Studies have suggested that the functional neuroanatomy of auditory hallucinations includes the primary auditory cortex (Reference Dierks, Linden and JandlDierks et al, 1999) as well as language-related temporal and frontal areas (Reference McGuire, Shah and MurrayMcGuire et al, 1993; Reference David, Woodruff and HowardDavid et al, 1996; Reference Lennox, Park and MedleyLennox et al, 2000; Reference Shergill, Brammer and WilliamsShergill et al, 2000). In particular, the activation of the primary auditory cortex was interpreted as an integrative component of the false perception representing its physical quality. However, later functional imaging studies showed inconsistent results concerning the involvement of this region (Reference Lennox, Park and MedleyLennox et al, 2000; Reference Shergill, Brammer and WilliamsShergill et al, 2000; Reference Copolov, Seal and MaruffCopolov et al, 2003). The activation of the primary auditory cortex contributes to the quality of the voices as not being self. It was recently shown, however, that such activation can be a consequence of selective auditory attention (Reference Petkov, Kang and AlhoPetkov et al, 2004). Thus, the question remained open whether the activation of the left primary auditory cortex is rather an epiphenomenon, i.e. an enhanced response to the scanner noise due to enhanced auditory attention during the hallucinations. Support for the view that activation of this cortical area can occur through routes other than external auditory stimulation is given from functional magnetic resonance imaging (fMRI) studies of silent lip-reading which showed auditory activation (Reference Calvert, Bullmore and BrammerCalvert et al, 1997; Reference MacSweeney, Amaro and CalvertMacSweeney et al, 2000).
Activation of the primary auditory cortex can be investigated with the N100 component of auditory evoked potentials (Reference Pantev, Bertrand and EulitzPantev et al, 1995). Attention to auditory input increases N100 amplitude (Reference Hillyard, Hink and SchwentHillyard et al, 1973; Reference Hari, Hamalainen and KaukorantaHari et al, 1989), whereas distracting stimuli reduces it (Reference Numminen, Salmelin and HariNumminen et al, 1999), presumably driven by thalamic modulation (Reference Frith and FristonFrith & Friston, 1996). Using evoked potentials elicited by well-defined tones, the responsivity of the primary auditory cortex can be investigated by analysing the N100 separately for periods with and without auditory hallucinations in an intra-individual design.
In this study we investigated the reaction of the cortical auditory system that physiologically responds to real, external, acoustic stimuli under both the physiological condition and the condition of auditory hallucinations. From prior studies it is known that increased general attention to auditory stimuli during such hallucinations enhances the response to the external stimulus. If the activation of the primary auditory cortex observed in fMRI during auditory hallucinations (Reference Dierks, Linden and JandlDierks et al, 1999) is solely due to directed auditory attention, the amplitude of the auditory evoked potentials should increase. In contrast, if the amplitude of the evoked potential during the hallucination is reduced, activation of this cortical region might be a constituent of the hallucination and distract auditory attention from external stimuli; in consequence, a reduced N100 amplitude would be found.
METHODS
Sample
We analysed data from 7 people out of a total of 11 we originally investigated, all of whom had a psychotic disorder with acute auditory hallucinations (two patients were withdrawn because the test procedure was interrupted, and in two cases there were too few segments for auditory evoked potential analysis). Diagnosis was ascertained according to ICD–10 criteria (World Health Organization, 1992), coded as F20 (n=6) and F23 (n=1) (Reference BramerBramer, 1988), on the basis of a semi-structured clinical interview as well as review of the patients’ case notes. All participants were right-handed and were receiving in-patient treatment at the University Hospital for Clinical Psychiatry in Bern, Switzerland. Only patients without relevant medical disorders (except for their psychiatric diagnosis) were included, on the basis of their medical history and medical and neurological examination. All patients reported normal hearing and showed normal binaural auditory thresholds. All patients included in the study were taking either a typical antipsychotic medication (2 patients: haloperidol) or an atypical antipsychotic (5 patients: olanzapine, risperidone or quetiapine) in conventional dosages. Five received additional benzodiazepine (diazepam, lorazepam) or benzodiazepine-like (zolpidem) treatment. Because we used an intra-individual design, we did not expect that differences in medication would affect the results. Potentially, psychopharmacological treatment may have modulating effects on focal attention. This might have had a general influence on the individual N100 amplitudes, but there is no evidence that this might account for an eventual difference between the two experimental conditions (hallucinations v. no hallucinations).
The investigation was conducted in accordance with the Declaration of Helsinki and approved by the governmental ethics committee (Kantonale Ethik-Kommission Bern). All patients gave their written informed consent before participating in the study.
Psychopathological assessment scales
The Clinical Global Impression scale (CGI; Reference GuyGuy, 1976) and the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) were used to assess psychopathology and the severity and acuity of the disease. Additionally, hallucinations were rated using the Oulis auditory hallucinations rating scale (Reference Oulis, Mavreas and MamounasOulis et al, 1995). The character of the hallucinations thus assessed fulfilled the criteria of the Schneiderian first-rank symptoms of voices referring to the patient in the second or third person or in the form of a commentary.
Experimental design and stimulus material
The participants were recorded in a silent, electrically shielded and slightly darkened room in a comfortable resting position. They were instructed to listen and attend to their voices and to indicate the beginning as well as the end of the hallucination period by pressing one button to indicate ‘begin’ and another button to indicate ‘end’. During the entire recording period of about 8 min, a series of tone pulses was presented to both ears using insert phones (Ear-Tone 3A Insert Phones, Indianapolis, Indiana, USA). These tones consisted of 1000 Hz sinusoidal tones with a duration of 70 ms (increase 10 ms, plateau 50 ms, decrease 10 ms) and were presented with an interstimulus interval of 1430 ms± 140 ms. All participants reported normal hearing. Auditory thresholds were assessed individually in each participant in the recording room, with the same apparatus used for the stimulation procedure. The tone intensity was then adjusted to be 60 dB above the ascertained threshold.
Electrophysiological recordings
The electroencephalogram (EEG) was recorded using silver/silver chloride electrodes attached to the scalp at 74 regularly spaced standard positions of the international ten-ten system. Impedances were kept below 10 kΩ. The recording reference electrode was at electrode position Cz. An electrocardiogram and electro-oculogram (1 cm below each eye) were recorded for artefact monitoring. All signals were amplified, band-pass filtered between 0.3 Hz and 70 Hz, digitised at 250 Hz and stored using a BrainScope EEG system (M&I, Prague, Czech Republic). The participants’ button presses and the onset of each 1000 Hz tone were digitally marked on the EEG.
Analysis of the auditory evoked potentials
On the basis of the participants’ button presses, the EEG recording period was divided into periods with and without hallucinations. In these periods, EEG epochs from 200 ms before tone onset to 500 ms post-onset were selected. An automatic artefact detection excluded epochs with amplitudes exceeding 100 μV from further analysis. Because some of the participants had produced considerable movement artefacts, a conservative band-pass filter (3–12 Hz), which allowed optimal focusing on N100 and P200, was applied, removing remaining baseline problems and muscle artefacts. To obtain the auditory evoked potentials the epochs were averaged separately for periods with and without auditory hallucinations and for each participant. Participants with fewer than 50 epochs in either condition were excluded (n=2). All averaged event-related potentials (ERPs) were recomputed to average reference. From the individual averaged auditory evoked potentials, grand mean ERPs were computed separately for both conditions.
Identification of auditory evoked potential components
The determination of the time windows for further analysis was based on the identification of microstates. Microstates in ERPs are periods with quasi-stable field topography; they are typically centred in time around a peak in map amplitude and separated by brief moments of rapid field configuration change (Reference Lehmann, Gevins and RémondLehmann, 2005). Microstates analysis minimises the problem of multiple testing by collapsing over periods that have a similar topography, and thus presumably similar neural generators. The latency range and topography of the N100 and P200 microstate were identified using the grand mean auditory evoked potential over participants and conditions: first, the global field power (GFP) curve and the global map dissimilarity curve (Reference Lehmann, Gevins and RémondLehmann, 2005) of the ERP were computed. The GFP is the time-varying standard deviation across all electrodes and indicates, moment by moment, the overall strength of the electric field. Global map dissimilarity is a time-varying index of change of the GFP-normalised electric field; high map dissimilarity indicates moments of rapid change of field configuration and thus determines the temporal borders of topographic ERP microstates (Reference Lehmann, Gevins and RémondLehmann, 2005). Prototypical N100 and P200 topographies were extracted from the grand mean ERP at the GFP peak around 100 ms (N100) and after 150 ms (P200). These two prototype topographies were normalised in amplitude for GFP=1. The onsets and offsets of the N100 and P200 microstates were defined by the peaks of the dissimilarity curve closest to the moment of the GFP maximum of the component (see Fig. 1).

Fig. 1 Global field power and global map dissimilarity curves. Upper graph: the global field power is the time-varying standard deviation across all electrodes. It indicates, moment by moment, the overall strength of the electric field of the auditory evoked potential (n=7, all epochs), here shown for the analysed first 500 ms after the stimulus (time 0). Lower graph: global map dissimilarity was used to compute a time-varying index of topographic change, comparing maps adjacent in time. Periods of low dissimilarity indicate adjacent maps have similar topography and belong to the same microstate. High dissimilarity indicates a rapid change of field configuration. The peaks of map dissimilarity were thus used to determine the onset and offset latencies of the auditory evoked potential N100 and P200 microstates. The N100 and P200 topographies are shown in the inserts.
Effect of hallucinations on auditory evoked potential amplitude
The amplitude and topography of the N100 and P200 microstates were compared between conditions (with and without hallucinations). The individual N100 and P200 amplitudes were quantified using a procedure similar to the topographic component recognition procedure (Reference Brandeis, Naylor and HallidayBrandeis et al, 1992): using the normalised topographies of the grand mean N100 and P200 microstates as templates, the time-varying spatial covariance of the individual auditory evoked potential with the two template topographies was computed in each participant and condition. Instead of considering a single, user-selected channel against an also pre-selected reference, this procedure is reference-independent and weights all measured channels according to their objective contribution to the component under investigation. The individual total amplitude of the two N100 and P200 microstates was then defined as the area under the covariance curve between the previously defined onset and offset of each component. The individual total amplitudes of the two components were then compared between conditions using two-tailed paired t-tests. By combining microstate and spatial covariance analysis, we were thus able to reduce the temporal and spatial redundancy of the data and extract and compare a single, representative quantifier for each ERP component of interest. Such data-driven approaches may not necessarily provide new information, but make the results unambiguous regarding brain function and increase their reproducibility (Reference Lehmann, Gevins and RémondLehmann, 2005).
Effect of hallucinations on auditory evoked potential topography
For the topographic comparisons, mean individual microstate maps were computed for both conditions by averaging the voltages over the microstate latency range of the N100 and P200 microstates. These individual mean microstate maps were normalised (divided) by the GFP. As in earlier studies of potential landscape differences (Reference Kondakor, Pascual-Marqui and MichelKondakor et al, 1995; Reference Strik, Fallgatter and BrandeisStrik et al, 1998), the tests for topographic differences between conditions used the program TANOVA, which is part of a software package for LORETA (http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm), according to the following procedure: first, the difference between the mean microstate maps of the two conditions was measured using the global map dissimilarity (Reference Lehmann and SkrandiesLehmann & Skrandies, 1980). Next, the distribution of this difference under the null hypothesis (i.e. assuming the difference is random) was established by repeatedly shuffling the microstate maps over the two conditions and computing the global map dissimilarity between the mean microstate maps of the two now randomly defined conditions (Reference EdgingtonEdgington, 1980; Reference ManlyManly, 1997). Finally, the statistical significance of the difference, based on the actual conditions, was obtained by comparing it with the distribution of the randomly obtained differences. In our analysis 5000 random permutations were used.
Source localisation
For the localisation of sources of the auditory evoked potential microstate topographies, the single stimulus-related epochs of all participants and both conditions were used. In each epoch, the mean topography of the N100 and P200 microstate time window was computed. Using these topographies, the strength and orientation of low-resolution electric tomography algorithm (LORETA) distributed sources was computed, confined to the cortical areas of the digitised brain atlas of the Montreal Neurological Institute at about 7 mm resolution (2394 voxels). In each voxel, the signal-to-noise ratio of the three-dimensional vectors representing the strength and orientation of the estimated sources was tested across the epochs using Hotelling's T 2 tests. Furthermore, in microstates with a significant difference in auditory evoked potential amplitude or topography, the individual mean microstate maps were used to compute individual voxel-wise LORETA current source density values for the two conditions. The difference of current density values between conditions was assessed and visualised using voxel-wise paired t-tests.
RESULTS
Clinical characteristics of the sample
The participants’ average age was 35 years, (range 21–54). There was one woman in the sample. The mean PANSS scores of the seven participants whose data were analysed were total score 81.3 (s.d.= 13.2), positive scale 24.4 (s.d.=4.5) and negative scale 16.9 (s.d.=2.7). The mean CGI score was 5.9 (s.d.=1.0), indicating a severe degree of acuity of symptoms. All the participants were convinced that the hallucinatory voices were real and reported that they could understand the verbal content of the voices clearly. Five of seven participants reported that the voices were coming from inside their head, whereas the other two experienced the voices as coming from outside their head. Five of seven patients perceived the voices to be as loud as real voices or even louder, whereas two reported that the voices were muted, like a whisper.
Auditory evoked potentials
Nine of the 11 patients completed the entire recording procedure. Data for two of these nine patients had to be excluded because of an insufficient number (<50) of artefact-free EEG segments for the average auditory evoked potential. A mean of 129 (s.d.= 90) segments were available for periods with auditory hallucinations; for the periods without hallucinations, the mean number of segments was 103 (s.d.=47). An average of 15 (range 4–24) periods with auditory hallucinations and 15 (range 3–21) periods without hallucinations were reported during the measurement time. In all patients, periods with hallucinations were consistently distributed during the measuring period without order effect. The GFP curve and the global map dissimilarity of the grand mean auditory evoked potential across participants and conditions are shown in Fig. 1. The microstate analysis identified the N100 component in a latency window from 64 ms to 132 ms and the P200 component in a latency window from 136 ms to 212 ms. The N100 topography was characterised by a symmetrical, frontocentral negative topography, whereas the P200 had a frontocentral positive topography.
Amplitude
In the N100 microstate the amplitude was smaller in periods with hallucination compared with periods without hallucinations (t=4.36, d.f.=6, P<0.01). The N100 amplitude reduction during hallucinations could be observed in every person tested (Fig. 2.). There was no difference for P200 amplitudes (t=0.23, d.f.=6, NS).
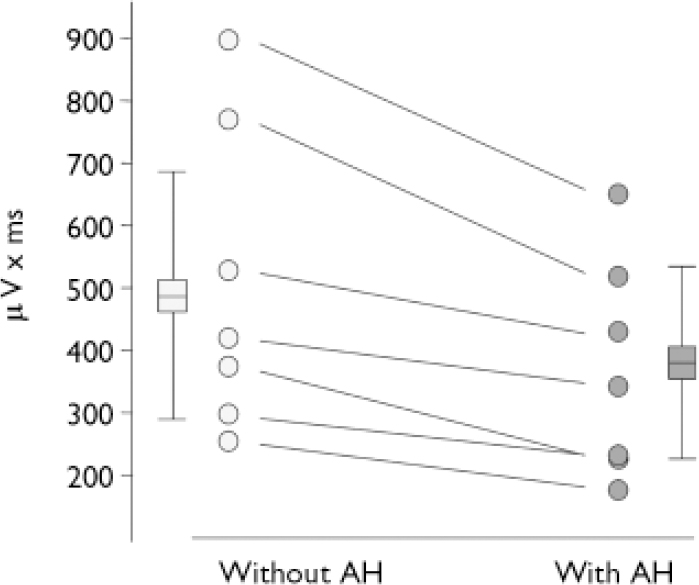
Fig. 2 Area under the curve of the N100 component. Individual values indicated by circles, boxes and whiskers indicate group statistics (mean, s.d.: n=7) (AH, auditory hallucinations).
Topography
The randomisation test of the N100 topography indicated a significant difference between evoked potentials recorded in periods with and without hallucinations (P=0.02; 100 out of 5000 randomisation runs yielded larger differences than the one actually observed). There was no such difference for the P200 topography (P=0.62; 3140 out of 5000 randomisation runs yielded larger differences than the one actually observed).
Sources
The LORETA source localisation statistics of the N100 indicated the strongest signal-to-noise ratios of the estimated sources bilaterally in the temporal cortex. Left hemispheric signals were stronger than right hemispheric signals (Fig. 3). In the estimated current source density of the averaged individual auditory evoked potentials, the largest differences of source strength between periods with and without hallucinations were located in the left temporal lobe, where smaller amplitudes during hallucinations were observed (Fig. 4).

Fig. 3 The low-resolution electric tomography algorithm (LORETA) solution of the N100. Activation was determined across all analysis epochs with and without auditory hallucinations and all participants (n=1854) using Hotelling'sT 2 tests. For visualisation, the source solution was mapped to the inflated cortical surface of one participant's anatomical MRI, left view. A colour version of this figure is presented as a data supplement to the online version of this paper.

Fig. 4 Glass-brain view of the location of decreased low-resolution electric tomography algorithm (LORETA) current source density in periods with auditory hallucinations in the N100. Black voxels indicate voxels with lower source amplitudes (t-statistics, n=7, P<0.01) in response to the stimulating beep tone during periods with competing internal voices compared with periods without auditory hallucinations.
DISCUSSION
Is the previously observed increased neuronal activity in the primary auditory cortex during hallucinations (Reference Dierks, Linden and JandlDierks et al, 1999) a neurophysiological correlate of the subjective experience of the inner voices as being alien and not-self generated? Alternatively, is it due to increased unspecific general auditory attention during listening to the external attributed internal voices, leading to increased neuronal activity in response to scanner noise during hallucination periods? This differentiation is of crucial importance for the development of a neurobiological model of the generation of hallucinations.
In this study, the responsiveness of the auditory cortex was assessed with auditory evoked potentials, the physiological brain electrical response to auditory stimuli. We found N100 and P200 components in all participants, configuring the expected topography (Reference Gomes, Dunn and RitterGomes et al, 2001); thus indicating that the methods applied were appropriate to measure responsiveness of auditory cortex. If the stimuli are attended to, the N100 response increases (Reference Hillyard, Hink and SchwentHillyard et al, 1973; Reference Hari, Hamalainen and KaukorantaHari et al, 1989). If attention is distracted from the stimulating tones, the N100 decreases (Reference Papanicolaou, Wilson and BuschPapanicolaou et al, 1988; Reference Corbetta, Miezin and DobmeyerCorbetta et al, 1990; Reference Numminen, Salmelin and HariNumminen et al, 1999; Ford et al, Reference Ford, Mathalon and Heinks2001a ,Reference Ford, Mathalon and Kalba b ,Reference Ford, Mathalon and Kalba c ).
Reduced N100 to external stimuli during hallucinations
The main finding of our study is the statistically significant reduction in the N100 to the tone pulses in the periods with auditory hallucinations compared with periods without such hallucinations in all patients; a result in accordance with an earlier descriptive report of disturbed N100 in auditory hallucinations in two patients (Reference Tiihonen, Hari and NaukkarinenTiihonen et al, 1992). The attentional task load was the same for periods with and without the hallucinations: participants were instructed always to attend to the voices, and never to the tones. Furthermore, as both the beginning of a hallucination period and the end of such a period (thus the beginning of a normal period) were indicated by a button press, these button presses were completely counterbalanced between the two conditions and are unlikely to account for the reported finding. Additional support for the hypothesis that the auditory hallucinatory state is associated with reduced activity in response to external speech stimuli in temporal cortical regions is given by another early fMRI study (Reference Woodruff, Wright and BullmoreWoodruff et al, 1997), in which speech-related activation in different patient groups with and without auditory hallucinations was analysed. Data were interpreted on the basis of a possible competition for common neurophysiological resources. Psychologically, this suggests that during periods with hallucinations selective attention is focused on the acoustic aspect of hallucination events. Accordingly, the presence of voices absorbs part of the available attention capacities of the primary auditory cortex and thus reduces the N100 to external stimuli in periods when hallucinations are present. In a more neurobiological interpretation, this would mean that reduced N100 during hallucinations is the neurophysiological correlate of competition for limited neuronal resources. However, it remains possible that the reduced N100 does in fact represent an inability to focus attention on auditory inputs, with the consequence of confusion between inner speech and hearing voices.
The later auditory component, the P200, seems not to be specific for an activation of primary auditory cortex, and in addition to temporal lobe sources (Reference Vaughan, Arezzo and PictonVaughan & Arezzo, 1988) a frontal involvement has been suggested (Reference McCarley, Faux and ShentonMcCarley et al, 1989). Furthermore, the P200 has been reported to be correlated with negative symptoms in schizophrenia (Reference Shenton, Faux and McCarleyShenton et al, 1989). Thus, we did not expect any specific effects in our study for this component, and this was supported by our results.
Hemispheric asymmetry with predominance of the left primary auditory cortex
The reduction of the N100 amplitude during auditory hallucinations was localised predominantly on the left side. Recent imaging studies point to a lateralisation of the auditory cortex associated with specific processing of speech. Thus, right cortical areas might be most important for direction discrimination (Reference Poeppel, Guillemin and ThompsonPoeppel et al, 2004; Reference Brechmann and ScheichBrechmann & Scheich, 2005), whereas the left hemisphere has a strong predisposition to process the verbal character of speech (Reference Maeder, Meuli and AdrianiMaeder et al, 2001; Reference Tervaniemi and HugdahlTervaniemi & Hugdahl, 2003) and lexical judgements (Reference Poeppel, Guillemin and ThompsonPoeppel et al, 2004). These findings argue for a view in which speech perception is mediated bilaterally in the auditory cortices, and the left-sided lateralisation described here is probably associated with processes subsequent to the auditory analysis of the verbal character of the hallucinations. The left auditory cortex is an essential part of the brain's language system, which further includes Wernicke's area and the motor speech areas of Broca, as well as their inter-connections. Given that activity of the left auditory cortex above a certain level signals external (predominantly verbal) input, the abnormal baseline activity of this area during hallucinations will potentially mislabel internally generated language content – such as inner speech – as alien. In terms of attention processes during inner speech, the focus of attention thus lies not on the language content (presumably represented in left frontal regions), but on the acoustic form (presumably represented in the left temporal cortex). This hypothesis can explain both why patients perceive something as real that does not physically exist and why they do not attribute the contents of their thoughts to their own mental activity.
Hallucinations and cortical language circuitry
Although all modules of the language system are likely to coactivate to some degree during language perception and production, the focus of activity within this network is assumed to represent the consciously experienced mode of operation. When Broca together with other frontal premotor and motor speech regions predominate, the individual is speaking and experiences her or his own speech (overt speech), whereas when Broca's area alone predominates, inner speech is perceived. High levels of activity in the auditory cortex and Wernicke's area indicate that processing within the language network is driven by external sources. Activation of the primary auditory cortex during auditory hallucinations coincides with the perception that a verbal stimulus is not self- but alien-generated. This suggests that the abnormal activation of this cortical region might be a mechanism to promote the sense of non-self attributed to verbal representations, which are self-generated by the brain's language system. The network involved in the generation of auditory hallucinations is probably more extended and has been well studied (Reference McGuire, Shah and MurrayMcGuire et al, 1993; Reference Shergill, Brammer and WilliamsShergill et al, 2000). However, this does not appear in our results because the probe design we used was selective for activation of the primary auditory cortex. Furthermore, our result gives support to an early hypothesis that hallucinations arise from disruptions in the speech processing neurocircuitry, rather than from non-language cognitive or pure attentional deficits (Reference Hoffman, Rapaport and MazureHoffman et al, 1999).
Endogenous activation of auditory cortex might constitute hallucinations
The reduced N100 component in periods with hallucinations compared with periods free from from hallucinations is not consistent with increased general attention to auditory stimuli. Rather, it indicates that the higher activation in the auditory cortex measured by fMRI is a specific activation that contributes essentially to the quality of the hallucinations. The cortical activation during the hallucination subtracts neural resources from sensory perception and, psychologically, distracts from external stimuli. The results support the interpretation that the abnormal coactivation of the primary auditory cortex during inner speech is a constituent of the hallucination. The endogenous activation of this area during auditory hallucinations (Reference Dierks, Linden and JandlDierks et al, 1999) seems to be structurally facilitated (Reference Hubl, Koenig and StrikHubl et al, 2004) by fibre tracts connecting language-related frontal and temporal regions with the auditory cortex. They may facilitate an abnormal coactivation in regions related to processing the acoustical features of language. Structural anomalies of Wernicke's region and of the arcuate fascicle, along with a pathological retrograde excitation of the primary auditory cortex, may thus constitute the pathophysiological basis of auditory hallucinations in schizophrenia (Reference Strik and DierksStrik & Dierks, 2004). The activation of the primary auditory cortex may represent a contemporaneous, more physical acoustic image of a verbal thought, providing the attribute of ‘being alien’ and the loss of its quality of being ‘self-generated’.
Acknowledgements
The authors thank Christine Hug, Xia-Xia Xhang and Yvonne Fontana for assistance with the EEG measurements. Funding support by the Swiss National Science Foundation (3200-059077.99 to T.D.) is gratefully acknowledged.







eLetters
No eLetters have been published for this article.