Weight gain is a major side-effect of antipsychotic drug treatment, contributing to morbidity and poor adherence to treatment. Several of the newer atypical antipsychotic agents have profound effects on weight, the greatest increases occurring with clozapine and olanzapine (Reference Allison, Mentore and HeoAllison et al, 1999). Obesity has its inevitable consequences, which are increasingly observed in patients receiving antipsychotic drugs; such consequences may include the ‘metabolic syndrome’ of hypertension, cardiovascular disease, dyslipidaemia and impaired glucose tolerance, which may lead to type 2 diabetes (Reference HendersonHenderson, 2002; Reference Koponen, Saari and SavolainenKoponen et al, 2002). This study aims to explore the effect of initial antipsychotic drug treatment on abdominal fat deposition, and how this increase in body fat relates to circulating leptin, insulin and lipid levels in drug-naïve patients with first-episode schizophrenia.
METHOD
Study sample
Participants in the study were in-patients of Chinese Han ethnicity referred to the Department of Psychiatry, Nanjing Brain Hospital and Nanjing Medical University, China. All patients met the criteria for a diagnosis of schizophrenia according to DSM–IV (American Psychiatric Association, 1994). These people were experiencing their first psychotic episode and had not previously received antipsychotic drug treatment. They had no other mental and neurological disease, eating disorder, diabetes mellitus, hypertension, or history of alcohol or other substance misuse. Antipsychotic treatment was not randomised but provided according to normal local clinical practice for 10 weeks. This involved treatment primarily with risperidone or chlorpromazine; diazepam, or anticholinergic drugs to control extrapyramidal side-effects were administered as required. All participants gave written informed consent for participation in the study, which had been approved by the hospital's ethics committee, in accordance with the Declaration of Helsinki.
A total of 46 patients were recruited (27 men and 19 women). All patients received dietetically balanced hospital meals (daily energy intake: men 10.5 MJ (2500 kcal), women 9.2 MJ (2200 kcal)), occasionally supplemented by gifts from relatives, and had the opportunity for an hour's physical exercise each day. All patients were rated using the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) for the assessment of clinical psychotic symptoms by the senior psychiatrist (Z.-J.Y.) trained in its use. A minimum PANSS score of 60 was acceptable on admission; improvement after antipsychotic drug treatment was expressed by percentage change over baseline PANSS scores.
Control group
The control group consisted of hospital staff well matched for age (26.9 years, s.d.=50) and gender (22 men, 16 women) with the patient group, and were all apparently healthy after assessment by physical examination, blood screening and urine test. None had any history of treatment for neuropsychiatric disorders.
Experimental measures
Body fat indicators
Weight, height, and waist and hip circumferences were measured, and the body mass index (BMI) and waist:hip ratio (WHR) were calculated for all patients on admission and weekly for 10 weeks. The same measurements were made on the control group members. Waist circumference was measured midway between the lower rib margin and the iliac crest, and hip circumference was measured at the level of the widest circumference. Measurement of abdominal subcutaneous fat (SUB) and intra-abdominal fat (IAF; visceral fat) was performed by magnetic resonance imaging (MRI) on subgroups of participants (40 patients and 22 controls) with a whole-body scanner (Siemens Magnetom 8) using a 0.2 T magnetic field and an inversion recovery pulse sequence (inversion time 0.25 ms, repetition time 500 ms, and echo time 20 ms; field of view 380 mm3, matrix 192 × 256). One single transverse scan was taken halfway between the lower rib margin and the iliac crest with the participant lying supine. This site was determined by palpation approximately at the level of L4/L5 vertebra. Slice thickness was 10 mm and one scan took approximately 6 min. Image analyses to determine the abdominal fat used the techniques described by Seidell et al (Reference Seidell, Bakker and van der Kooy1990). Scans were taken within 36 h of admission and after 10 weeks.
Biochemical indicators
Blood was taken for testing from the patients on admission and after 10 weeks of antipsychotic treatment. Whole venous blood samples were taken into tubes containing ethylenediamine tetra-acetic acid (EDTA) at approximately 06.30 h following overnight fasting, and 2 h after breakfast for non-fasting plasma glucose measurement. After centrifugation at 2500 g at 4°C for 5 min, plasma supernatants were collected and immediately stored at – 80°C prior to assay. The samples were processed randomly and masked to diagnosis. Plasma glucose concentration was assayed by an automated glucose oxidase method. Measures of lipids – total high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol and triglycerides – were determined by standard clinical biochemistry laboratory assays. Fasting plasma insulin was measured with a 125I-insulin radioimmunoassay kit (National Health Institute, Beijing, China) in duplicate for each participant. The intra- and interassay coefficients of variation were below 3%. A leptin immunoradiometric kit (ACTIVE Human Leptin Coated-Tube Immunoradiometric Assay Kit DSL-23100, Diagnostic Systems Laboratories, Webster, TX, USA) was used to determine total fasting plasma leptin levels in duplicate. The intra- and interassay coefficients of variation were below 4%.
Data analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences, version 10.0. Initial analysis involved univariate analysis of variance (ANOVA) to compare the study groups, and repeated-measures analysis to determine the effects of treatment on body fat and biochemical indicators. Except where significant interaction terms were observed, the results are reported as t-tests. Values are given as mean (standard deviation). Significance levels are for two-tailed tests; P values below 0.05 were regarded as significant. Antipsychotic dose was converted to chlorpromazine equivalents (risperidone dose × 100). Correlations between biochemical indicators and clinical parameters and features were examined by Spearman rank correlation. Stepwise multiple regression was used for analysis of the influence of weight and fat indicators and clinical features on plasma leptin levels.
RESULTS
Patient treatment and clinical response
The age, treatment details and symptom scores for the patient groups are shown in Table 1. There was no significant difference between male and female patients in age or symptom score at baseline and after treatment (all P>0.05, data not shown). Thirty patients received risperidone at an initial daily dose of 1 mg, increasing over 10 days to a mean dose of 4.77 mg (s.d.=0.86): 5.64 mg (s.d.=0.67) for 11 men, 4.26 mg (s.d.=0.45) for 19 women. Another 15 male patients received chlorpromazine at an initial daily dose of 50 mg, increasing to an average dose of 480 mg (s.d.=122.2). In addition, one male patient was treated with quetiapine at an initial dose of 25 mg, increasing to 600 mg over 1 week. Male patients had a higher daily dose of antipsychotic: chlorpromazine equivalents for males, 518.5 mg (s.d.=108.4); for females, 426.3 mg (s.d.=45.2); t=3.956, P=0.0001.
Table 1 Clinical features and symptom assessment in patients with first-episode schizophrenia
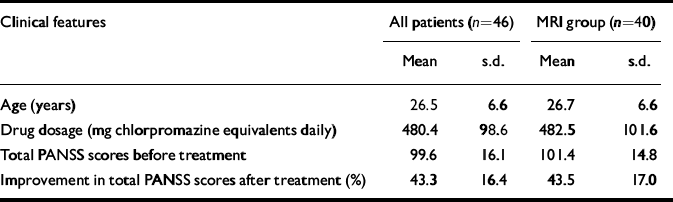
| Clinical features | All patients (n=46) | MRI group (n=40) | ||
|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |
| Age (years) | 26.5 | 6.6 | 26.7 | 6.6 |
| Drug dosage (mg chlorpromazine equivalents daily) | 480.4 | 98.6 | 482.5 | 101.6 |
| Total PANSS scores before treatment | 99.6 | 16.1 | 101.4 | 14.8 |
| Improvement in total PANSS scores after treatment (%) | 43.3 | 16.4 | 43.5 | 17.0 |
Weight and fat indicators
Initial comparisons between the control and patient groups showed no significant difference between the two. Values for the control group were: average weight 60.7 kg (s.d.=9.6); BMI 21.6 kg (s.d.=2.3); WHR 0.81 (s.d.=0.07); SUB 102.9 cm2 (s.d.= 51.6); and IAF 47.3 cm2 (s.d.=13.8). The general absence of any gender–group interaction indicated that this effect held true within the genders except for a significant interaction term for WHR (F=6.03, P=0.016), reflecting a lower WHR in female controls 0.77 (s.d.=0.06) compared with female patients (t=2.469, P=0.019). There were significant increases in all weight and fat indicators after 10 weeks’ antipsychotic treatment in the patient group (Table 2). This showed significant interactions with gender for SUB (F=5.675, P=0.022), and particularly for IAF (F=13.727, P=0.001) and WHR (F=11.574, P=0.001), but not for BMI, reflecting substantially greater changes in the male group than in the female group. Thus no significant change in WHR and SUB was apparent for the female patients after treatment; significant differences between males and females were found for the changes in WHR (t=3.496, P=0.001), SUB (t=2.2, P=0.038) and IAF (t=3.941, P=0.0001).
Table 2 Effect of antipsychotic drug treatment on body fat indicators in patients with first-episode schizophrenia
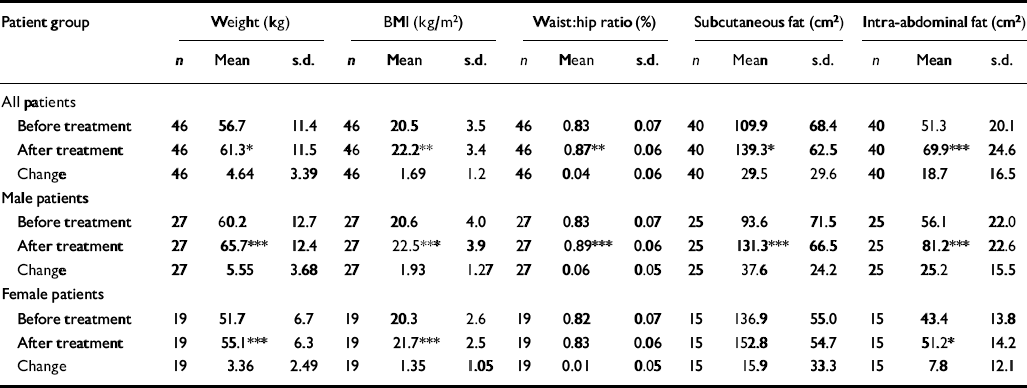
| Patient group | Weight (kg) | BMI (kg/m2) | Waist:hip ratio (%) | Subcutaneous fat (cm2) | Intra-abdominal fat (cm2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | |
| All patients | |||||||||||||||
| Before treatment | 46 | 56.7 | 11.4 | 46 | 20.5 | 3.5 | 46 | 0.83 | 0.07 | 40 | 109.9 | 68.4 | 40 | 51.3 | 20.1 |
| After treatment | 46 | 61.3* | 11.5 | 46 | 22.2** | 3.4 | 46 | 0.87** | 0.06 | 40 | 139.3* | 62.5 | 40 | 69.9*** | 24.6 |
| Change | 46 | 4.64 | 3.39 | 46 | 1.69 | 1.2 | 46 | 0.04 | 0.06 | 40 | 29.5 | 29.6 | 40 | 18.7 | 16.5 |
| Male patients | |||||||||||||||
| Before treatment | 27 | 60.2 | 12.7 | 27 | 20.6 | 4.0 | 27 | 0.83 | 0.07 | 25 | 93.6 | 71.5 | 25 | 56.1 | 22.0 |
| After treatment | 27 | 65.7*** | 12.4 | 27 | 22.5*** | 3.9 | 27 | 0.89*** | 0.06 | 25 | 131.3*** | 66.5 | 25 | 81.2*** | 22.6 |
| Change | 27 | 5.55 | 3.68 | 27 | 1.93 | 1.27 | 27 | 0.06 | 0.05 | 25 | 37.6 | 24.2 | 25 | 25.2 | 15.5 |
| Female patients | |||||||||||||||
| Before treatment | 19 | 51.7 | 6.7 | 19 | 20.3 | 2.6 | 19 | 0.82 | 0.07 | 15 | 136.9 | 55.0 | 15 | 43.4 | 13.8 |
| After treatment | 19 | 55.1*** | 6.3 | 19 | 21.7*** | 2.5 | 19 | 0.83 | 0.06 | 15 | 152.8 | 54.7 | 15 | 51.2* | 14.2 |
| Change | 19 | 3.36 | 2.49 | 19 | 1.35 | 1.05 | 19 | 0.01 | 0.05 | 15 | 15.9 | 33.3 | 15 | 7.8 | 12.1 |
Biochemical indicators
No significant difference in fasting plasma leptin or fasting plasma insulin levels was found between the control group and patients on admission: controls, plasma insulin 17.19 mIU/l (s.d.=18.84), t=0.295, P=0.769; plasma leptin 11.07 ng/ml (s.d.=11.39), t=71.546, P=0.127. Insulin, however, showed a significant gender–group interaction (F=8.664, P=0.004), reflecting the higher values observed in female patients (t=2.056, P=0.049).
There was a substantial elevation in plasma leptin in the patient group after 10 weeks’ treatment as well as within gender subgroups (Table 3). No significant difference in plasma insulin was found after 10 weeks of treatment. There was no significant difference in fasting plasma glucose levels before and after treatment either in all patients or in the gender subgroups. However, after 10 weeks of treatment, a significant increase in non-fasting plasma glucose concentration was found. Substantial and significant differences were also observed in total and LDL cholesterol and triglycerides. No clear gender difference in response to treatment was apparent in glucose measures, leptin, insulin or lipid markers, as there was no significant interaction. Similarly, the lack of significant treatment–drug type interactions in ANOVA indicated no significant difference between risperidone and chlorpromazine in their effects on any of these measures or on body fat indicators. However, there were significant gender differences in leptin levels in the control group at baseline assessment (males 5.77 ng/ml (s.d.=4.99) v. females 18.35 ng/ml (s.d.=13.69); t=–3.511, P=0.003) and in the patient group both at baseline (t=–5.061, P=0.0001) and after treatment (t=–3.107, P=0.004).
Table 3 Effect of antipsychotic drug treatment on biochemical indicators in patients with first-episode schizophrenia

| Biochemical indicator | All patients (n=46) | Male patients (n=25) | Female patients (n=19) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |||||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Fasting glucose (mmol/l) | 5.01 | 0.63 | 4.91 | 0.82 | 5.02 | 0.49 | 4.87 | 0.90 | 5.0 | 0.80 | 4.95 | 0.72 |
| Non-fasting glucose (mmol/l) | 6.34 | 1.61 | 7.18** | 1.65 | 6.37 | 1.91 | 7.09* | 1.47 | 6.30 | 1.12 | 7.31* | 1.92 |
| Fasting insulin (mIU/l) | 18.65 | 25.65 | 22.45 | 24.44 | 11.62 | 6.63 | 20.4 | 26.63 | 30.53 | 39.06 | 25.92 | 20.56 |
| Fasting leptin (ng/ml) | 7.68 | 7.71 | 21.61*** | 17.13 | 3.79 | 4.67 | 16.08*** | 16.39 | 14.25 | 7.45 | 30.95** | 14.4 |
| Cholesterol (mmol/l) | 4.08 | 0.83 | 4.58*** | 0.95 | 4.07 | 0.93 | 4.56** | 0.98 | 4.1 | 0.65 | 4.62** | 0.92 |
| Triglyceride (mmol/l) | 1.15 | 0.60 | 1.66*** | 0.67 | 1.18 | 0.70 | 1.66* | 0.73 | 1.10 | 0.41 | 1.66*** | 0.56 |
| HDL (mmol/l) | 1.24 | 0.28 | 1.23 | 0.27 | 1.19 | 0.29 | 1.17 | 0.26 | 1.33 | 0.23 | 1.35 | 0.26 |
| LDL (mmol/l) | 2.19 | 0.96 | 2.63*** | 0.85 | 2.30 | 1.04 | 2.68* | 0.90 | 1.99 | 0.80 | 2.53* | 0.77 |
Correlation of body fat indicators with clinical features, outcome and biochemical indicators
Within the MRI-determined baseline body fat indicators, only IAF was positively correlated with age (r=0.463, P=0.003) in the patient group but not significantly in the control group (r=0.201, P=0.37). There was no significant correlation between the changes in body fat and clinical features, including age and antipsychotic dose. The biochemical markers also showed no significant correlation with these clinical features (data not shown). Patients showed a substantial decrease in PANSS scores over 10 weeks’ treatment (see Table 1); however, the change in BMI was not significantly correlated with this clinical improvement (r=0.223, P=0.137).
In the control group there was no significant relationship between plasma leptin levels and body fat, although a trend to increased SUB with increased leptin was apparent (r=0.384, P=0.078). In patients, initial leptin concentrations correlated significantly with BMI at baseline (r=0.33, P=0.03), and with SUB but not IAF measurements at baseline (r=0.73, P=0.0001). Leptin, both on admission and after 10 weeks, correlated inversely with the percentage change in SUB (r=–0.505, P=0.002; r=–0.467, P=0.005) but not with that in IAF. Moreover, this change in SUB but not in IAF was also strongly correlated with fasting insulin concentrations at baselineline base (r=–0.523, P=0.001) and after 10 weeks’ treatment (r=–0.537, P=0.001). To elucidate further the influences on plasma leptin in schizophrenia, stepwise multiple regression was performed with body fat indicators, gender, age, drug type and antipsychotic dose included as independent variables; only gender and baseline SUB were significantly associated with initial leptin (β=–0.64, t=4.928, P=0.0001), whereas after 10 weeks leptin was only significantly related to gender.
DISCUSSION
Weight gain and fat deposition in the treatment of schizophrenia
To our knowledge, this is the first investigation into the initial effects on weight and regional fat distribution measured by MRI following antipsychotic treatment of patients with a first episode of schizophrenia. The MRI technique is a precise and reliable means of determining the two fat measures, with better resolution than computed tomography (CT) (Reference Seidell, Bakker and van der KooySeidell et al, 1990). We obtained no substantial evidence of a difference in weight or fat deposition between the control group and the patient group, although slight elevations in fat indicators (despite lower mean BMI values) were apparent in the patients in comparison with control participants. This is in contrast to the findings of Thakore et al (Reference Thakore, Mann and Vlahos2002), who reported that IAF but not SUB (determined by CT) was significantly increased in patients with schizophrenia compared with age-matched normal controls, and that this was unrelated to antipsychotic drug treatment. However, differences in social factors between Thakore's sample of patients and the Chinese patients investigated here, as well as the presence of previously treated drug-free patients in the former study, might have contributed to the apparent discrepancy with the results presented here.
It is well established that antipsychotic drug treatment can induce substantial weight gain, with different drugs having greater or lesser effects, and the greatest increases occurring with atypical antipsychotics (reviewed by Reference Allison, Mentore and HeoAllison et al, 1999; Reference Taylor and McAskillTaylor & McAskill, 2000). Inevitably, most studies undertaken in the context of drug trials have assessed patients who had previously received antipsychotic drug treatment. Such treatment confounds the effects of drugs over the period of the study by elevating values of baseline weight and related metabolic measures by amounts dependent on the drug (or drugs) previously received. In addition, the difficulty in distinguishing correlates of disease from the consequences of drug treatment means that it is possible that schizophrenia itself can be a risk factor for the metabolic syndrome (Reference Ryan and ThakoreRyan & Thakore, 2002). Hence the importance of studies in drug-naïve patients. We observed that initial antipsychotic treatment has a substantial effect on weight and fat deposition.
The mechanisms underlying this antipsychotic-drug induced weight gain are likely to be multifactorial. Antagonism of the serotonin receptor 5-HT2C, for which several antipsychotic drugs, particularly olanzapine and clozapine, have high affinity, is a strong candidate as the receptor is known to influence appetite and thereby weight gain (Reference Nonogaki, Strack and DallmanNonogaki et al, 1998). Association of a polymorphism of the 5-HT2C receptor gene with antipsychotic drug-induced weight gain provides further evidence of the importance of the receptor in this process (Reference Reynolds, Zhang and ZhangReynolds et al, 2002). However, effects at other receptors, including dopamine D2, α-adrenoceptors and particularly the histamine H1 receptor (Reference Kroeze, Hufeisen and PopadakKroeze et al, 2003), have also been implicated.
Leptin changes
Leptin is a hormone secreted by adipose tissue to have effects at receptors in the hypothalamus that result in the control of food intake. Deficits in leptin or leptin receptors result in overeating and obesity, indicating the importance of the hormone in body mass homoeostasis. The increase in fat deposition observed here occurred despite a substantial elevation in levels of circulating leptin, indicating that leptin control of fat deposition in patients receiving antipsychotic treatment is awry. This is likely to occur because the drugs block other receptors, e.g. 5-HT2C, that interact with the effects of leptin in the hypothalamus (Reference Nonogaki, Strack and DallmanNonogaki et al, 1998). However, the inverse correlation between SUB increases and leptin levels suggests that some remaining influence of leptin on subcutaneous fat deposition is present. These results are certainly consistent with previous findings of elevated leptin concentrations in antipsychotic-treated patients with schizophrenia (Reference Herran, Garcia-Unzueta and AmadoHerran et al, 2001) and appear likely to be a consequence of subcutaneous fat deposition, over which leptin normally imparts most regulatory control (Reference Cnop, Landchild and VidalCnop et al, 2002).
Weight gain and hyperglycaemia
The accumulation of intra-abdominal fat seen in patients in this study may well be the first indication of increasing risk of diabetes, since this measure is correlated with insulin resistance (Reference Cnop, Landchild and VidalCnop et al, 2002). Certainly there is growing evidence that the treatment of schizophrenia, particularly with clozapine and olanzapine, may impair glucose metabolism and increase the risk of diabetes (Reference HendersonHenderson, 2002; Reference Newcomer, Haupt and FucetolaNewcomer et al, 2002). We observed that an increase in non-fasting glucose concentration emerged after 10 weeks’ treatment. This finding may provide the first indications of impaired glucose utilisation, which has been reported in patients treated chronically with risperidone, as well as more profound effects following olanzapine and clozapine (Reference Newcomer, Haupt and FucetolaNewcomer et al, 2002). More speculatively, this developing insulin resistance, along with the observations of central fat deposition and the substantial and significant increases in blood lipid levels, may reflect early signs of progression towards the metabolic syndrome.
Drug-induced weight gain and clinical improvement
The changes in weight and/or body fat indicators do not appear to be related to clinical improvement in this study. Our finding is consistent with the absence of any significant relationship between weight gain and symptom improvement in another, larger, sample of similar patients (Reference Zhang, Yao and ZhangZhang et al, 2003), despite reports that weight gain is positively correlated with symptom improvement following clozapine and olanzapine (Reference Czobor, Volavka and SheitmanCzobor et al, 2002). However, consistent with our findings, the latter report found no relationship between weight gain and symptom improvement in patients receiving risperidone. In addition, it is difficult to eliminate artefactual results from such studies; poor nutrition and low weight may be a consequence of more severe symptoms, and such patients may show greater increases in both symptom improvement and weight gain following treatment.
Although the findings reported here describe the consequences of just 10 weeks’ treatment with common antipsychotics, they do provide indicators of a developing morbidity associated with weight gain. Such studies need to be extended to determine the longer-term consequences of antipsychotic drug treatment in previously drug-naïve patients, and to differentiate better the effects of different drugs. However, the potential that substantial subcutaneous and visceral fat deposits (particularly the latter) may have in developing insulin resistance leading to the metabolic syndrome suggests that all should be done to minimise this drug-induced weight gain and its impact. This may include dietary counselling (Reference MeltzerMeltzer, 2001), routine screening for diabetes (Reference HendersonHenderson, 2002) or even, in the future, genetic testing for liability (Reference Reynolds, Zhang and ZhangReynolds et al, 2002). Consideration of the different severities of weight gain associated with different drugs (Reference Allison, Mentore and HeoAllison et al, 1999) should, of course, be an important factor in choosing appropriate drug treatments.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Antipsychotic drug treatment rapidly induces abdominal fat deposition and hyperlipidaemia, with inevitable consequences on morbidity in schizophrenia.
-
▪ This effect occurred along with increased leptin secretion, indicating that during antipsychotic drug treatment the normal inhibitory control of leptin on body weight is dysfunctional.
-
▪ Drug-induced weight gain is not a correlate of symptom improvement, at least in patients in this study receiving risperidone or chlorpromazine.
LIMITATIONS
-
▪ The study did not include patients receiving drugs with greatest reported effects (i.e. clozapine and olanzapine) or with minimal effects on weight gain, and thus the findings cannot easily be generalised to all antipsychotic drug treatments.
-
▪ A relatively small series of patients was studied, limiting the opportunity to investigate subgroups with adequate statistical power.
-
▪ Patients were studied for only 10 weeks; whether these findings reach a plateau, continue to increase or even eventually diminish cannot be determined from them.
Acknowledgements
This study was partly supported by a grant from the China National Natural. Science Foundation and an unrestricted educational grant from Pfizer UK.






eLetters
No eLetters have been published for this article.