The neurotransmitter 5-hydroxytryptamine (5-HT, serotonin) may be necessary for the pharmacological action of lithium (Reference Wood and GoodwinWood & Goodwin, 1987). Studies in healthy volunteers suggest that lithium enhances brain 5-HT function (Reference McCance, Cohen and CowenMcCance et al, 1989) and thus augments antidepressant effects in unipolar disorder (Reference Cowen, McCance and WareCowen et al, 1991). However, it is not clear whether or not brain 5-HT function also mediates the effects of lithium in bipolar disorder, including prevention of relapse and reduction of suicidality (Reference Goodwin and GhaemiGoodwin & Ghaemi, 1998). Acute tryptophan depletion has been used to examine the role of brain 5-HT function in various conditions and also in the mechanism of action of antidepressants (Reference Reilly, McTavish and YoungReilly et al, 1997). The purpose of this study was to investigate the role of brain serotonin in the mechanism of action of lithium. To do this, we examined the effects of acute tryptophan depletion on mood and suicidal ideation in bipolar patients who were symptomatically stable on lithium.
METHOD
Subjects
Nineteen patients fulfilling DSM—IV (American Psychiatric Association, 1994) diagnostic criteria for bipolar I disorder in full remission were recruited from out-patient clinics in the Newcastle area. All patients had been euthymic for at least 3 months and were receiving lithium carbonate at therapeutic doses. The lithium was not interrupted during the experimental procedure. Subjects were reviewed by a research psychiatrist to confirm the diagnosis and remission status, and we excluded any concurrent Axis I diagnosis and significant physical illness. Mean fullscale IQ was estimated by the National Adult Reading Test (Reference NelsonNelson, 1982). Written informed consent from each patient was obtained and the study received full approval from the local ethics committee.
Experimental design
The experiment was a within-subject, double-blind, placebo-controlled, counter-balanced crossover design study with at least 7 days' washout between experimental sessions. In the case of female subjects, the experimental sessions took place in the follicular phase of the menstrual cycle with only one procedure per cycle. Subjects presented following an overnight fast to the Department of Psychiatry at 08.30 h and underwent baseline assessments. The experimental drink was then administered. Four hours after consumption of the drink, mood and suicidality were rated. Following the experimental session, subjects returned home and underwent a further assessment of mood and suicidality, which was carried out the following day at 28 hours after consumption of the drink.
Mood rating scales
Objective mood ratings
The Hamilton Rating Scale for Depression, a 21-item standardised scale for the measurement of the severity of depressive symptoms, was used (Reference HamiltonHamilton, 1960), with subjects rated at baseline, 4 hours and 28 hours.
The Young Mania Rating Scale, an 11-item measure of the severity of manic symptoms developed by Young et al (Reference Young, Biggs and Ziegler1978), was used. Subjects were interviewed and rated at baseline, 4 hours and 28 hours.
Subjective mood ratings
The Internal State Scale, a self-report instrument for the simultaneous assessment of the severity of depressive and manic symptoms, was used (Reference Bauer, Crits-Christoph and BallBauer et al, 1991). Visual analogue line format was used for the 17 items, with the anchor points coding for severity and frequency of symptoms. Items are grouped in scoring to four subscales: Activation, Well-being, Perceived Conflict and Depression Index. Subjects were asked to make a vertical mark at the appropriate point on the line which corresponded with the way they felt at the time of rating and this point was measured from the left-hand anchor point as the zero.
The Carroll Bipolar Visual Analogue Scale consists of a 23-item scale based on the Carroll—Klein model of manic—depressive illness (Reference Carroll, Carroll and BarrettCarroll, 1991). Each item was presented as a visual analogue scale on a 100-mm long line with appropriate anchor statements describing the manic and depressive extremes of each item. The midpoint denotes the euthymic state and a score of zero, with the extreme depressive response scoring ‒50 and the extreme manic response scoring +50. Of the 23 items, seven items each represented consummatory reward, central pain and psychomotor regulation, and two items represented incentive reward (major components of the Carroll—Klein model). Subjects were asked to make a vertical mark at the appropriate point on the line which corresponded with the way they felt at the time of rating and this point was measured from the zero midpoint.
The Beck Depression Inventory, a 21-item assisted self-rating inventory used to assess the severity of depressive states, was used (Reference Beck, Ward and MendelsonBeck et al, 1961). Each item was graded on a 4-point scale of 0-3, with the total score being used to assess the degree of depression (the higher the score the worse the depression). The total score was used in the analysis.
The Profile of Mood States is a multiple affective adjective checklist with 67 items rated by the subject on a 5-point scale (0-4 inclusively), developed by McNair et al (Reference McNair, Lorr and Droppleman1992). Subjects were asked to rate how they felt ‘right now’ at baseline and at 4 and 28 hours post-drink by reading the adjective list and assigning the relevant score. The six factor scores Tension—Anxiety, Depression—Dejection, Anger—Hostility, Vigour—Activity, Fatigue—Inertia and Confusion—Bewilderment were then calculated and the composite score for total mood generated.
The Hourly Visual Analogue Rating, a further visual analogue scale of five 100-mm lines marked with ‘very’ and ‘not at all’ at respective anchor points for each of five categories (Sadness, Anxiety, Irritability, Difficulty concentrating and Energy) was used. Subjects rated themselves prior to ingestion of the drink, at hourly intervals throughout the experimental session and during the following day.
A suicidality rating was generated by combining the individual items in the Beck Depression Inventory and the Hamilton Rating Scale for Depression. Suicidality was evaluated at baseline and at 4 hours and 28 hours after the drink.
Drink composition
Both drinks were of identical composition, with the exception of the addition of 2.3 g tryptophan to the control drink. A 100 g amino acid drink was used containing L-alanine 5.5 g, L-arginine 4.9 g, L-cysteine 2.7 g, L-glycine 3.2 g, L-histidine 3.2 g, L-isoleucine 8 g, L-leucine 13.5 g, L-lysine monohydrochloride 11 g, L-methionine 3 g, L-phenylalanine 5.7 g, L-proline 12.2 g, L-serine 6.9 g, L-threonine 6.5 g. L-tyrosine 6.9 g, L-valine 8.9 g. This was mixed in 300 ml water, flavoured with blackcurrant and sweetened.
Biochemical measures
On four separate occasions, 20 ml venous blood was taken during each experimental session. A sample was taken before the amino acid drink (at time zero), before symptomatic evaluation (after 240 minutes), after symptomatic evaluation (after 330 minutes) and the following day (after 28 hours). Plasma was immediately separated by refrigerated centrifugation and a sample for free tryptophan was further centrifuged using an ultrafiltrate tube. All samples were stored at ‒20°C until assay. Plasma total and free tryptophan were determined by high-pressure liquid chromatography by the method of Marshall et al (Reference Marshall, Kennedy and Eccleston1987). Serum lithium was determined using atomic absorption spectrophotometry on the remainder of the sample in the local biochemistry laboratory.
Statistical analysis
SPSS for Windows, Release 7 (SPSS, 1998) was used for statistical analysis. The Kolmogorov—Smirnov test and, where necessary, non-parametric statistical methods were used. Biochemical and mood rating scale scores were analysed using repeated measures analysis of variance (ANOVA) with drink condition (tryptophan depletion or placebo) and time as within-subject variables and order as a between-subjects variable. Reported P values were corrected using the Huynh—Feldt correction factor when the sphericity assumption was not met. For clarity, uncorrected degrees of freedom are reported. Psychological variables were analysed using post-hoc paired t-tests (two-tailed). These data are quoted as means (s.e.m.) with 95% confidence intervals.
RESULTS
Nineteen patients were recruited to the study; of these, 15 completed the entire protocol. In the group that did not complete the protocol, three were male and one was female; all did not complete the study because of intolerance of the drink. The characteristics of those remaining in the study are shown in Table 1.
Table 1 Patient characteristics
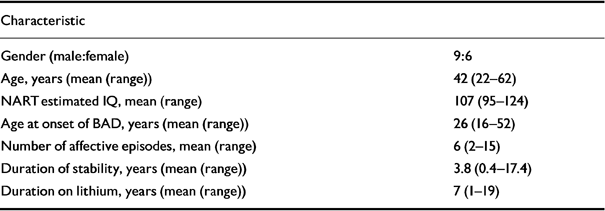
| Characteristic | |
|---|---|
| Gender (male:female) | 9:6 |
| Age, years (mean (range)) | 42 (22-62) |
| NART estimated IQ, mean (range) | 107 (95-124) |
| Age at onset of BAD, years (mean (range)) | 26 (16-52) |
| Number of affective episodes, mean (range) | 6 (2-15) |
| Duration of stability, years (mean (range)) | 3.8 (0.4-17.4) |
| Duration on lithium, years (mean (range)) | 7 (1-19) |
Biochemistry
Tryptophan levels
Tryptophan depletion was generally well tolerated in those who remained in the study. There was a significant effect of depleting drink compared to control drink on both free and total tryptophan concentration, with 83% depletion of free tryptophan (paired t-test, P=0.016) and 84% depletion of total tryptophan (paired t-test, P=0.001; see Fig. 1).
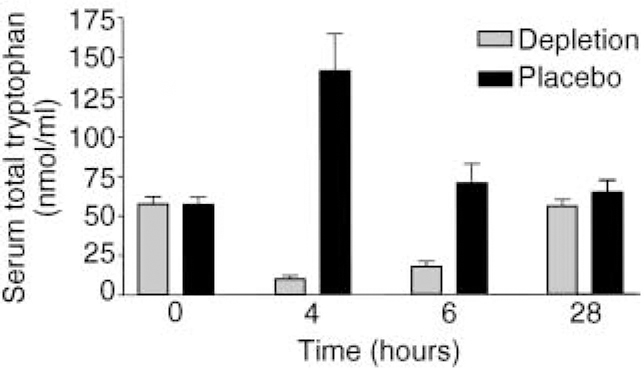
Fig. 1 Effects of depletion and placebo drinks on serum total tryptophan, showing significant reduction in total tryptophan at 4 and 6 hours following depleting drink.
Serum lithium levels
Serum lithium fell during the test day and this reached statistical significance (F 3,42=27.99, P<0.001; see Fig. 2). However, there was no significant difference between the two groups (depletion/non-depletion) at any of the time points (paired t-test, P>0.2) and no significant difference between levels prior to testing within each trial (paired t-test, P>0.6).
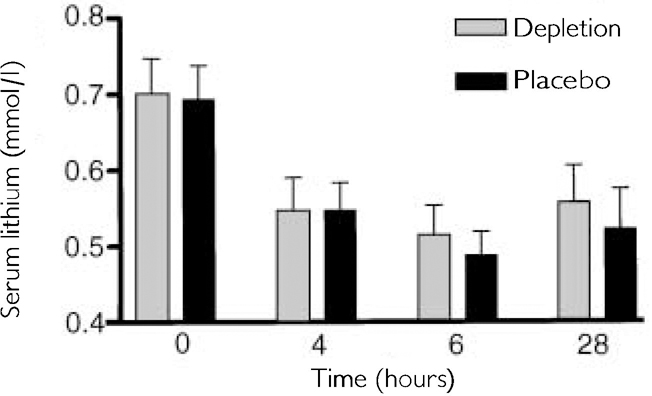
Fig. 2 Effects of time on serum lithium between the depleted and non-depleted condition, showing changes over time as predicted by lithium's pharmacokinetics.
Mood rating scales
Objective mood ratings
On the Hamilton Rating Scale for Depression the mean baseline score did not differ between the two conditions (placebo=2; active=2). There was no effect of depletion (F 1,14=0.2, P=0.665) or time (F 2,28=0.39, P=0.684) and no interaction between drink and time (F 2,28=0.04, P=0.956).
On the Young Mania Rating Scale, the mean baseline score also did not differ between the two conditions (placebo=<1, active=<1). There was no effect of depletion (F 1,14=2.95, P=0.108) or time (F 2,28=2.63, P=0.09) and no interaction between drink and time (F 2,28=0.11, P=0.892).
Subjective mood ratings
On the Internal State Scale there was a significant effect of drink on the depressive index sub-scale, with subjects receiving the balanced drink reporting decrease in the depressive index (less depressed) rating at 4 hours but not at 28 hours (F 1,14=4.72, P=0.048), whereas the depleting drink caused no change in mood. There was no other significant effect of the drink on any other sub-scale (see Table 2).
Table 2 Subjective mood rating scale scores; mean (s.e.m.)
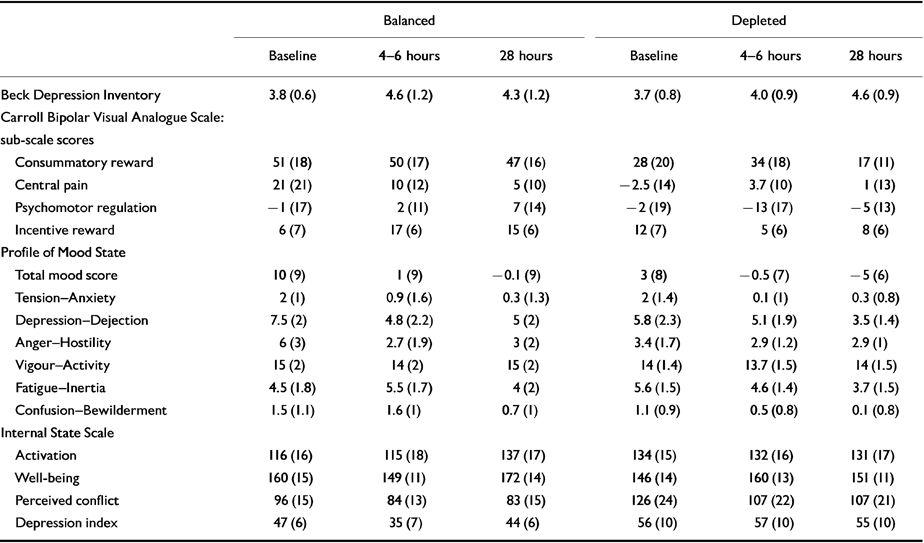
| Balanced | Depleted | |||||
|---|---|---|---|---|---|---|
| Baseline | 4-6 hours | 28 hours | Baseline | 4-6 hours | 28 hours | |
| Beck Depression Inventory | 3.8 (0.6) | 4.6 (1.2) | 4.3 (1.2) | 3.7 (0.8) | 4.0 (0.9) | 4.6 (0.9) |
| Carroll Bipolar Visual Analogue Scale: sub-scale scores | ||||||
| Consummatory reward | 51 (18) | 50 (17) | 47 (16) | 28 (20) | 34 (18) | 17 (11) |
| Central pain | 21 (21) | 10 (12) | 5 (10) | -2.5 (14) | 3.7 (10) | 1 (13) |
| Psychomotor regulation | -1 (17) | 2 (11) | 7 (14) | -2 (19) | -13 (17) | -5 (13) |
| Incentive reward | 6 (7) | 17 (6) | 15 (6) | 12 (7) | 5 (6) | 8 (6) |
| Profile of Mood State | ||||||
| Total mood score | 10 (9) | 1 (9) | -0.1 (9) | 3 (8) | -0.5 (7) | -5 (6) |
| Tension—Anxiety | 2 (1) | 0.9 (1.6) | 0.3 (1.3) | 2 (1.4) | 0.1 (1) | 0.3 (0.8) |
| Depression-Dejection | 7.5 (2) | 4.8 (2.2) | 5 (2) | 5.8 (2.3) | 5.1 (1.9) | 3.5 (1.4) |
| Anger-Hostility | 6 (3) | 2.7 (1.9) | 3 (2) | 3.4 (1.7) | 2.9 (1.2) | 2.9 (1) |
| Vigour—Activity | 15 (2) | 14 (2) | 15 (2) | 14 (1.4) | 13.7 (1.5) | 14 (1.5) |
| Fatigue—Inertia | 4.5 (1.8) | 5.5 (1.7) | 4 (2) | 5.6 (1.5) | 4.6 (1.4) | 3.7 (1.5) |
| Confusion—Bewilderment | 1.5 (1.1) | 1.6 (1) | 0.7 (1) | 1.1 (0.9) | 0.5 (0.8) | 0.1 (0.8) |
| Internal State Scale | ||||||
| Activation | 116 (16) | 115 (18) | 137 (17) | 134 (15) | 132 (16) | 131 (17) |
| Well-being | 160 (15) | 149 (11) | 172 (14) | 146 (14) | 160 (13) | 151 (11) |
| Perceived conflict | 96 (15) | 84 (13) | 83 (15) | 126 (24) | 107 (22) | 107 (21) |
| Depression index | 47 (6) | 35 (7) | 44 (6) | 56 (10) | 57 (10) | 55 (10) |
On the Carroll Bipolar Visual Analogue Scale the baseline scores for the individual items (consummatory reward, central pain, psychomotor regulation and incentive reward) were not significantly different between the two conditions. There was no effect of depletion or time and no interaction between drink and time for all the individual items (see Table 2).
On the Beck Depression Inventory there was no significant difference between baseline scores for depleted (mean=3.7) and placebo (mean=3.8) and no effect of depletion or time and no interaction between drink and time for this item (see Table 2).
On the Profile of Mood States there was no significant difference at baseline between the two conditions on both the total score and the individual sub-scales (see Table 2). The depleting drink did not alter the total score or the individual sub-scales significantly. There was a significant decrease in the total mood score (F 2,28=3.31, P=0.05), and the Anger—Hostility score (F 2,28=4.28), P=0.024) with both depleting and balanced drinks.
On the Hourly Visual Analogue Rating, tryptophan depletion produced no significant effect on any of the measures. There was a significant effect of time on both Anxiety (F 7,98=2.25, P=0.038) and Sadness (F 7,98=3.5, P=0.002). Patients were less anxious and less sad at the end of each testing day irrespective of the drink taken.
On the Suicidality Rating, there was no effect of depletion on this composite score (F 1,14=0.27, P=0.61) and no change over time (F 2,28=1.56, P=0.229) or any drink by time interaction (F 2,28=0.48, P=0.062).
DISCUSSION
Our results indicate that acute tryptophan depletion does not alter mood or suicidal ideation in bipolar patients who are well on lithium carbonate. These findings, taken with a previous report (Reference Benkelfat, Seletti and PalmourBenkelfat et al, 1995), strongly suggest that both the mood-stabilising effects and the beneficial effects on suicidal ideation of lithium in these patients are not reversed by perturbations of brain 5-HT levels or turnover. When evaluating the effects of tryptophan depletion, the timing of the measures is critical. We attempted to control for this by performing ratings at two time points, both on the day of drink consumption and the day following. However, we cannot rule out an effect of depletion at another time.
Acute tryptophan depletion has been performed using a variety of experimental protocols in previous studies (for review see Reference Reilly, McTavish and YoungReilly et al, 1997) and produces a depressive mood shift in patients recovered from unipolar depression (Reference Smith, Fairburn and CowenSmith et al, 1997). Our study, in common with this latter study, used a well-established paradigm and produced a large drop in plasma tryptophan, and thus brain 5-HT levels. This study did not employ a low tryptophan diet preceding depletion, but rather used an overnight fast. Low tryptophan diet is used to minimise diet-induced fluctuations in baseline tryptophan levels (Reference Reilly, McTavish and YoungReilly et al, 1997). However, in this study, baseline free tryptophan levels did not show a difference.
5-HT and lithium
Despite our findings, the pharmacological locus of lithium's effects in bipolar disorder may still be within the brain 5-HT system. Serotonergic neurons arise from the dorsal and median raphe nuclei in the brain-stem and project widely throughout the brain and spinal cord. Multiple 5-HT receptors have been described, with the 5-HT1A and 5-HT2 receptor subtypes being most clearly implicated in mood disorders (Reference Cowen, Feighner and BoyerCowen, 1996). Importantly, these receptors appear to play different roles in mood disorders and lithium has distinct effects on each receptor subtype. The 5-HT1A receptor has been implicated in the mechanism of action of antidepressants, in the neurobiology of unipolar disorder and in augmentation of antidepressants by lithium (Reference Cowen, McCance and WareCowen et al, 1991; Reference Cowen, Feighner and BoyerCowen, 1996). Previous studies have found that lithium enhances the neuro-endocrine response to the 5-HT precursor L-tryptophan, an effect that is mediated by the 5-HT1A receptor (Reference McCance, Cohen and CowenMcCance et al, 1989; Reference Smith, Ware and CowenSmith et al, 1991). Studies in depressed patients suggest that the pharmacological basis of lithium's augmentation of antidepressant action in unipolar disorder is by an action on 5-HT1A-mediated neurotransmission (Reference Cowen, McCance and WareCowen et al, 1991). The effect of lithium on 5-HT1A-receptor-mediated neurotransmission is probably dependent on 5-HT availability, as lithium has no effect on 5-HT1A receptor sensitivity (Reference Walsh, Ware and CowenWalsh et al, 1991). Acute tryptophan depletion has been shown to reverse the action of antidepressants (Reference Delgado, Charney and PriceDelgado et al, 1990), suggesting that this procedure alters neurotransmission through the 5-HT1A receptor subtype. Our findings suggest that the beneficial actions of lithium on mood and suicidal ideation in bipolar disorder are not mediated by an effect on 5-HT1A receptor neurotransmission and are not acutely dependent on 5-HT availability.
The molecular effects of lithium on 5-HT2 receptor numbers and linked second messenger systems are complex (Reference Moorman and LeslieMoorman & Leslie, 1998), but lithium has been shown to increase slow-wave sleep in normal volunteers, an effect mediated by 5-HT2 receptor function (Reference Friston, Sharpley and SolomonFriston et al, 1989). D-fenfluramine, the 5-HT-releasing agent, appears to act via 5-HT2 postsynaptic receptors, as judged in serotonin neuroendocrine tests (Reference CowenCowen, 1993). Lithium does not alter this neuroendocrine response to the 5-HT (Reference Power, Dorkins and CowenPower et al, 1993), suggesting that the effect of lithium on 5-HT2-receptor-mediated neurotransmission is not dependent on 5-HT availability but is a direct effect on the receptor or post-receptor mechanisms.
Lithium prevents suicide in bipolar affective disorder (Tondo et al, Reference Tondo, Baldessarini and Floris1997a ,Reference Tondo, Jamison and Baldessarini b ) and may differ from other mood stabilisers, such as carbamazapine, in this property (Reference Thies-Flechtner, Muller-Oerlinghausen and SeibertThies-Flechtner et al, 1996). The 5-HT2 receptor subtype has also been implicated in mood disorders and in suicide (Reference Ferrier and PerryFerrier & Perry, 1992) and this receptor has been postulated to be involved in the beneficial effects of lithium on suicidality (Reference Goodwin and GhaemiGoodwin & Ghaemi, 1998). In keeping with these observations, our results do not support a mechanism of action acutely dependent on central 5-HT availability acting through post-synaptic 5-HT1A receptors, but support the alternative proposition that the effects of lithium are upon 5-HT2 receptor numbers and related cellular signalling mechanisms. Future studies of lithium's mechanism of action on mood and suicidal ideation in bipolar disorder should therefore focus on its effects on the 5-HT2 receptor.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Acute reduction of brain 5-hydroxytryptamine (5-HT, serotonin) levels does not alter mood in bipolar patients symptomatically stable on lithium.
-
▪ Similarly, acute reduction of brain 5-HT levels does not alter suicidality in bipolar patients symptomatically stable on lithium.
-
▪ Acute tryptophan depletion is a useful method for examining brain 5-HT function in bipolar disorder.
LIMITATIONS
-
▪ The paradigm of acute tryptophan depletion used may not lower central tryptophan levels enough to provoke symptoms.
-
▪ The effect of acute tryptophan depletion on brain 5-HT levels was not directly measured in these patients.
-
▪ The number of patients studied was relatively small.
ACKNOWLEDGEMENTS
This work was made possible by support from the Theodore and Vada Stanley Foundation. We thank Professor I. N. Ferrier for his helpful advice on this manuscript.







eLetters
No eLetters have been published for this article.