 |
| –40 | 2 | 26 | Left precentral gyrus | 6 | 0.004024 | 0.000804 |
| –4 | –12 | 14 | Left thalamus | 0.004315 | 0.000437 | |
| –30 | –14 | –20 | Left hippocampus | 0.005559 | 0.000034 | |
| –22 | –62 | –42 | Left cerebellum | 0.00675 | 0.000003 | |
| –2 | –80 | –14 | Cerebellum/lingual gyrus | 18 | 0.004632 | 0.000229 |
| 4 | 26 | –8 | Subgenual | 32 | 0.004791 | 0.000166 |
| 8 | –24 | 10 | Right thalamus | 0.004738 | 0.000183 | |
| 26 | –74 | –8 | Right fusiform gyrus | 18 | 0.0054 | 0.000047 |
| Schizophrenia > controls | ||||||
| 28 | –8 | –10 | Right anterior medial temporal lobe | 0.004105 | 0.000004 | |
| Retrieval: high > low | ||||||
| Controls > schizophrenia | ||||||
| 24 | –28 | 0 | Right posterior hippocampus | 0.003679 | 0.000014 | |
| Schizophrenia > controls | ||||||
| –8 | 30 | 36 | Anterior cingulate | 32 | 0.004212 | 0.000011 |
| 38 | –16 | –34 | Right parahippocampal gyrus | 20 | 0.002659 | 0.000508 |
| 44 | –54 | 52 | Right superior parietal lobule | 40 | 0.002510 | 0.000718 |
After applying this procedure for all 228 foci regardless of the tasks, we grouped the individual contrasts into independent categories:
-
(a) memory encoding v. low-level baseline (8 studies);
-
(b) memory retrieval v. non-episodic retrieval comparison tasks (11 studies);
-
(c) high v. low retrieval conditions (6 studies).
Only two studies used contrasts that did not fit into any of these categories (see Table 1).
For the studies using high v. low levels of retrieval conditions, some between-group contrasts were performed using the within-group contrast in one direction (high retrieval > low retrieval), whereas others were in the other direction (low retrieval > high retrieval). To combine the different studies while taking the direction of the group difference into account, within-group contrasts that were in the low > high retrieval direction (Reference Crespo-Facorro, Wiser and AndreasenCrespo-Facorro et al, 2001; Reference Weiss, Zalesak and DeWittWeiss et al, 2004) were reversed by also reversing the group comparison. (Between-group functional neuroimaging analyses are in fact group by task interactions, i.e. a contrast of contrasts. Within-subject contrasts have to be performed first and the output of these contrasts are then used for the between-group comparisons: it is a difference of differences. For two groups (A and B) and two tasks (1 and 2), we can say that mathematically (A1-A2)-(B1-B2)=(B2-B1)-(A2-A1) and (A2-A1)-(B2-B1)= (B1-B2)-(A1-A2). It is thus possible to reverse a within-subject contrast by also reversing the group contrast.) Three-dimensional maps of smoothed foci were then created and thresholded for each of the three categories and each between-group contrast direction (Table 2) with the same valid method that could also be used for combining within-group contrasts.
RESULTS
Overall meta-analysis
When all foci were included in the meta-analysis, the areas of significant agglomeration were located in the left inferior prefrontal cortex, bilateral medial frontal cortex, anterior cingulate, left precentral/middle frontal gyrus, bilateral anterior medial temporal lobe, right posterior hippocampus, left superior temporal gyrus, bilateral fusiform gyrus and left cerebellum (Table 3, Fig. 1).
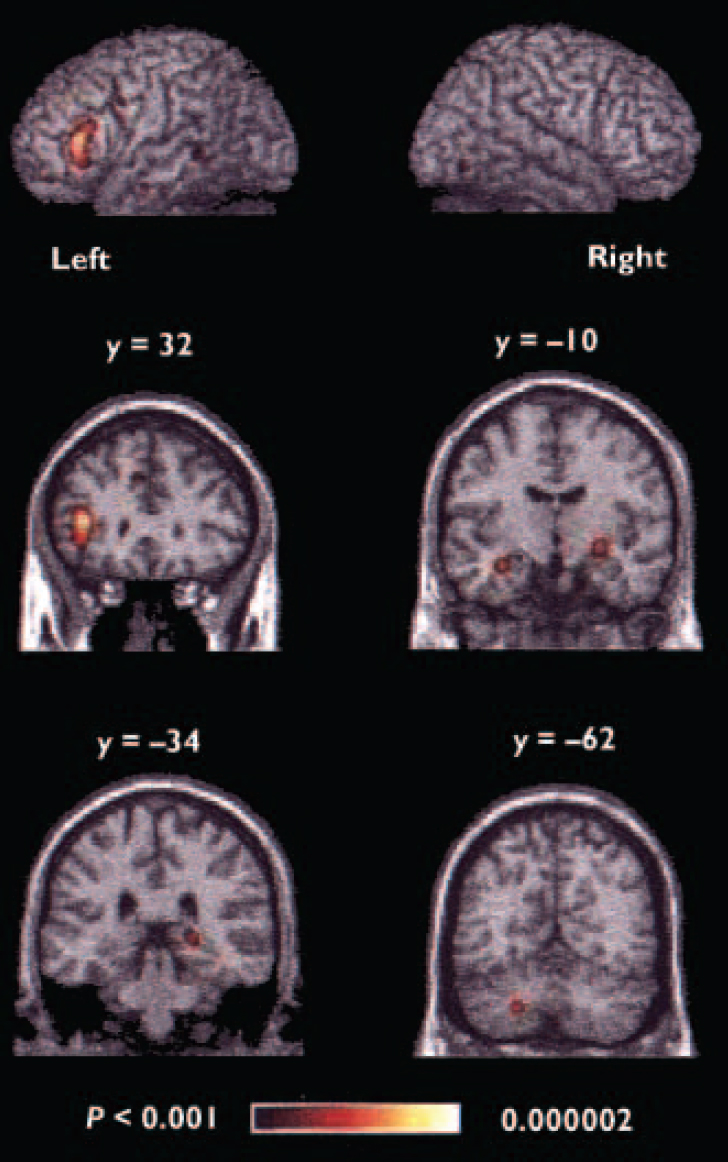
Fig. 1 Regions of significant agglomeration of foci in the overall meta-analysis. The y values given for the coronal slices denote the coordinates in the Talairach/MNI system; the colour range represents the above threshold voxel probability.
Specific contrasts
The clustering into three categories allowed us to identify the regions that were either more or less active in the schizophrenia group relative to the control group.
For the encoding v. low-level baseline category (Table 4, Fig. 2a), the control group exhibited greater activation across studies in the left inferior prefrontal cortex, right anterior middle frontal gyrus, right medial frontal gyrus and right posterior hippocampus relative to people with schizophrenia. No region showed significant agglomeration for the schizophrenia group relative to controls.

Fig. 2 Regions of significant agglomeration of foci in category of contrasts: (a) encoding v. low-level baseline; (b) retrieval v. non-episodic retrieval baseline; (c) high v. low retrieval. The y and x values denote the coordinates in the Talairach/MNI system for the coronal slices and sagittal slices, respectively; the colour ranges represent the above-threshold voxel probability.
For the retrieval v. non-episodic retrieval comparison tasks category (Table 4, Fig. 2b), control participants had clusters of greater activation in the left inferior prefrontal cortex, left middle frontal gyrus, left medial frontal gyrus, right subgenual region, left precentral gyrus, bilateral thalamus, left anterior hippocampus, right fusiform gyrus and bilateral cerebellum. The only region to show significant agglomeration for people with schizophrenia relative to controls was the right anterior medial temporal temporal lobe (border between hippocampus and amygdala).
For the high retrieval v. low retrieval category (Table 4, Fig. 2c), a cluster of greater activation for the control participants was observed in the right posterior hippocampus. For participants with schizophrenia, clusters were observed in the cingulate gyrus, right parahippocampal gyrus and right superior parietal cortex.
DISCUSSION
Using a new meta-analytic approach, we performed a quantitative review of functional neuroimaging studies of episodic memory in schizophrenia. This meta-analysis revealed a set of brain regions showing consistent patterns of differential activation between people with schizophrenia and healthy individuals during the performance of diverse memory tasks. Consistent with previous models of brain dysfunctions in schizophrenia and qualitative reviews on this same topic (Reference Weiss and HeckersWeiss & Heckers, 2001), the regions that showed the most consistent differences between people with schizophrenia and healthy participants were located in the prefrontal cortex and in the temporal lobe. Hypotheses relating to the potential causes of this differential frontal and temporal activity in schizophrenia are discussed below. Foci of consistent differential activation between groups were also observed in the thalamus and cerebellum and provide support for abnormalities in the cortical-thalamic-cerebellar-cortical circuit in schizophrenia.
Left inferior prefrontal cortex
The left inferior prefrontal cortex was the main region that distinguished between control and schizophrenia groups in this meta-analysis. This region showed the most significant agglomeration of foci in the overall meta-analysis, and was found to be consistently more active in controls relative to people with schizophrenia during both encoding and retrieval relative to baseline conditions. The region is involved in elaborative semantic or phonological processing and in the implementation of strategies (e.g. organisation) during episodic memory encoding (Reference Dempster and BrainerdDempster & Brainerd, 1995; Reference Simons and SpiersSimons & Spiers, 2003); it is also active during strategic search, the maintenance of successfully retrieved information and response selection during retrieval (Reference Badre and WagnerBadre & Wagner, 2002; Reference PetridesPetrides, 2002; Reference Petrides, Alivisatos and FreyPetrides et al, 2002; Reference Simons and SpiersSimons & Spiers, 2003). Interestingly, it has been postulated that during encoding, people with schizophrenia fail to engage spontaneously in efficient elaborative processing. Ragland et al (Reference Ragland, Gur and Raz2001) have suggested that people with schizophrenia process stimuli on a more superficial level and do not spontaneously use semantic processing to guide memory encoding and retrieval. None the less, it seems that people with schizophrenia can use specific cues or strategies to improve their performance when they are provided with them (Reference Kubicki, McCarley and NestorKubicki et al, 2003; Reference Ragland, Moelter and McGrathRagland et al, 2003). For example, Ragland et al (Reference Ragland, Moelter and McGrath2003) used a level of processing paradigm (Reference Craik and LockhartCraik & Lockhart, 1972) to study verbal recognition memory in schizophrenia. During encoding, participants were instructed to make either ‘upper case’ v. ‘lower case’ judgements (shallow encoding) or ‘concrete’ v. ‘abstract’ judgements (deep encoding). On a subsequent recognition memory test there was no significant difference in performance between control participants and those with schizophrenia, and both groups benefited from deeper encoding. Similarly, Paul et al (Reference Paul, Elvevåg and Bokat2005) observed a modulation of recognition memory performance by the level of processing in schizophrenia, although in their study patients performed more poorly on the recognition tasks relative to the control group. It follows that it is not primarily the capacity to use elaborative processing to improve memory performance that is affected in schizophrenia, but rather the tendency to use a deep elaborative encoding strategy when no specific instructions are provided.
In healthy participants a relationship between left inferior prefrontal cortex activity and the use of deeper processing during encoding has been extensively reported (e.g. Reference Kapur, Craik and TulvingKapur et al, 1994; Reference Kubicki, McCarley and NestorKubicki et al, 2003; Reference Petersson, Sandblom and ElfgrenPetersson et al, 2003). This same relationship was examined in people with schizophrenia by Kubicki et al (Reference Kubicki, McCarley and Nestor2003), who contrasted the neural correlates of elaborative semantic processing with those related to perceptual processing. Relative to controls, people with schizophrenia showed reduced activation of the left inferior prefrontal cortex for the contrast between semantic and perceptual encoding, despite their normal performance and despite the normal modulation of memory performance by level of processing (Reference Craik and LockhartCraik & Lockhart, 1972). This difference in activation may stem from a between-group difference in stimulus processing, reflecting either relatively reduced processing during semantic encoding or relatively increased processing during perceptual encoding, even if this difference does not affect subsequent recognition performance.
It has been suggested that some of the cognitive impairments in schizophrenia might be secondary to patients’ impaired ability to use organisational strategies to maximise performance (Reference BaumanBauman, 1971; Reference Koh, Kayton and PetersonKoh et al, 1976; Reference Iddon, McKenna and SahakianIddon et al, 1998; Reference Hazlett, Buchsbaum and JeuHazlett et al, 2000). For instance, people with schizophrenia do not spontaneously use semantic clustering as a strategy to improve their memory performance (Reference Gold, Randolph and CarpenterGold et al, 1992; Reference Paulsen, Heaton and SadekPaulsen et al, 1995; Brebion et al, Reference Brebion, Amador and Smith1997, Reference Brebion, David and Jones2004; Reference Hazlett, Buchsbaum and JeuHazlett et al, 2000; Reference Nohara, Suzuki and KurachiNohara et al, 2000). Organisational strategies are thought to rely at least in part on the left inferior prefrontal cortex during both encoding and retrieval (Reference Fletcher, Shallice and DolanFletcher et al, 1998; Reference Wagner, Koutstaal and SchacterWagner et al, 1999; Reference Simons and SpiersSimons & Spiers, 2003). With regards to retrieval, Nohara et al (Reference Nohara, Suzuki and Kurachi2000) have observed a significant correlation between categorical clustering and the activation in this cortical region in control participants that was not observed in people with schizophrenia. Taken together, these results suggest that the differential activation of this region in people with schizophrenia could be related to their inability to use efficient strategies spontaneously during both encoding and retrieval.
Another possibility is that the decreased activation of the left inferior prefrontal cortex is related to memory performance because this region has also been thought to be involved in retrieval success (Reference Habib, Lepage and TulvingHabib & Lepage, 2000; Reference Konishi, Wheeler and DonaldsonKonishi et al, 2000). The region was, however, observed to be deactivated in schizophrenia even when there was no significant difference in performance between groups (Reference Andreasen, O'Leary and CizadloAndreasen et al, 1996; Reference Wiser, Andreasen and O'LearyWiser et al, 1998; Reference Ragland, Gur and RazRagland et al, 2001; Reference Hofer, Weiss and GolaszewskiHofer et al, 2003a ; Reference Kubicki, McCarley and NestorKubicki et al, 2003). Even if it has been shown that people with schizophrenia are less likely to base their recognition judgements on conscious recollection relative to control participants (Reference Huron, Danion and GiacomoniHuron et al, 1995; Reference Danion, Rizzo and BruantDanion et al, 1999), it is unlikely that the deactivation of this cortical region observed in this group relates to the reduced proportion of successfully or consciously recollected items, because there was no significant clustering of differential activation between groups in that region for the contrast between high and low retrieval conditions.
Medial temporal lobes
Functional neuroimaging studies in healthy individuals have consistently implicated the medial temporal lobes in both memory encoding and memory retrieval (Reference Lepage, Habib and TulvingLepage et al, 1998; Reference Schacter and WagnerSchacter & Wagner, 1999). During encoding, the hippocampal formation is thought to support the encoding of information in a meaningful way, such as creating new associations (Reference Vandenberghe, Price and WiseVandenberghe et al, 1996; Henke et al, Reference Henke, Buck and Weber1997, Reference Henke, Weber and Kneifel1999; Reference Lepage, Habib and CormierLepage et al, 2000; Reference Davachi, Maril and WagnerDavachi et al, 2001; Reference Davachi and WagnerDavachi & Wagner, 2002; Reference Achim and LepageAchim & Lepage, 2005b ). Medial temporal activation during encoding has also been shown to predict subsequent recognition success (Reference Brewer, Zhao and DesmondBrewer et al, 1998; Reference Wagner, Schacter and RotteWagner et al, 1998; Reference Davachi, Mitchell and WagnerDavachi et al, 2003; Reference Chua, Rand-Giovannetti and SchacterChua et al, 2004; Reference Jackson and SchacterJackson & Schacter, 2004), supporting the implication of this structure in the creation of durable memory traces. Our meta-analysis revealed a significant agglomeration of foci of reduced activation in people with schizophrenia in the right hippocampus during encoding, possibly reflecting the less efficient or less associative encoding strategies used by people with schizophrenia.
Different lines of evidence suggest that during memory retrieval the hippocampus supports conscious recollection of information from memory, whereas the parahippocampal gyrus is involved in familiarity assessment (Reference YonelinasYonelinas, 2002). It has also been suggested that people with schizophrenia could have a specific deficit in conscious recollection (Reference Huron, Danion and GiacomoniHuron et al, 1995; Reference Danion, Rizzo and BruantDanion et al, 1999; Reference Weiss, Schacter and GoffWeiss et al, 2003). The results from our meta-analysis support this idea by showing that people with schizophrenia present a deactivation of the region implicated in conscious recollection (i.e. the hippocampus) when exposed to conditions favouring the recovery of information from memory (high v. low retrieval). Moreover, this hippocampal deactivation is accompanied by an overactivation of the region implicated in familiarity assessment (i.e. the parahippocampal gyrus), suggesting that people with schizophrenia use familiarity assessment rather than conscious recollection as a basis for retrieval.
Another interesting observation is a focus of agglomeration of greater activation in schizophrenia relative to controls during memory retrieval v. baseline, centred in the right anterior medial temporal lobe. In contrast, control participants show greater left lateralised activation in this region for the same comparison. This pattern of activation is consistent with the idea that people with schizophrenia show less lateralised medial temporal activation as a function of stimulus type (verbal and non-verbal) (Reference Crow, Ball and BloomCrow et al, 1989; Reference Gur, Jaggi and ShtaselGur et al, 1994), because all studies included in the retrieval v. baseline category used verbal stimuli.
Other frontal regions
A few other prefrontal regions showed significant agglomeration of foci in our meta-analysis. For instance, the left middle frontal gyrus showed significant agglomeration of foci in the controls relative to people with schizophrenia for the retrieval v. baseline contrast. This region is known to support post-retrieval monitoring during episodic memory retrieval, with activation typically in the right middle frontal gyrus for more simple monitoring and additional involvement of the left middle frontal gyrus for more complex tasks (Reference Achim and LepageAchim & Lepage, 2005a ). The observation that the left middle frontal gyrus shows a greater activation in controls suggests a specific impairment of more complex post-retrieval monitoring processes in schizophrenia, a view consistent with the finding that more complex tasks reveal greater memory impairment (Reference Danion, Rizzo and BruantDanion et al, 1999).
The anterior medial prefrontal cortex also showed significant agglomeration in the general meta-analysis as well as in encoding v. baseline and retrieval v. baseline. This region is thought to be involved in the retrieval of self-relevant or self-generatedgenerated self-information (Reference Simons and SpiersSimons & Spiers, 2003; Reference Fossati, Hevenor and LepageFossati et al, 2004), although the specific mechanisms remain to be understood. Failure to activate these regions in people with schizophrenia could reflect their difficulty in using sources of information other than the ones provided by the task.
The anterior cingulate also showed significant agglomeration in both the overall meta-analysis and the high v. low retrieval category. Paus et al (Reference Paus, Koski and Caramanos1998) have suggested that the anterior cingulate has a role in memory and cognitive effort. It could be the case that this activity reflects the greater effort needed by people with schizophrenia to perform these memory tasks.
Other regions: cerebellum, thalamus
The cerebellum projects to motor and prefrontal regions of the cerebral cortex through synapses in the thalamus. Through this circuit, the cerebellum is thought to modulate and coordinate both motor and cognitive functions (Andreasen et al, Reference Andreasen, O'Leary and Cizadlo1996, Reference Andreasen, Paradiso and O'Leary1998, Reference Andreasen, Nopoulos and O'Leary1999). Converging evidence has pointed to dysfunctions of this cortical-cerebellar-thalamic-cortical circuit in schizophrenia, and our findings of significant agglomerations of foci observed in the cerebellum and thalamus are consistent with this notion. The specific neural mechanisms underlying the coordination of cognitive activity by the cerebellum, however, remain to be clarified. It has been proposed that the cerebellum could modulate memory processing through a mechanism for correction of discrepancies (Reference SchmahmannSchmahmann, 1991; Reference Fink, Markowitsch and ReinkemeierFink et al, 1996; Reference Greve, Stanford and SuttonGreve et al, 1999) or through a mechanism for coordination between different pieces of information (Reference FabbroFabbro, 2000).
Studies without Talairach/MNI coordinates
Five functional neuroimaging studies of memory in schizophrenia could not be included in the meta-analysis because no Talairach/MNI coordinates were reported (Reference Ragland, Gur and GlahnRagland et al, 1998; Reference Shihabuddin, Buchsbaum and HazlettShihabuddin et al, 1998; Hazlett et al, Reference Hazlett, Buchsbaum and Byne1999, Reference Hazlett, Buchsbaum and Jeu2000; Reference Nohara, Suzuki and KurachiNohara et al, 2000). Two of these studies used restricted regions of interest either in the thalamus (Reference Hazlett, Buchsbaum and ByneHazlett et al, 1999) or in the striatum (Reference Shihabuddin, Buchsbaum and HazlettShihabuddin et al, 1998) and observed decreased activation in these regions in schizophrenia relative to controls. The other three studies examined the entire brain and, consistent with our results, reported reduced activation in participants with schizophrenia in the left inferior frontal gyrus and other frontal regions (Reference Ragland, Gur and GlahnRagland et al, 1998; Reference Hazlett, Buchsbaum and JeuHazlett et al, 2000; Reference Nohara, Suzuki and KurachiNohara et al, 2000) as well as in the temporal lobe (Reference Ragland, Gur and GlahnRagland et al, 1998; Reference Hazlett, Buchsbaum and JeuHazlett et al, 2000) during episodic memory tasks. Overall these results are consistent with those that reported Talairach/MNI coordinates for peaks of activation, making it unlikely that the exclusion of these five studies from our meta-analysis biased our results.
Future research
In summary, the quantitative aspect of this meta-analytic method represents an improvement over current qualitative methods because it offers an objective way of regrouping studies and summarising results. However, it must be noted that most of the studies reviewed here compared a memory task (encoding or retrieval) with a low-level baseline. Such contrasts are not ideal for discriminating the neural correlates of specific memory processes and instead are likely to reflect several cognitive and perceptual processes (Reference Stark and SquireStark & Squire, 2001). More sophisticated experimental designs would increase our chances of identifying selective deficient processes in schizophrenia and ultimately the neural correlates of these abnormal processes.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ People with schizophrenia have abnormal patterns of brain activation during both memory encoding and retrieval.
-
▪ Several brain regions, including the prefrontal cortex and hippocampus, are implicated in these abnormal memory functions.
-
▪ One challenge to the cognitive remediation of memory problems in schizophrenia will be to take into consideration these multiple faulty memory processes and possibly executive dysfunctions when devising an intervention.
LIMITATIONS
-
▪ It was not possible to integrate the results from studies that did not report specific coordinates for their activation foci.
-
▪ The results obtained using this meta-analytic approach might have been biased by the inclusion of studies that used regions of interest (e.g. the hippocampus).
-
▪ The number of studies in each category was insufficient to assess the effect of important variables such as medication status or age on the pattern of agglomeration of activation foci.
Acknowledgements
This study was supported by the Canadian Institutes of Health Research (CIHR; grant 53280), Fonds de Recherche en Santé du Québec and the National Alliance for Research on Schizophrenia and Depression, and by a CIHR studentship to A.M.A. We thank Jorge Armony and André Achim for technical support and Matt Menear for editing the manuscript.









eLetters
No eLetters have been published for this article.