Antidepressants such as the selective serotonin reuptake inhibitors (SSRIs) are widely used to treat major depression. Although they have reasonable efficacy they also produce adverse effects, of which the best known include headache, changes in sleep pattern, changes in gastrointestinal function, and changes in sexual functioning. 1 Worsened anxiety and agitation may be seen in the first few days of treatment. Other subjective side-effects are not usually considered by healthcare professionals, yet ‘blunting of emotions’ is mentioned by some people who take SSRIs, in clinic and on web forums. They report that, although they feel less emotional pain than before, they also experience a restricted range of other emotions that are a normal part of everyday life. It is unclear whether these experiences relate to the mode of action of the antidepressants. Although some research reports have emerged that may be relevant to such complaints, Reference Hoehn-Saric, Lipsey and McLeod2–Reference Opbroek, Delgado, Laukes, McGahuey, Katsanis and Moreno4 there has been no systematic investigation of people's experiences of this phenomenon.
We aimed to understand, from the patient's perspective, the phenomenon of SSRI-associated emotional blunting. Furthermore, we aimed to use this understanding to develop an item bank that would inform the development of a reliable and valid questionnaire measure of this phenomenon.
Method
Study design
This qualitative study used two different data sources (interview participants from local recruitment in Oxfordshire and anonymous data sources posting on web forums) and two different data-gathering methods (individual interviews and group interview) to understand the phenomenon. Ethical approval for the interview study was obtained from Oxfordshire REC A (06/Q1604/184).
Data sources
Interviews
Participants were recruited in three ways: introduction by general practitioners (GPs) (three Oxfordshire general practices searched their database for people with recent SSRI prescriptions and mailed study details to 220 individuals); introduction by Oxfordshire psychiatrists; and a recruitment poster. Inclusion criteria were: aged 18 years or over; fluent in spoken and written English; had taken an SSRI regularly for any reason; and attributed undesirable emotional symptoms to the SSRI. The sample therefore included some participants no longer taking an SSRI, in order to include people who were non-adherent because of emotional side-effects. Purposive sampling was used, to ensure that sufficient variation was present in the sample, i.e. different genders, age groups, diagnoses (currently depressed v. non-depressed) and SSRI adherence.
Web forums
Four openly accessible public web forums were systematically searched for evidence (Appendix 1).
Data gathering
Interviews
Participant data were gathered, including date of birth; gender; ethnic group; employment status; marital status; indication(s) for SSRI prescription; duration of indication(s); details of SSRI – name, dose, duration, time since stopping (if any); details of any other psychotropic medication taken; medical comorbidity; and psychoactive substance misuse (alcohol and illicit drugs) in the last week. All participants completed the Beck Depression Inventory–II (BDI–II) Reference Beck, Steer, Ball and Ranieri5 before interview.
Thirty-eight semi-structured individual interviews were conducted, as part of a continuous process of data gathering, data analysis and ongoing refinement of our understanding of emotional side-effects. Interviews were conducted by V.C., who introduced herself as a researcher from a team interested in depression, its treatments and their side-effects. Interviews were audio-recorded. Participants were asked to comment on their experiences, good and bad, of SSRIs; to comment specifically on adverse experiences; and finally to expand on emotional effects of SSRIs, including ‘emotional blunting’. As understanding increased, more specific questions were added.
A single group interview was then conducted with a subsample of eight currently depressed participants, in order to refine our understanding of emotional blunting in that group. The focus group was facilitated by J.P. and V.C. This interview was both audio- and video-recorded.
Finally, once the main framework from qualitative analysis was formulated, the main themes and subthemes from the framework were formed into items for a draft questionnaire of emotional side-effects of antidepressants. A further series of 11 individual interviews were conducted with a subsample of participants, in order to validate the findings (‘respondent validation’) and field test the draft questionnaire.
Web forums
The forums were searched systematically for relevant posts. All posts of possible relevance were retrieved, along with any descriptive characteristics, such as age and gender, of the posters. Posts relating to any antidepressant were included.
Data analysis
Quantitative data relating to interview participants were summarised using simple approaches. Raw BDI–II scores were categorised into minimal (0–13), mild (14–19), moderate (20–28), and severe (29–63) depression. Reference Beck, Steer, Ball and Ranieri5 A proprietary computer program XSight version 2 running on Windows XP was used for qualitative data management (www.qsrinternational.com). Data gathered on audiotape were transcribed verbatim by an experienced transcriber, reviewed by V.C., and uploaded to XSight. Statements and linked descriptive data retrieved from web forums were also uploaded to XSight. Qualitative analysis used a simple method, the ‘framework technique’. Reference Ritchie, Spencer, Bryman and Burgess6 Participant data were interpreted and summarised, leading to a framework of specific phenomena that appeared increasingly likely to describe the range of emotional side-effects of SSRIs. Most of the analysis was conducted by V.C., but J.P. co-analysed some data and met regularly with V.C. to discuss emerging findings and refine the emerging framework. Several methods were used to reduce the impact of researcher bias, including awareness of preconceptions; sharing preconceptions between researchers; ensuring that all interpretations were supported by participant-derived data; and respondent validation.
Results
Interview participants
Ninety-two individuals contacted the research team with a view to participating in this study. Of these, 24 did not fulfil inclusion criteria, usually as a result of their lack of emotional side-effects of antidepressants; 17 received study information but did not pursue their interest; 8 contacted the team towards the end of the study and did not fulfil sampling requirements; and 2 actively declined to participate. Of the 41 individuals who agreed to participate, 3 subsequently withdrew from the study prior to interview. The final sample therefore consisted of 38 participants, 19 of whom were recruited via poster, 18 via GP mailing, and 1 via psychiatrists.
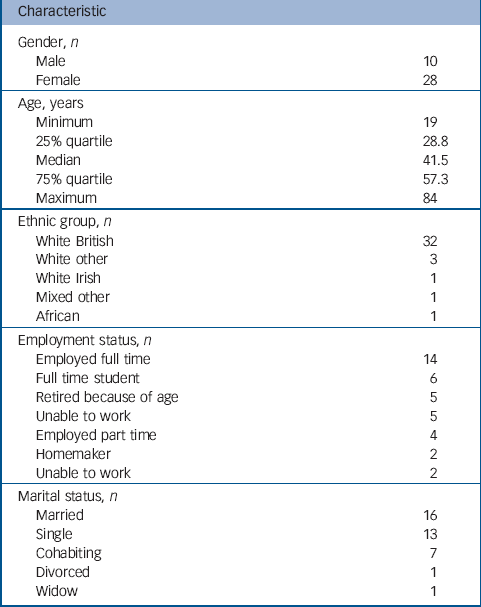
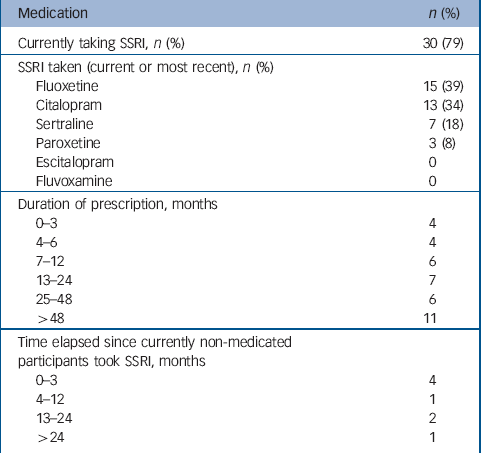
Demographics of the sample are summarised in Table 1. Diagnoses of participants, derived from self-report and BDI–II scores are summarised in Table 2. Just over half of participants had BDI–II scores within the range for ‘minimal’ depression, and over one-quarter had scores of five or less. Current or most recent antidepressant medication taken by participants is summarised in Table 3. Eight of the sample were not taking an SSRI currently, but participated in the study because they reported emotional side-effects of SSRIs and had coherent descriptions of those side-effects. Participants had taken the SSRI for a median duration of 23 months.
Table 1 Demographic characteristics of interview participants
| Characteristic | |
|---|---|
| Gender, n | |
| Male | 10 |
| Female | 28 |
| Age, years | |
| Minimum | 19 |
| 25% quartile | 28.8 |
| Median | 41.5 |
| 75% quartile | 57.3 |
| Maximum | 84 |
| Ethnic group, n | |
| White British | 32 |
| White other | 3 |
| White Irish | 1 |
| Mixed other | 1 |
| African | 1 |
| Employment status, n | |
| Employed full time | 14 |
| Full time student | 6 |
| Retired because of age | 5 |
| Unable to work | 5 |
| Employed part time | 4 |
| Homemaker | 2 |
| Unable to work | 2 |
| Marital status, n | |
| Married | 16 |
| Single | 13 |
| Cohabiting | 7 |
| Divorced | 1 |
| Widow | 1 |
Table 2 Diagnosis and current depression score of interview participants

| n | |
|---|---|
| Diagnosis from participant report | |
| Depressive disorder only, total n | 23 |
| Single depressive episode | 4 |
| Recurrent depressive episode | 8 |
| Prevention of recurrent depressive episode | 11 |
| Anxiety disorder and depressive disorder | 8 |
| Anxiety disorder only | 2 |
| Obsessive—compulsive disorder only | 2 |
| Chronic pain and depressive disorder | 2 |
| Anxiety disorder and eating disorder | 1 |
| Beck Depression Inventory—II scoresa | |
| Minimal (0-13) | 21 |
| Mild (14-19) | 4 |
| Moderate (20-28) | 6 |
| Severe (29-63) | 7 |
Table 3 Current or most recent antidepressant medication taken by interview participants
| Medication | n (%) |
|---|---|
| Currently taking SSRI, n (%) | 30 (79) |
| SSRI taken (current or most recent), n (%) | |
| Fluoxetine | 15 (39) |
| Citalopram | 13 (34) |
| Sertraline | 7 (18) |
| Paroxetine | 3 (8) |
| Escitalopram | 0 |
| Fluvoxamine | 0 |
| Duration of prescription, months | |
| 0-3 | 4 |
| 4-6 | 4 |
| 7-12 | 6 |
| 13-24 | 7 |
| 25-48 | 6 |
| >48 | 11 |
| Time elapsed since currently non-medicated participants took SSRI, months | |
| 0-3 | 4 |
| 4-12 | 1 |
| 13-24 | 2 |
| >24 | 1 |
Framework themes
Eight key framework themes were identified, each of which contained multiple subthematic categories. These key themes are summarised in Appendix 2, and described below. The description of each theme is supported by quotations from participant interviews, either embedded within the text descriptions of each theme and denoted by italics (e.g. participants reported that their emotions were ‘more thoughts than feelings’) or as standalone quotations in the online supplement.
General effects on all emotions
Most participants described a general reduction in the intensity of all the emotions that they experienced, so that all their emotions felt flattened or evened out, and their emotional responses to all events were toned down in some way. Very common descriptions of this phenomenon included feelings of emotions being ‘dulled’, ‘numbed’, ‘flattened’ or completely ‘blocked’, as well as descriptions of feeling ‘blank’ and ‘flat’. A few participants described a more extreme phenomenon, in which they did not experience any emotions at all. Others felt that they often experienced their emotions as thoughts rather than as feelings, as if their emotional experience had become more ‘cognitive’ or ‘intellectual’. Some participants were able rationally to recognise situations in which they should feel a certain way, and yet the actual emotional response was not there or was altered in some way. Alternatively, some participants could still respond to emotional situations in an appropriate way, but without what they felt was real feeling.
Many participants described improved control over their emotions, so that what they considered to be excessive emotional reactions were reduced and more appropriate. This meant that they could more readily deal with or let go of certain emotions, and some participants described improved control over fear. Some participants described a difficulty in understanding or being in tune with what they were feeling, as if their own emotions were less clear to them. A few participants described that at times those emotions that were present seemed ‘unreal’, ‘fake’ or ‘artificial’.
Reduction of positive emotions
Almost all participants described a reduction in their positive emotions, which they attributed to their SSRI antidepressant. This reduction was manifest as both reduced intensity and reduced frequency of these emotions. Participants reported reduction in a wide range of positive emotions, including happiness, enjoyment, excitement, anticipation, passion, love, affection and enthusiasm.
Most participants reported that the intensity of positive emotions was ‘dampened down’ or ‘toned down’, such that participants did not experience the same emotional ‘lift’ or ‘high’. Many participants reported that they experienced positive emotions less often, and a few participants described that they were almost absent. Many participants described reduced enjoyment of, for example, social situations, hobbies or interests, beauty and nature, and music and other emotional media. Some participants reported that excitement and anticipation were reduced. They had, for example, lost the rush of excitement as an event approached, or no longer looked forward to things in the same way. Some participants felt reduced love or affection towards others and, in particular, reduced attraction towards their partner or reduced feelings of love or pride towards their family. Some participants described reduced passion, zest and enthusiasm for life and its components.
Reduction of negative emotions
All participants experienced a reduction of intensity or frequency of negative emotions, which they attributed to their SSRI antidepressant. Most participants considered that at some stage the reduction in negative emotions was beneficial to them, bringing relief from distressing negative emotions, and allowing normal daily life to resume. Some participants reported that negative emotions had been removed almost entirely. The negative emotions commonly described as reduced included sadness; emotional pain or distress; anger, irritability or aggression; and anxiety, worry or fear. Other negative emotions such as fear and surprise, embarrassment, guilt and shame, and disappointment were also mentioned to a degree. Although a reduction in these negative emotions was usually at some stage a benefit or relief, for many participants it had became an unwanted side-effect, impairing their quality of life. Participants described the need to be able to feel negative emotions when appropriate, such as grief or concern. Some were unable to respond with negative emotions, such as being unable to cry when this would have been appropriate or respond appropriately to bad news.
Emotional detachment
Most participants described feeling emotionally detached or disconnected, and attributed this to their SSRI antidepressant. Some participants described being detached from their surroundings, and described feelings of being ‘in limbo’, of ‘unreality’ or ‘disconnection’ and of feeling as though they were a ‘spectator’ rather than a participant. Some participants described functioning like a ‘zombie’ or ‘robot’, with reduced or absent emotional responses. Some participants described feeling detached from their own emotions and instincts. Most participants described that this emotional detachment extended to a detachment from other people. Specifically, they felt reduced sympathy and empathy, and felt detached during social interactions. In particular, many participants described an emotional detachment from their friends and family, including their partner or children. Participants' attitudes towards emotional detachment from other people were mixed. Although this was often seen as an undesirable side-effect of antidepressants, it was also sometimes seen as beneficial, by allowing disengagement from others' problems, others' negative emotions and highly charged situations that would otherwise be upsetting.
Just not caring
Almost all participants described not caring about things that used to matter to them and attributed this change to their SSRI antidepressant. They cared less about themselves, about other people and about the consequences of their actions. Not caring could have both helpful and unhelpful consequences, reducing the sense of pressure and stress that some participants felt in their daily lives, yet increasing the likelihood that important tasks were neglected.
Many participants described a general feeling of indifference to things in life that used to matter to them. Many participants described feeling apathetic and unmotivated, despite their illness having improved and attributed this apathy to their antidepressant. Some participants felt that their sensible, safety-conscious, side had diminished and they just did not care as much about the consequences to themselves of their behaviour. As a result, they might behave in a less careful, considered way. A few participants went further, mentioning thoughts of self-harm or suicide that they related, at least in part, to feelings of emotional detachment and emotional numbness. One participant had started to self-harm in an effort to feel emotion. Many participants reported not caring as much about others, such as during social interaction, by being less sensitive or courteous towards other people. In addition, many described reduced concern for others' feelings, and reduced concern about other peoples' opinions of them. Some participants described being less concerned or even unable to care about responsibilities in their everyday lives, such as at home, in their finances or at work, and might include, for example, a lack of urgency or need to complete tasks.
Changed personality
Some participants felt their personality had changed in some way, or been lost, leaving them ‘like a shell’. In some ways, they were not the person that they used to be. Participants reported that specific aspects of their personality, and, in particular, emotional aspects, had been changed or lost, such that they were a different person. These changes were attributed by participants to their SSRI antidepressant. Some participants believed that at times their antidepressant had made them behave quite out of character. One participant believed that the medication had changed their personality permanently, having a lasting effect beyond finishing their medication.
Effects on everyday life (helpful and unhelpful)
The impact of the above phenomena on participants' daily lives varied widely, both in extent and in perceived helpfulness.
-
(a) Unhelpful effects. Some participants were concerned that blunting of their emotions and, thereby, of their day-to-day concerns, might mask or hide problems. Concerns were expressed that this might prevent them resolving their own emotional issues, prevent them engaging with other problems or issues requiring their attention, and ‘cover up’ who they really were. ‘Just not caring’ had an unhelpful effect on everyday responsibilities, resulting in financial problems, and problems at work or college. Emotional detachment from family and reduced emotional responsiveness had an unhelpful impact on family life, and on perceived quality of parenting. Reduced inspiration, imagination, motivation and passion for and enjoyment of creative activities had adversely affected some participants' creativity. In some participants, emotional side-effects had led to reduced sociability. Emotional flattening, emotional detachment from other people, and reduced concern for other people's needs and feelings had unhelpful effects on relationships within families, with a significant other and at work. A few participants suggested that the emotional detachment and reduced anxiety arising from taking antidepressants was of concern when trying to make important life decisions, especially those with an emotional component.
-
(b) Helpful effects. However, some participants described helpful effects on their everyday lives that they attributed to emotional side-effects. For example, the reduction of certain emotional responses, such as anger, aggression or worry, could have a beneficial effect on personal relationships. Many participants believed that the emotional detachment and reduced anxiety arising from taking antidepressants had improved their ability to take a step back from their situation, and thereby to think more clearly and objectively. This helped them in making good decisions day to day, helped them to deal more successfully with other people, and improved their self-confidence.
It's because of my pills!
The emotional effects summarised in the above key themes were attributed by participants to their SSRI antidepressant, either in total or in part. Of note, some of the emotional effects are similar to symptoms of depression – for example, reduced positive emotions, reduced interest and reduced motivation. Some participants remarked on this difficulty. Indeed, some participants described similarities between the SSRI-induced state and depression itself, and suggested that the medication might increase or induce a kind of depression. Other participants were uncertain whether taking an antidepressant or a change in their life circumstances was the cause of changes in their emotional experiences. However, most participants felt able to distinguish emotional side-effects from their depression or other illness, for several reasons, including the following.
-
(a) Presence or persistence of an emotional syndrome when the participant perceives their illness to have improved or resolved. Some participants described the presence or persistence of an emotional syndrome although they perceived their illness to have improved or resolved completely. These participants stated that their lack of interest and lack of caring, combined with reduced positive emotions, were present despite the absence of any feelings of emotional pain or depression. Rather, all feelings and emotions were reduced.
-
(b) SSRI antidepressants making emotions feel ‘chemical’. A few participants felt able to distinguish emotional side-effects from their depression or other indication because of what they described as ‘a chemical feeling’, in which their emotions were experienced as ‘chemical’ or ‘artificial.’
-
(c) Effects of changes in dose or changes in SSRI antidepressant on the emotional syndrome. Some participants made specific reference to changes in their emotional experiences in relation to changes in medication type or dose. It was not possible to identify from the data whether specific SSRIs were more likely to have an emotional syndrome attributed to them, but some participants reported more emotional side-effects on specific SSRIs.
-
(d) Effects of discontinuation of SSRI antidepressant on the emotional syndrome. Some participants who had experienced periods of being on and off medication had noticed reduced emotional experience while on their SSRI, and a return of their emotional experiences upon discontinuation.
-
(e) The time course of the emotional syndrome. Many participants reported two distinct time courses of the emotional syndrome. A few participants described that they experienced these effects briefly during the early stages of taking their medication, and that subsequently it diminished. However, many participants noticed these emotional side-effects later on, often as they started to recover from their illness. Some participants considered that the ‘flattening’ was helpful, providing relief from their emotional distress early on. However, it became an unwanted side-effect as their emotional state improved, and they were left being more able to cope but with persistent flattening.
As they attributed their emotional syndrome to their SSRI antidepressant, many participants had considered whether they should stop taking the medicine. Some reported weighing up pros (treatment benefits) and cons (emotional side-effects and others, if they existed). Participant attitudes to continuing treatment at a time when they considered themselves to be suffering emotional side-effects from an antidepressant were mixed. Many participants viewed their emotional side-effects as undesirable, but were still willing to continue taking their SSRI, because of the perceived reduction in risk of relapse. They therefore viewed the side-effects as preferable to the illness for which they were being treated. However, some participants did express a strong preference to be able to feel the full range of emotions. Consequently, some participants reported that the emotional syndrome was one reason for them considering stopping or actually stopping their SSRI.
Respondent validation
The eleven participants who attended validation interviews expressed strong support for the overall study findings, and for the statements used in a draft questionnaire. A few participants felt that their responses would differ according to their emotional experiences at different time points and with different antidepressants, suggesting that the draft questionnaire was sensitive to change in the phenomenon under study. Supporting quotations are provided in the online supplement.
Data from web forums
Two hundred and seventy-two relevant posts were included, of which 32% were from www.depressionforums.org, 27% from www.about.com, 22% from www.google.groups.co.uk and 18% from www.socialaudit.org.uk. Data relating to individual posters, including gender, antidepressant indication, antidepressant type and current medication use, are summarised in the online data supplement Table DS1.
This evidence provided additional support for the eight key themes, although some differences were noted from the interview-derived data. These included:
-
(a) descriptions on web forums of longstanding adverse effects, months or years after the antidepressant was stopped;
-
(b) descriptions on web forums of complaints of doctors ‘misunderstanding’ the patient's emotional side-effects, and attributing them to depressive relapse;
-
(c) more florid descriptions on web forums of emotional side-effects; and
-
(d) less prominence on the web forums of reports of reduced positive emotions.
Finally, it was notable that descriptions of emotional side-effects were not limited to SSRIs, and were often associated with other commonly prescribed medications, including serotonin–noradrenaline reuptake inhibitors (such as venlafaxine) and mood stabilisers (such as lithium salts).
Discussion
This study provides robust evidence that some individuals taking SSRI antidepressants experience significant emotional symptoms that they strongly attribute to their antidepressant. It also helps us to characterise and understand these emotional symptoms, by providing detailed insights into patient experiences. Participants reported emotional symptoms that clustered into six key themes, and described the associated impact on their daily functioning (seventh theme). Participants' robust attribution of these emotional symptoms to their SSRI, and their presentation of a range of evidence to support this belief (eighth theme), indicates that these phenomena may well be emotional side-effects of SSRIs.
Participant attitudes towards these side-effects were not simply negative, suggesting that they could be evaluated as part of the cost–benefit associated with taking the medicine. Some participants felt that, although unhelpful, the side-effects were better than the possibility of relapse of their illness. Others reported that these side-effects were, in part, a reason for wanting to stop taking their antidepressant, or for having already stopped taking it. Notably, emotional side-effects had an impact on perceived quality of parenting and were occasionally linked to thoughts of self-harm or suicide.
The study has three key strengths. First, its qualitative methods allowed an understanding of the phenomenon of emotional side-effects from the patients' perspective, rather than from that of research or clinical ‘experts’. Second, the patient-derived data provide a guide to the actual language used by patients, so that the questionnaire derived from the data can contain appropriately worded items. Finally, the independent confirmation of the main themes by participants interviewed independently supports the content validity of our themes.
The main limitation of the study is the self-selecting nature of the sample: most interview participants responded to a poster or invitation letter indicating the nature of the research, and the content will have influenced perceptions of relevance. Hence, we currently have no way of knowing how common or uncommon are emotional side-effects and how far the experiences of this sample are representative or unrepresentative of the experiences of all people prescribed SSRIs. Equally, contributors to web forums may well be unrepresentative of the more general population of antidepressant users. Furthermore, our interview sample was skewed towards long-term consumers of SSRIs, and we therefore know little about similar experiences in the much larger population of short-term users. Finally, it is impossible to draw robust conclusions regarding causality from an observational, cross-sectional study such as this, where recall bias, for example, may have an effect. There is a clear need for further research, of a quantitative nature, to confirm and expand upon these early findings.
This is the first qualitative study of patient experiences of emotional side-effects of SSRIs. The relevant non-qualitative literature is limited, but our results fit well with that limited body of research. One observational study of SSRI-related emotional side-effects has been conducted, Reference Opbroek, Delgado, Laukes, McGahuey, Katsanis and Moreno4 in which 18 aspects of ‘emotional intensity’ were compared in people with major depression reporting SSRI-induced sexual dysfunction and in controls. The SSRI group reported significant reductions in 12 of the 18 aspects, including ability to cry, irritation, care about others' feelings, sadness, creativity, surprise, anger, expression of their feelings and worry, which fits well with our results. Our finding that the intensity of emotions, including negative emotions, is reduced, and that emotional responses are accordingly more easily controlled, fits with reports of SSRI-induced inability to cry, Reference Oleshansky and Labbate3 reduction in irritability, aggression and negative affect Reference Knutson, Wolkowitz, Cole, Chan, Moore and Johnson7 and reduced emotional lability resulting from cerebrovascular accident Reference Panzer and Mellow8 or other brain injury. Reference Seliger, Hornstein, Flax, Herbert and Schroeder9 In addition, our key theme of ‘no longer caring’ fits with several case reports and one case–control study of SSRI-induced changes, including apathy, indifference, and reduced motivation, in children, adolescents, adults and older adults. Reference Hoehn-Saric, Lipsey and McLeod2,Reference Barnhart, Makela and Latocha10–Reference Wongpakaran, van Reekum, Wongpakaran and Clarke14
Clinicians should consider adding emotional side-effects to those common antidepressant side-effects that they routinely mention to people starting treatment, such as headache, anxiety and gastrointestinal disturbance. Of note, although in some individuals these emotional side-effects are early, in many they appear to be a late phenomenon, emerging following partial or full recovery from the index illness. Clinicians should, therefore, also ask routinely about emotional side-effects when they are assessing progress on antidepressants. This might comprise asking a broad screening question, and then, if necessary, more specific questions to characterise the nature and extent of the problem, the extent to which the individual attributes the problems to their antidepressant and its contribution to their decision-making regarding ongoing adherence.
Appendix 1
Web forums
about.com/health
Hosted in the USA. Run by New York Times Co. Advertisement supported. Aims to provide information to the public from ‘experts’ in variety of fields. Contains a wide range of health-related forums.
depressionforums.org
Hosted in the USA. Independent volunteer-run bulletin board/support site. Aims to provide peer support for individuals suffering from mental illness.
groups.google.co.uk
Hosted in the UK. Advertisement supported. Contains many discussion board topics, including a Depression and Mood Disorders board.
socialaudit.org.uk
Hosted in the UK. Run by a charity, the Public Interest Research Centre Ltd. Contains public information and discussion boards relating to pharmaceutical medicine, including ‘The Antidepressant Web’ discussion board.
Appendix 2
Emotional side-effects of SSRIs: key themes
General effects on all emotions
Reduced intensity, or even absence of emotions, which are flattened, numbed, dulled or blanked; thoughts rather than feelings; difficulty understanding emotions; emotions feel fake or artificial; improved emotional control
Reduction of positive emotions
Reduced intensity and frequency of e.g. excitement, enjoyment, happiness, love, affection, passion, enthusiasm
Reduction of negative emotions
Reduced intensity and frequency of e.g. sadness, anger, aggression, anxiety and worry. Reduced ability to cry
Emotional detachment
Detachment or disconnection from the environment; from the self; and from other people, including children, partner and friends
Just not caring
Not caring about self, about others, about responsibilities; apathy; reduced interest and motivation; disinhibition; thoughts of self-harm
Changed personality
Aspects of personality are altered or removed; behaviour is out of character
Effects on everyday life
Helpful and/or unhelpful; often helpful initially, but becoming unhelpful as recovery occurs; masking or hiding problems; impact on responsibilities, on own behaviour, on relationships with others, on creativity, on judgement and decision-making
It's because of my pills!
Emotional side-effects are distinct from emotional illness; vary with dose, with specific medicine, and with adherence; vary with time; and may have an impact on treatment adherence
Funding
Servier, the funders, were able to comment on initial study design, but had no role in the collection, analysis and interpretation of data, and no role in the writing of the manuscript. Servier have a research programme for the development of psychotropic compounds, including antidepressants. Although they were able to comment on the final manuscript, no changes were introduced as a result of their comments, and they had no influence on the decision to submit the paper for publication. The researchers were, therefore, independent of the funders.
Acknowledgements
The study was sponsored by the University of Oxford. We are grateful to the interview participants, for giving their time and their views; and to clinicians and support staff who assisted with recruitment. We are also grateful to Servier company for funding this study.






eLetters
No eLetters have been published for this article.