Acute behavioural disturbance including verbal aggression and violence is a common occurrence in acute mental health units, with the majority of cases resulting from acute psychosis or substance misuse. Reference Atakan and Davies1,Reference McAllister-Williams and Ferrier2 Most patients settle with verbal de-escalation or agree to take oral medication. Reference McAllister-Williams and Ferrier2 Such approaches fail in a proportion of patients whose behaviour escalates from verbal abuse to physical violence. These people often require physical restraint and involuntary parenteral sedation, to prevent harm to other patients, staff and property. The aim in these cases is for the patient to be rapidly tranquillised or sedated. Reference McAllister-Williams and Ferrier2,Reference Huf, Coutinho and Adams3 Despite the extent of the problem there is little consensus on the most effective and safest drugs for sedation, and there is limited evidence. Reference Powney, Adams and Jones4 Clinical practice is generally guided by individual or institutional experience. Guidelines recommend an antipsychotic or benzodiazepine as a single agent or in combination, or an antipsychotic with promethazine. Reference McAllister-Williams and Ferrier2,5,Reference Lepping6 Common choices are haloperidol alone; haloperidol and lorazepam; haloperidol with promethazine; or a newer antipsychotic such as olanzapine. Reference Huf, Coutinho and Adams3,5,Reference Pilowsky, Ring, Shine, Battersby and Lader7 Droperidol has been used less commonly after a Food and Drug Administration (FDA) boxed warning in 2002, but recently has been shown to be effective and safe in studies in emergency departments. Reference Isbister, Calver, Page, Stokes, Bryant and Downes8,Reference Knott, Taylor and Castle9 Benzodiazepines are problematic in non-critical care settings because they may cause respiratory depression, and a recent systematic review found that adding a benzodiazepine to haloperidol afforded no benefit but carried the risk of harm. Reference Powney, Adams and Jones4 The same review concluded that independent trials with simple outcomes are required to improve the evidence for rapid tranquillisation, because there are significant risks associated with many recommended drugs. Reference Powney, Adams and Jones4 We aimed to compare the effectiveness and safety of droperidol with haloperidol for the sedation of patients with acute behavioural disturbance in an acute mental health unit.
Method
We undertook a masked, randomised controlled study of intramuscular haloperidol (10 mg) v. intramuscular droperidol (10 mg) for the rapid tranquillisation of patients with acute behavioural disturbance in a psychiatric intensive care unit. The primary outcome was the time to sedation. The study was undertaken from August 2011 to June 2013 in the psychiatric intensive care unit of a large tertiary specialist mental health facility in Australia. The psychiatric emergency care centre of this facility receives over 4400 presentations per year. Patients are generally referred from general practitioners, regional hospitals, other units within the institution or from the community – usually by the community mental health teams or the ambulance or police service. Ethical approval was obtained from the local human research ethics committee. Consent was waived because of patients’ lack of decision-making capacity to consent to medical treatment as duty of care. The trial was registered with the Australian New Zealand Clinical Trial Registry (ACTRN12611000565943).
Study participants
All patients with agitation or aggression who were admitted involuntarily to the psychiatric intensive care unit from the psychiatric emergency care centre were eligible for inclusion in the study. They were managed according to a standardised sedation protocol including the use of a purpose-designed acute behavioural disturbance chart. Participants were all adults (> 18 years of age) with acute behavioural disturbance who required parenteral medication for sedation and in whom verbal de-escalation and/or oral medication had failed. We excluded patients willing to take oral medication for sedation without physical restraint or seclusion and patients under 18 years old.
Interventions
Patients were identified for the study from either the psychiatric emergency care centre or the in-patient psychiatric intensive care unit. Once patients were recruited to the study they were escorted to the psychiatric intensive care unit and physically restrained with the assistance of security staff to allow the administration of intramuscular medication to the gluteal region. Patients were then either taken to their own room or placed in a seclusion room. They were not physically restrained once the medication had been administered. There was access to resuscitation equipment at all times, and staff had regular training in basic cardiopulmonary resuscitation. The psychiatric intensive care unit is staffed with four nurses for eight patients during daylight hours and the evening, and three nurses overnight. A psychiatrist and a psychiatric registrar are on site in working hours, and a medical officer is available in the hospital after hours.
Pre-packed treatment kits were available in the psychiatric intensive care unit; these had been produced by the Calvary Mater Newcastle pharmacy in conjunction with Richard Stenlake Compounding Chemist, Sydney, Australia. Each kit contained either droperidol (10 mg in 2 ml) or haloperidol (10 mg in 2 ml). Droperidol was purchased from Phebra Ltd (Sydney, Australia); the haloperidol was purchased from Fagron Ltd (Sydney, Australia) and transferred into vials identical to those containing the droperidol formulation. This was done under aseptic conditions by Richard Stenlake Compounding Pharmacy. The 10 mg droperidol dose was based on a similar study in the emergency department and 10 mg of both droperidol and haloperidol are equivalent doses used in rapid tranquillisation in psychiatry.
Block randomisation was used. Microsoft Excel was used to randomly create blocks of four (ABAB, AABB, etc.) or six (ABABAB, AAABBB, etc.). The use of different block sizes meant that it was impossible to predict the next treatment. Each A or B allocation was then assigned a study code. The list of study codes with allocations was generated by a research assistant and supplied to the Calvary Mater Newcastle pharmacy, so that the investigators and treating staff remained unaware of the allocations. The pharmacy relabelled the vials of haloperidol or droperidol with study numbers based on the list of allocations. The vials were then supplied to the psychiatric intensive care unit in sequential order. The psychiatric intensive care unit was kept stocked with treatment kits for the duration of the study. Patients were administered the trial drug and then observed in the seclusion room. Vital signs and the level of agitation and sedation were recorded at 10 min intervals after the trial drug for at least 1 h or until the patient settled. Additional sedation was recommended if the patient showed no sign of settling 30 min after the initial sedation, but this was given at the discretion of the treating physician.
Data collection and processing
A previously developed acute behavioural disturbance chart was introduced into the psychiatric intensive care unit 1 year prior to the trial commencing. Reference Calver, Downes, Page, Bryant and Isbister10 During this introductory year the chart was used to record prospectively the level of agitation and sedation in all patients, using the Sedation Assessment Tool (SAT). Reference Calver, Stokes and Isbister11 The SAT (see Appendix) scores the patient from +3 (physically violent) to –3 (unconscious) and allows rapid assessment before and after sedative medication is given. This initial year familiarised the staff with scoring the SAT, developing confidence in its utility and reliability, and ensured that both SAT scores and vital signs were recorded in all patients correctly for the trial. Reference Calver, Drinkwater and Isbister12 A baseline SAT score was recorded when the patient was recruited to the study; SAT scores and vital signs were then recorded every 10 min after the trial drug for the first hour, then half-hourly until the patient settled. A number of the observations were initially recorded remotely from outside the seclusion room, including respiratory rate and SAT score. Remote observations were commenced from the onset of the acute behavioural disturbance until it was considered safe to approach the patient and record vital signs including heart rate, blood pressure, oxygen saturation and respiratory rate. Adverse events and staff injury were recorded along with any observed extrapyramidal side-effects. Additional medications were given at the discretion of the treating doctors, recorded on the data sheet and charted on the medication chart.
Outcome measures
The primary outcome was the time to sedation, defined as time from the administration of the trial drug until the SAT score decreased by 2 or more or the score was 0 (calm and alert). Reference Calver, Stokes and Isbister11,Reference Calver, Drinkwater and Isbister12 Failed sedation was defined as the patient not being sedated within 120 min. Adverse drug effects were defined as a respiratory rate less than 12 breaths/min, systolic blood pressure less than 90 mmHg, heart rate less than 60 beats/min, oxygen saturation less than 90% or the presence of extrapyramidal side-effects. The use of additional sedation was any medication administered within 60 min of the time of the study drug being given. Successful sedation was defined post hoc as patients sedated within 120 min who did not require additional sedation and had no adverse effects.
Statistical analysis
The sample size was calculated to be 230 so as to detect a difference in the time to sedation of 20 min between groups, assuming a within-group standard deviation of 30 min (based on a retrospective audit of psychiatric intensive care unit patients). Because time to sedation was likely to be a non-parametric continuous variable the sample size was calculated using the t-test (α = 0.01, β = 0.9) and 15% added. At the completion of the study one investigator (G.I.) still masked to the allocation audited all primary and secondary outcomes using the original data sheets. Another investigator not involved in recruiting patients or coordination of the study (C.P.) was then given the masked data, and separately the group allocations as either A or B by the pharmacy. At this time only the study labels A or B and not the drug names were known to the investigator. This investigator analysed the data independently and presented this to the other investigators. Only then did the pharmacy reveal whether A or B was haloperidol or droperidol.
Medians, interquartile ranges (IQRs) and ranges are reported for continuous variables. Percentages are reported for dichotomous outcomes with 95% confidence intervals. The continuous primary outcome was analysed using the Mann–Whitney test because the data were non-parametric. Dichotomous secondary outcomes were analysed using a two-tailed Fisher’s exact test. A significance level of P<0.05 was used. All analyses and graphics used GraphPad Prism version 6.02 for Windows (www.graphpad.com).
Results
There were 584 sedation episodes during the 23-month study period and of these 356 were not included in the analysis because the treating clinician elected to give labelled parenteral sedation

Fig. 1 Patient recruitment and allocation to haloperidol or droperidol.
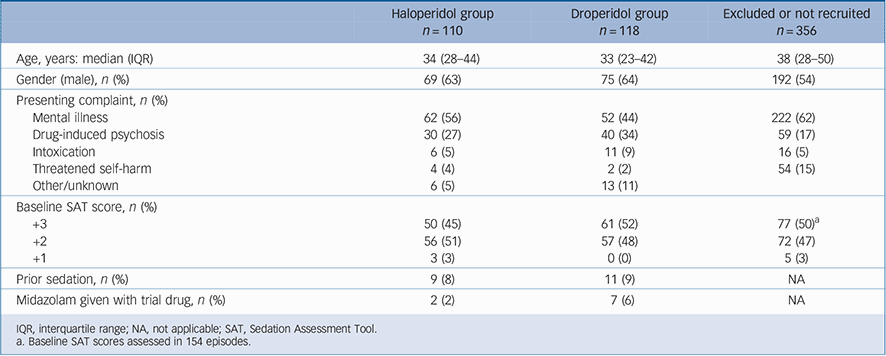
(most commonly droperidol 10 mg) or a lower or higher dose of labelled parenteral medication, or it was recognised that the patient had already been recruited to the study (Fig. 1). An acute behavioural disturbance chart was filled out for 165 of these 356 episodes (46%), and the baseline characteristics of the patients did not differ from those recruited to the study (Table 1). In 112 of the 356 episodes (31%) an initial SAT score was recorded which was similar to those of the study participants (Table 1).
There were 228 episodes of acute behavioural disturbance in 206 patients recruited to the study, with 5 being sedated on three occasions, 12 on two occasions and 189 on one occasion. In all 228 episodes the patients were randomised: 110 were allocated
Table 1 Baseline characteristics of the patients
| Haloperidol group n = 110 |
Droperidol group n = 118 |
Excluded or not recruited n = 356 |
|
|---|---|---|---|
| Age, years: median (IQR) | 34 (28–44) | 33 (23–42) | 38 (28–50) |
| Gender (male), n (%) | 69 (63) | 75 (64) | 192 (54) |
| Presenting complaint, n (%) | |||
| Mental illness | 62 (56) | 52 (44) | 222 (62) |
| Drug-induced psychosis | 30 (27) | 40 (34) | 59 (17) |
| Intoxication | 6 (5) | 11 (9) | 16 (5) |
| Threatened self-harm | 4 (4) | 2 (2) | 54 (15) |
| Other/unknown | 6 (5) | 13 (11) | |
| Baseline SAT score, n (%) | |||
| +3 | 50 (45) | 61 (52) | 77 (50) Footnote a |
| +2 | 56 (51) | 57 (48) | 72 (47) |
| +1 | 3 (3) | 0 (0) | 5 (3) |
| Prior sedation, n (%) | 9(8) | 11 (9) | NA |
| Midazolam given with trial drug, n (%) | 2 (2) | 7 (6) | NA |
IQR, interquartile range; NA, not applicable; SAT, Sedation Assessment Tool.
a. Baseline SAT scores assessed in 154 episodes.
haloperidol and 118 allocated droperidol. The median age was 33 years (range 16–71, IQR 27–43) and 144 were male (63%). All were involuntary patients; 114 (50%) had a primary diagnosis of mental illness and 70 (31%) had been admitted with acute behavioural disturbance due to psychostimulant drugs. Baseline SAT scores were +3 in 111 episodes (49%), +2 in 113 episodes (50%), +1 in 3 episodes and not recorded in one. One hundred and ten patients entered into the study needed to be placed in the seclusion room for the protection of themselves and others. Demographic data, cause of acute behavioural disturbance and baseline SAT scores were similar in the treatment groups except that more patients with primary mental illness received haloperidol
Table 2 Primary and secondary outcomes
| Haloperidol group n = 110 |
Droperidol group n = 118 |
Excluded or not recruited n = 356 |
|
|---|---|---|---|
| Time to sedation, min (IQR) | 20 (15–30) | 25 (15–30) | 20 (20–30) |
| Sedated within 120 min, n (%) | 101 (92) | 109 (92) | |
| Additional sedation, n (%) | 14 (13) | 6 (5) | |
| Adverse effects, n (%) | |||
| Hypotension | 1 (1) | 3 (3) | |
| Hypotension/desaturation | 0 (0) | 1 (1) | |
| Extrapyramidal side-effects | 0 (0) | 1 (1) | |
| Oversedation | 0 (0) | 1 (1) | |
| Staff injuries, n (%) | 5 (5) | 3 (3) | |
| Midazolam given with trial drug, n (%) | 2 (2) | 7 (6) | NA |
IQR, interquartile range; NA, not applicable.
and more with psychostimulant effects received droperidol (Table 1). In breach of the study protocol midazolam was given nine times simultaneously with the study drug, twice in the haloperidol group and seven times in the droperidol group.
Primary outcome
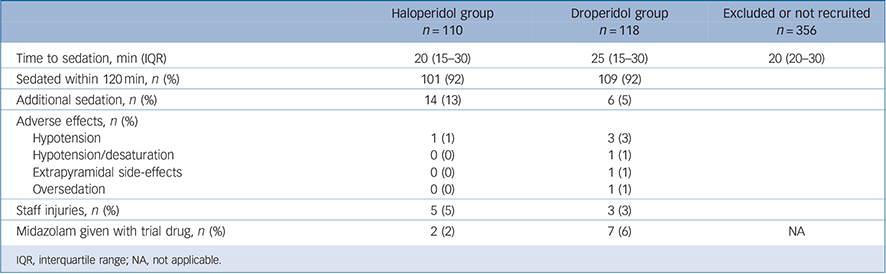
Effective sedation was achieved in 210 of 228 episodes (92%) with 9 patients receiving haloperidol and 9 patients receiving droperidol not sedating within 120 min (Table 2). The median time to sedation was 20 min (IQR 15–30, range 10–75) for haloperidol compared with 25 min (IQR 15–30, range 10–115), which was not statistically significantly different (P = 0.89) (Figs 2, 3). The median time to sedation in 126 of the 356 episodes not included in the trial was 20 min (IQR 20–30, range 10–70).
Secondary outcomes
Additional sedation was required in 20 of 228 episodes: 14 (13%) after haloperidol was given and 6 (5%) after droperidol was given (difference 7.6%, 95% CI 0.3–15; P = 0.059). Three of the 18 patients (17%) not sedated were given additional sedation. Adverse effects occurred in seven episodes (3%), one in the haloperidol group and six in the droperidol group: 1 of 110 (1%) v. 6 of 118 (5%); P = 0.12 (see Table 2). Staff injuries resulted from assaults prior to the administration of parenteral sedation and often occurred while the patient was being restrained. There were 44 staff injuries due to acute behavioural disturbance in the psychiatric intensive care unit during the 2-year study period and only eight were recorded on the study data sheets.

Fig. 2 Time to sedation for intramuscular haloperidol (10 mg) v. droperidol (10 mg).
Discussion
We found droperidol and haloperidol to be equally effective for the sedation of patients with acute behavioural disturbance in the acute mental health setting. The time to sedation for both drugs was similar and an equal proportion of patients were sedated within 120 min (92%) with a median time to sedation of 20–25 min. Although not statistically significant, there were more adverse effects in the patients given droperidol, mainly hypotension, but in no case was treatment required. Conversely, more additional sedation was required in patients given haloperidol, which again was not a statistically significant finding. The study suggests that either haloperidol or droperidol is suitable for the rapid tranquillisation of agitated and aggressive patients in an acute psychiatric unit, half of whom had a primary mental illness.
The most commonly used and recommended drugs for acute behavioural disturbance are benzodiazepines and antipsychotics. Reference Lepping6 A recent study reviewing the current trends in the UK reported lorazepam as the most recommended medication followed by haloperidol, and concluded that multiple agents and combinations are commonly used. Reference Innes and Sethi13 In contrast to clinical practice, the systematic review by Powney et al found limited evidence to support the use of haloperidol alone, better evidence to support haloperidol with promethazine (to decrease the rate of extrapyramidal side-effects) and no evidence to support the combination of haloperidol and benzodiazepines – and probable harm in the latter combination. Reference Powney, Adams and Jones4 In addition, no study has found that other antipsychotics are superior to haloperidol. Reference Powney, Adams and Jones4 Our study adds to this and provides further evidence that haloperidol alone is effective and that droperidol is similar and not more effective.

Fig. 3 Cumulative proportion of patients sedated v. time after drug administration
Previous studies
Only two small trials have previously directly compared droperidol with haloperidol, one in the emergency department and one in an acute psychiatric unit. Reference Thomas, Schwartz and Petrilli14,Reference Resnick and Burton15 Both trials found that intramuscular droperidol required less additional sedation compared with haloperidol, but were small and of low quality. Reference Powney, Adams and Jones4,Reference Thomas, Schwartz and Petrilli14,Reference Resnick and Burton15 There has been a resurgence of the use of droperidol in Australia in the past few years owing to its success for sedation of violent and agitated patients in the emergency department. Reference Isbister, Calver, Page, Stokes, Bryant and Downes8,Reference Knott, Taylor and Castle9,Reference Richards, Derlet and Duncan16,Reference Martel, Sterzinger, Miner, Clinton and Biros17 The validity of the ‘black box’ warning has been questioned by a systematic review, Reference Kao, Kirk, Evers and Rosenfeld18 and there are large studies of the safe use of droperidol for sedation prior to the warning. Reference Chase and Biros19 This has, in turn, increased the use of droperidol in some mental health units. Haloperidol has remained the major conventional antipsychotic used to treat undifferentiated acute behavioural disturbance in acute psychiatric units. It has been the most studied antipsychotic and has been used as a comparator in countless trials in mental health. Reference Powney, Adams and Jones4 Haloperidol has been shown to be as effective as atypical antipsychotics, Reference Wang, Woo and Bahk20 but has a higher propensity for extrapyramidal side-effects and has a well-defined association with QT prolongation and torsades de pointes in large doses. Reference Sharma, Rosman, Padhi and Tisdale21,Reference Muzyk, Rayfield, Revollo, Heinz and Gagliardi22
There are numerous studies of sedation or tranquillisation of patients with agitation or aggressive behaviour in the mental health setting, but few of these focus on highly agitated and aggressive patients who require physical restraint and parenteral sedation. For such patients rapid sedation rather than ‘tranquillisation’ is required, which means patients need to be contained within 30–60 min. Most studies in the mental health setting have outcomes at 2 h, 4 h, 6 h and 24 h, Reference Wilhelm, Schacht and Wagner23–Reference Meehan, Wang, David, Nisivoccia, Jones and Beasley25 which are clearly not appropriate for this type of patient. In contrast, a previous study of haloperidol used a primary outcome of sedation within 20 min and had similar times to sedation to our study. Reference Huf, Coutinho and Adams3 In addition, many other studies exclude intoxicated patients and those with substance misuse, Reference Meehan, Zhang, David, Tohen, Janicak and Small24,Reference Meehan, Wang, David, Nisivoccia, Jones and Beasley25 making generalising their results to clinical practice difficult because drug and alcohol use and intoxication are common in acute mental health admissions. Our study was restricted to a population of patients with severe agitation and aggression that required parenteral medication, and did not exclude any patient with a drug and alcohol history, making it more applicable to clinical practice.
Undertaking randomised controlled trials in this cohort of patients is difficult for many reasons, not least being the ethical issues surrounding consent for research involving these people. We have demonstrated in Australia that it is possible to undertake a controlled trial of medication without consent in this patient group. Reference Wilhelm, Schacht and Wagner23 The local human research ethics committee agreed parenteral sedation with physical restraint was already being used without patient consent as a duty of care for treatment of these patients. The committee therefore allowed us to waive consent for a study that compared two treatments that were already given as part of standard clinical care. Most controlled trials have required consent for patient recruitment, Reference Meehan, Zhang, David, Tohen, Janicak and Small24–Reference Breier, Meehan, Birkett, David, Ferchland and Sutton28 which has meant that the trials excluded the majority of severely agitated and violent patients, who were included in our study. Such studies are less useful for defining treatment in patients with severe acute behavioural disturbance, whereas our study provides important evidence about commonly used drugs to inform clinical guidelines for these high-risk and difficult to manage patients.
Adverse events
The large number of staff injuries reported over the study period confirms the severity of the behaviour in these patients. However, all injuries to staff were from assaults prior to parenteral sedation or were sustained in the process of restraining the patient. This suggests that the rapid parenteral sedation of these patients with haloperidol or droperidol prevented further injury to staff. The strict monitoring of adverse effects in this study allowed accurate assessment during the initial period following the onset of sedation. The most common adverse effect was hypotension, which was transient and did not require intervention. Hypotension occurred more commonly with droperidol. It is therefore important that there is routine assessment of blood pressure in patients given droperidol when it is safe to do so. Both droperidol and haloperidol are known to cause extrapyramidal side-effects but in this study there was only one episode of dystonia reported which quickly resolved with oral benztropine.
Limitations
A potential limitation of our study was the number of episodes where patients were not eligible to be recruited, which may have resulted in selection bias. In almost a third of these epsodes the patient was excluded from the study because they had already been recruited. The remaining patients were not recruited because of the clinicians’ preference for a particular agent, a different dose of the drugs or a combination of drugs. However, the baseline characteristics of excluded patients were no different from those recruited to the study, including the baseline SAT scores. In addition, the excluded patients had a similar median time to sedation, suggesting that the use of higher doses and combinations was no better than haloperidol or droperidol alone. A major limitation of our study was that extrapyramidal side-effects might have occurred after the 120 min observation period. This is the most likely reason for the low rate of extrapyramidal side-effects reported, because such effects often occur many hours after the drug is administered. Numerous studies of haloperidol v. other drugs and/or placebo clearly show that extrapyramidal side-effects are more common with haloperidol. Reference Powney, Adams and Jones4 The difference in previous studies is that they were over longer periods and were therefore more likely to report extrapyramidal side-effects.
Finally, the study was not powered to detect differences in the secondary outcomes including adverse effects and additional sedation. Larger studies are now required to determine whether droperidol or haloperidol are associated with a greater risk of adverse effects or requirement for additional sedation.
Appendix
Sedation Assessment Tool

| Score | Responsiveness | Speech |
|---|---|---|
| +3 | Combative, violent, out of control | Continual loud outbursts |
| +2 | Very anxious and agitated | Loud outbursts |
| +1 | Anxious/restless | Normal/talkative |
| 0 | Awake and calm/cooperative | Speaks normally |
| –1 | Asleep but rouses if name is called | Slurring or prominent slowing |
| –2 | Responds to physical stimulation | Few recognisable words |
| –3 | No response to stimulation | None |







eLetters
No eLetters have been published for this article.