The persistent course and enduring disability commonly associated with psychosis make imperative the continuing development and evaluation of new treatments. Reference Craig, Garety, Power, Rahaman, Colbert, Fornells-Ambrojo and Dunn1–Reference Jones, Barnes, Davies, Dunn, Lloyd, Hayhurst, Murray, Markwick and Lewis5 Systematic reviews have concluded that combining medication with psychological interventions, specifically cognitive–behavioural therapy (CBT) and family intervention, improves clinical outcomes. Reference Pilling, Bebbington, Kuipers, Garety, Geddes, Orbach and Morgan6–Reference Pfammatter, Junghan and Brenner9 The evidence for relapse reduction is robust for family intervention, Reference Pilling, Bebbington, Kuipers, Garety, Geddes, Orbach and Morgan6,Reference Pfammatter, Junghan and Brenner9–Reference Pharoah, Mari, Rathbone and Wong11 with reductions around 20%. Reference Bustillo, Lauriello, Horan and Keith12 However, its implementation in routine service settings is poor and it is inapplicable for the many patients without close carers. There is consistent evidence that CBT reduces psychotic symptoms in people with medication-resistant symptoms, Reference Pilling, Bebbington, Kuipers, Garety, Geddes, Orbach and Morgan6–Reference Pfammatter, Junghan and Brenner9 and early indications that it may also reduce relapse Reference Gumley, O'Grady, McNay, Reilly, Power and Norrie13 and emotional distress. Reference Trower, Birchwood, Meaden, Byrne, Nelson and Ross14 The population for which the evidence for the effectiveness of CBT is most robust comprises patients in a stable phase of illness selected by referrers for the presence of persistent, medication-unresponsive and distressing positive symptoms. However, even for positive symptom reduction, effect sizes were small, at only 0.35 and 0.37 in recent meta-analyses. Reference Zimmermann, Favrod, Trieu and Pomini8,Reference Tarrier and Wykes15 Tarrier & Wykes Reference Tarrier and Wykes15 have queried methodological standards in CBT studies. Nevertheless, recent guidelines, particularly those of the UK National Institute for Health and Clinical Excellence (NICE), 16 have recommended that both CBT and family intervention should be made more widely available and that the effectiveness of CBT for relapse should be further researched.
The trial reported here was motivated by the need for methodologically secure information on the effectiveness of CBT, particularly in relation to relapse reduction. This required inclusion of patients with a recent relapse of established non-affective psychosis who were at risk of further relapse, rather than including patients more traditionally treated with CBT, namely those in a stable illness phase with distressing symptoms. We were also interested in comparing CBT with an equivalent, manualised treatment, in addition to potential differences in the mechanisms of action of CBT and family intervention. We therefore randomised between treatment as usual, treatment as usual plus CBT, and treatment as usual plus family intervention. We avoided methodological limitations of earlier trials, specifically lack of assessor masking, sample attrition and inadequate statistical methods for handling missing data. Reference Tarrier and Wykes15
Methods
Study design
This multicentre randomised controlled trial (ISRCTN83557988) comprised two pathways with separate randomisation. The first pathway (‘no carer pathway’) included those without carers randomly allocated to two groups: both groups received good standard care (treatment as usual), with the addition of CBT to one of the groups. In the second pathway (‘carer pathway’), those with carers were allocated to three groups (CBT plus treatment as usual; family intervention plus treatment as usual; or treatment as usual alone). Randomisation was also stratified within each of the five participating centres and within in-patient or out-patient status at the time of relapse. Randomisation schedules were independently generated by a trial randomisation service in a separate location from all trial centres (accessed by telephone), using randomised permuted blocks with a block size randomly varying between two and ten for the no carer pathway and three and nine for the carer pathway. Each patient's first assessment was completed after screening and informed consent for inclusion, but before randomisation.
Participants
Participants were recruited by approaching consecutive patients who had recently relapsed, whether or not they had been admitted. After this index relapse, patients were screened and invited to take part as soon as they were thought able to give informed consent. The inclusion criteria were:
-
(a) a current clinical diagnosis of non-affective psychosis (ICD–10 category F2 and DSM–IV);
-
(b) age 18–65 years;
-
(c) a second or subsequent psychotic episode starting not more than 3 months before they agreed to enter the trial;
-
(d) a rating of at least 4 (moderate severity) for at least one positive symptom on the Positive and Negative Syndrome Scale (PANSS). Reference Kay17
Criteria for exclusion from the trial were:
-
(a) a primary diagnosis of alcohol or substance dependency, organic syndrome or intellectual disability;
-
(b) a command of spoken English inadequate for engaging in psychological therapy;
-
(c) unstable residential arrangements such that the likelihood of being available for the duration of the trial was low.
Participants provided informed consent under protocols approved by the South Thames Multi-Centre Research Ethics Committee and the local research ethics committees of each of the participating centres.
Allocation to no carer and carer pathway
If patients had no carer with whom they had close contact, they were invited to participate in the no carer pathway. If patients identified a carer, a relative or friend with whom they lived or were in close contact for at least 10 h each week, the patient was asked to give informed consent for the carer pathway study. Once the patient had consented, the carer was also approached for consent. At the trial recruitment mid-point, it had become apparent that otherwise eligible patients with carers had been excluded from the study because they or their carers had refused to allow carer participation. A protocol change was made with the approval of the ethical committees mentioned earlier and the Trial Steering Committee: from that point, in cases where patients or carers refused carer participation, participants with carers were offered the opportunity to enter in the trial in the no carer pathway, with random allocation to CBT plus treatment as usual or treatment as usual alone. A total of 32 such participants were subsequently included. All analyses incorporated separate testing for any effects of this sub-group on outcomes.
Recruitment
Recruitment to the trial occurred between January 2002 and July 2004. A total of 683 patients meeting inclusion criteria were identified; 301 patients provided informed consent (44%), of whom 218 entered the no carer pathway, and 83 entered the carer pathway (Fig. 1). In addition, 382 patients withheld consent to the trial. Those who consented did not differ in age from those who did not, but they were more likely to be male (χ2=8.23, d.f.=1, P=0.004). In the no carer pathway, 106 participants were allocated to CBT plus treatment as usual and 112 to treatment as usual, while in the carer pathway, 28 participants were allocated to family intervention plus treatment as usual, 27 to CBT plus treatment as usual, and 28 to treatment as usual. For each patient in the carer pathway, there was one corresponding main carer from whom data were collected: there were, thus, 83 carers.

Fig. 1 Diagram of the flow of participants through the trial.
Participants were assessed at baseline before randomisation, and at 3, 6, 12 and 24 months. The intervention treatments were all completed by 12 months, but treatment as usual continued throughout.
Settings
The trial was set in five local mental health services in London and East Anglia: two in inner London, one in suburban London, one in Norwich and one at a centre in rural Norfolk. These settings differ in levels of social deprivation, in the proportion of patients with carers and in their ethnic composition.
Treatments
Cognitive–behavioural therapy and family intervention were both delivered for 9 months with a planned minimum of 12 and a maximum of 20 sessions.
Cognitive–behavioural therapy
The therapy was an adaptation of our generic CBT for psychosis manual Reference Fowler, Garety and Kuipers18 specifically aimed to target key aspects of relapse prevention highlighted by our cognitive model. Reference Garety, Kuipers, Fowler, Freeman and Bebbington19 The first stage focused on engagement and assessment, with the key task of fostering and maintaining a good psychotherapeutic relationship with people who would initially be in an acute psychotic state. A central focus of the work was developing a shared formulation of relapse. This was done by exploring people's understanding of triggers and risks of relapse and, where appropriate, by developing a new model of disorder emphasising alternatives to delusional thinking. Reference Freeman, Garety, Fowler, Kuipers, Bebbington and Dunn20 Therapists then attempted to target the key problems associated with vulnerability to relapse, as identified by the personal formulation. Targets would often include persistent negative beliefs about self and others, characteristic reasoning styles such as jumping to conclusions and distressing emotional reactions to events and anomalous experiences. The last stage involved developing a set of self-regulatory strategies to manage relapse. This would include a pragmatic relapse management plan and the identification of particular behavioural strategies to manage risk situations and early signs as they emerged.
Family intervention
Family intervention followed the manual of Kuipers et al Reference Kuipers, Leff and Lam21 with an emphasis on improving communication, offering discussion of up-to-date information about psychosis, problem-solving, reducing criticism and conflict, improving activity, and the emotional processing of grief, loss and anger. All family members who were willing and available were invited to participate in sessions. Sessions focused on one problem at a time and were aimed at an individual formulation of each family's problems as they defined them. There was a particular focus on relapse prevention, including how family members might understand warning signs and agree on appropriate intervention, including medication. Sessions were collaborative, involved two therapists and usually took place at home. Therapy sessions were tape-recorded (with permission) and therapists were monitored for adherence and competence.
Trial therapists, training and monitoring of adherence and competence
Cognitive–behavioural therapy
Five lead trial therapists, all doctorate level or equivalent clinical psychologists employed full time on the trial, provided CBT for 96 individuals (72% of the total). A further 37 participants receiving CBT were seen by therapists employed by the local mental health services. These therapists were a mix of doctoral clinical psychologists and nurses who had received specialist training in CBT. All therapists were required to demonstrate competence in CBT before recruitment. This included submitting tapes of therapy that passed standards of competence in CBT. Lead therapists were also required to demonstrate key techniques in role-play as part of their recruitment. This was followed by a period of intensive training in workshops with both the expert CBT therapists on the trial (D.F., P.G. and E.K.) and external experts. These workshops continued throughout the trial.
Lead therapists from each centre met monthly with the expert CBT therapists to discuss each patient and for supervision. A total of 106 patients (80%) consented to sessions being tape-recorded. Supervision consisted of regular discussions and reviews about each individual, using the taped sessions. Therapists were monitored for key CBT competencies with regard to structure, therapy skills and collaboration, and the use of CBT techniques, as suggested in the Cognitive Therapy Scale (CTS). Reference Young and Beck22 They were, however, allowed flexibility with regard to agenda-setting and homework in order to take account of client sensitivities. Formal monitoring of tapes was carried out using the Cognitive Therapy for Psychosis Adherence Scale (CTPAS) Reference Startup, Jackson and Pearce23 and the CTS Reference Young and Beck22 throughout the trial, with lead therapists monitoring samples of tapes from other centres. A total of 185 tapes from 66 patients (62% of the total treated) were sent to another centre for formal monitoring. These ratings indicated that in 90% of the sample the therapy delivered in taped interviews was adherent and competent CBT (i.e. above the standard CTS cut-off for competent CBT and adherent on the CTPAS). In eight patients (10%), the therapy was regarded as supportive work rather than CBT. To check on the anchoring of these internal ratings, a randomly selected sub-sample of 18 tapes was sent to an external expert rater during the trial, and a further 18 tapes were sent to three other external experts in CBT in psychosis after the trial. Of these tapes, 34 had been rated as adherent and competent CBT by the internal raters, and all were confirmed as such by the external raters. The internal rating of one therapy session as supportive therapy was confirmed, and one session was likewise agreed to be CBT of minimal quality.
Family intervention
Family intervention involved a lead and a co-therapist working together. The five lead trial therapists for CBT also acted as lead family intervention therapists to all 28 participants.
As part of the recruitment process, all lead therapists were required to show in-depth knowledge of evidenced-based family intervention in psychosis, and to demonstrate key techniques in role-play. They also attended intensive training from an expert family intervention therapist (E.K.). All co-therapists attended family intervention training workshops or received individual training from a trial lead therapist. As with the CBT, the local therapists were a mix of doctoral level clinical psychologists and nurses who had received training in family intervention.
The trial lead therapists were provided with specialist expert monthly supervision throughout the trial and attended advanced skills workshops by E.K. and another expert. The lead therapists also met fortnightly for peer supervision and case presentations. A total of 82% (n=23) of 28 participants provided consent to tape record the therapy sessions. Supervision involved listening to complete tape-recorded sessions or long excerpts.
The formalised monitoring of recorded therapy sessions, internally and externally, was undertaken using an additional section on Family Intervention incorporated into the CTPAS Reference Startup, Jackson and Pearce23 and parts of the Family Intervention Competence Rating Scale developed in 1992 (D. Lam, personal communication, 2007). For internal monitoring, E.K. and one of the lead therapists checked the therapy quality of a sample of 13 tapes. These ratings indicated that all therapy provided was both adherent and competent. As a further external check of therapy fidelity, a random selection of 11 therapy tapes (39% of 28 patients) were sent to an external expert rater. She rated 100% of the randomised tapes as adherent, and confirmed that family intervention did not overlap in techniques with CBT intervention.
Control condition
Treatment as usual consisted of good standard care delivered according to national and local service protocols and guidelines, including the prescription of antipsychotic medication. The frequency and nature of service contacts was monitored, as was the prescription of medication. Treatment as usual did not preclude the provision of psychological interventions, although in practice this was relatively rare, as reported below.
Reliability of research assessments
Baseline assessments were conducted by a trial research worker, after patient consent had been obtained. The aim was to complete the assessment within a 3-week period. Interviews were tape-recorded for reliability and quality control purposes. Research workers met regularly throughout the trial to maintain reliability of procedures and ratings. Reliability of interview ratings was assessed using the PANSS positive symptom score. At least one other assessor (selected from a panel of 15 raters – excluding the rater responsible for the initial assessment) re-rated 55 assessments. The number of re-ratings varied between 1 and 6, and the total number of ratings made by the 15 raters varied between 2 and 27.
A linear one-way random effects model (with participant identification as the explanatory factor) was fitted by restricted maximum likelihood using Stata's xtreg procedure (version 8 for Windows) and yielded an intraclass correlation of 0.88 (95% CI 0.82–0.92). This indicates very acceptable interrater reliability.
Masking procedures
Trial research assessors were independent of treatment delivery and every effort was made to ensure they were kept masked to allocation. The primary outcome variable, relapse, was assessed by masked panel evaluation following the procedure described by Craig et al Reference Craig, Garety, Power, Rahaman, Colbert, Fornells-Ambrojo and Dunn1 and Bebbington et al. Reference Bebbington, Craig, Garety, Fowler, Dunn, Colbert, Fornells-Ambrojo and Kuipers24 In order to test the success of masking with regard to the primary outcome, panel assessors guessed whether each participant had been allocated to receive CBT, family intervention or treatment as usual. The raters had a bias towards guessing that patients were in the treatment as usual condition. Thus, in the no carer pathway, averaging across four raters, CBT was guessed correctly in 22% of patients and treatment as usual was guessed correctly in 73% of patients. This indicates that information on the receipt of psychological treatment was successfully removed from the data used to rate relapse.
Secondary outcomes were rated by research assessors in interviews and considerable effort was made to achieve masked ratings. The following strategies were used:
-
(a) research workers were not involved in the randomisation process;
-
(b) therapists were encouraged to consider room use and diary arrangements in the light of potential breaks of masking;
-
(c) patients were reminded by the assessors not to talk about treatment allocation;
-
(d) after the initial assessment, the assessor did not look at the patient's clinical notes until the last of their ratings had been collected;
-
(e) in the few cases where masking was broken, another rater assessed the patient for the final assessment;
-
(f) on the occasions when masking was broken again by patients with the second rater, this was noted in the data file;
-
(g) in all cases, after patients had completed the final assessment, the assessor was instructed to make an allocation guess.
Of the 24-month assessments, 88% were completed masked (i.e. the allocation of the patient had not been revealed to the assessor). Of these masked rater assessments, the assessors guessed CBT allocation correctly for 54%, treatment as usual allocation correctly for 63% and family intervention correctly for 42%, similar to what would be expected by chance.
Diagnostic verification
Induction was based on a clinical diagnosis of non-affective psychosis. In order to consider whether this diagnosis was sustainable at the end of the trial, we used detailed descriptions of clinical progress to amplify the information about symptoms obtained at baseline by using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN). 25 This resulted in some diagnostic shift. Thus, at follow-up, 91% of participants continued to fulfil criteria for non-affective psychosis (ICD–10 F2), whereas 9% were diagnosed as having affective psychosis.
Demographic and clinical data
Data on age, gender, ethnicity, admissions, contact with services, and medication were taken from clinical notes. Adverse events were also recorded.
Primary outcome measures
Remission and relapse ratings were made using a published method employed in a previous randomised controlled trial. Reference Craig, Garety, Power, Rahaman, Colbert, Fornells-Ambrojo and Dunn1,Reference Bebbington, Craig, Garety, Fowler, Dunn, Colbert, Fornells-Ambrojo and Kuipers24 Consensus ratings are made by paired members of the research team using manualised a priori operationalised definitions, a method with moderate to good reliability (kappa values of 0.56 and 0.71 for the identification of remission and relapse respectively between paired raters) and good validity (independent PANSS ratings were strongly related to the remission/relapse ratings of participants). Reference Bebbington, Craig, Garety, Fowler, Dunn, Colbert, Fornells-Ambrojo and Kuipers24 Ratings are based on changes in positive psychotic symptoms. Evidence is required of improvement in (for partial remission) or absence of (for full remission) positive psychotic symptoms continuing for at least 4 weeks. Relapse ratings are based on evidence of the re-emergence of, or significant deterioration in, positive psychotic symptoms of at least moderate degree persisting for at least 2 weeks. Reference Bebbington, Craig, Garety, Fowler, Dunn, Colbert, Fornells-Ambrojo and Kuipers24
In the present study, the ratings were applied to detailed extracts of the clinical case notes. These consisted of monthly reports over 24 months on mental state and service interventions, from which all information that might provide clues as to whether the patient was being seen for CBT or family intervention had been removed. Group allocation remained concealed until all the ratings were complete.
Data on all hospital admissions were collected through the hospital administration systems.
Secondary outcome measures
Psychotic symptom measures
The PANSS is a 30-item, 7-point (1–7) rating instrument developed for the assessment of phenomena associated with schizophrenia. Reference Kay17 Symptoms over the past week are rated. Four scores are obtained: total (30 items), positive scale (7 items), negative scale (7 items) and general psychopathology (16 items).
The Psychotic Symptom Rating Scales (PSYRATS) is a 17-item, 5-point scale (0–4) multidimensional measure of delusions and hallucinations. Reference Haddock, McCarron, Tarrier and Faragher26 Symptoms are rated over the previous week. Two items each from the delusions scale (conviction and distress) and from the hallucinations scale (frequency and distress) were recorded.
Measures of affect
The Beck Depression Inventory Second Edition (BDI–II) is a self-report 21-item, 4-point scale (0–3) for the assessment of depression. Reference Beck, Steer and Brown27 Depression is assessed over the previous fortnight. A total score is usually used.
The Beck Anxiety Inventory (BAI) is a self-report 21-item, 4-point scale (0–3) for the assessment of anxiety. Reference Beck, Epstein, Brown and Steer28 Anxiety is assessed over the previous week. A total score is usually used.
Social functioning
The time-budget measure is an interview measure of social functioning, designed to be sensitive to changes in activity levels. Reference Jolley, Garety, Ellett, Kuipers, Freeman, Bebbington, Fowler and Dunn29 Time spent by the interviewees participating in activities four times a day (morning, lunch, afternoon, evening) over the previous 7 days are assessed and rated on a 0–4 scale. If necessary, a more typical week in the recent past is used for the assessment period. Increasing scores reflect increasingly demanding activities (in terms of both time occupied and complexity of task). Particular care is taken during the interview to elicit social activities. A total score of activity over the week is derived; additionally, the combined total score of ratings of 0 and 1 provides a measure of time spent in no or minimal activity. The scale has good interrater reliability and validity.
Social and occupational functioning is rated on a scale of 0–100 by the assessor using the Social and Occupational Functioning Assessment Scale (SOFAS). 30
Service receipt
Service use is measured for a retrospective 6-month period using the Client Service Receipt Inventory (CSRI). Reference Beecham, Knapp, Thornicroft, Brewin and Wing31 The CSRI covers services provided by the National Health Service, other health and social care agencies, the criminal justice system and informal carers. Data are collected from clinical notes, patients, carers and case managers.
Measures of therapy process
The following measures of therapy process were used.
-
(a) The Scale to Assess Unawareness of Mental Disorder. Reference Amador, Strauss, Yale, Flaum, Endicott and Gorman32 This scale is a multidimensional measure of current and past insight.
-
(b) The Illness Perception Questionnaire, which has been adapted for use in psychosis (as reported by Watson et al Reference Watson, Garety, Weinman, Dunn, Bebbington, Fowler, Freeman and Kuipers33 ), is a self-rated measure of the subjective perception of illnesses, in five dimensions: subjective symptoms, causes, cure/control, consequences, timeline. Reference Weinman, Petrie, Moss-Morris and Horne34 It has good reliability and validity.
-
(c) The Brief Core Schema Scales is a rapid self-rated measure of negative and positive evaluations of self and others, Reference Fowler, Freeman, Smith, Kuipers, Bebbington, Bashforth, Coker, Hodgekins, Gracie, Dunn and Garety35 with good reliability and validity.
-
(d) Reasoning – three key aspects of reasoning in delusions were assessed: jumping to conclusions; Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler, Kuipers and Dudley36 belief flexibility (Maudsley Assessment of Delusions Schedule); Reference Wessely, Buchanan, Reed, Cutting, Everitt, Garety and Taylor37 and alternative explanations for delusional experiences (Explanations of Experience Interview). Reference Freeman, Garety, Fowler, Kuipers, Bebbington and Dunn20
Intellectual functioning
The Quick Test provides an estimate of current intellectual functioning. Reference Ammons and Ammons38
Carer measures
The Camberwell Family Interview was administered by trained research workers and was subsequently used to assess levels of expressed emotion (EE): the number of critical comments, hostility, emotional overinvolvement, number of positive comments, and warmth. Reference Vaughn and Leff39 High EE is rated if a respondent scores 3 or above on emotional overinvolvement, 1 or above on hostility, or makes 6 or more critical comments. All raters were trained to criterion by Dr Christine Vaughn.
The self-report questionnaire Experience of Care-giving Inventory is designed to assess the experience of caring for a relative with a serious mental illness. Reference Szmukler, Burgess and Herrman40 The 66-item questionnaire has 10 sub-scales: difficult behaviour, negative symptoms, stigma, problems with services, effects on the family, the need to provide back-up, dependency, loss, rewarding personal experiences, and good aspects of the relationship with the patient.
Another self-report screening questionnaire, the General Health Questionnaire–28, is aimed at detecting those with a diagnosable psychiatric disorder. Reference Goldberg and Hillier41 There are four sub-scales: somatic symptoms, anxiety and insomnia, social dysfunction and severe depression.
Hypotheses concerning outcome
The primary outcome hypothesis for the no carer pathway was that CBT, when added to good standard care (treatment as usual), would reduce the rates of relapse and total days in hospital at 2-year follow-up, compared with treatment as usual alone. For the carer pathway, it was hypothesised that both CBT and family intervention, when added to good standard care (treatment as usual), would reduce the rates of relapse and total days in hospital at 2-year follow-up, compared with treatment as usual alone.
With regard to secondary outcomes, it was hypothesised that over both trial pathways CBT and family intervention would reduce relapse and psychotic and emotional symptoms at 12 months (end of treatment); that CBT but not family intervention would reduce psychotic and emotional symptoms at 24 months compared with treatment as usual; and that family intervention but not CBT would improve social functioning at 24 months compared with treatment as usual.
The treatment mediator hypotheses were that CBT but not treatment as usual would lead to improvements in schemas, insight and illness perceptions, and reasoning; and that family intervention but not treatment as usual would lead to improvements in EE, burden and mental health in carers.
Statistical analysis
Power
The following sample size calculations are from the original protocol. They were based on the primary outcome measure of relapse rate. Those with treatment as usual were predicted to have a relapse rate of 50% at 2 years. Those who had CBT were predicted to have a relapse rate of 30%. A total of 140 participants per cell of the no carer pathway would give 90% power to detect this difference using a simple χ2 test at 5% significance. We predicted that the main effect of family intervention was slightly better – a 25% relapse rate at 2 years. We would have around 90% power in the carer pathway with 75 participants per cell. All power calculations were carried out using nQuery Advisor. Reference Elashoff42 Although the protocol specified that the effect of CBT should be estimated by a joint analysis of the data from both pathways, the possibility of gaining extra power in this way was not explicitly considered at that stage.
Following the mid-trial protocol changes to the pathway recruitment criteria (allowing those with non-consent for carer involvement to be randomised into the no carer pathway), sample size and power considerations were revisited. The revised recruitment targets required randomising at least 100 participants to each of the two treatment arms in the no carer pathway and about 33 participants to each of the three arms of the carer pathway. Thus there would be about 133 participants receiving CBT and a similar number receiving treatment as usual. Provided that the analysis of the data from the two pathways was undertaken jointly, the power to detect a clinically significant difference in relapse rates (30% v. 50%, as before) would be for all practical purposes the same as that originally planned for the no carer pathway. It was explicitly acknowledged, however, that there would be inadequate power for the evaluation of family intervention, but a decision was taken to continue with recruitment to this pathway because it would provide valuable information on symptom severity and mediating variables.
Data analysis
All analyses reported here were based on the intention-to-treat principle, with due consideration being given to potential biases arising from loss to follow-up. The main analyses were aimed at estimating treatment effects using data from both pathways simultaneously. This resulted in estimates of the effects of CBT (relative to treatment as usual) that were common to both pathways; estimates of the effect of family intervention (relative to treatment as usual) were, of course, applicable only to the carer pathway. Multiple regression models (or equivalent logistic models, in the case of binary data) were fitted to estimate separate treatment effects for outcomes at 12 and 24 months, controlling for pathway (two levels), treatment centre (five levels), in-patient status (two levels) and, where relevant and available, the corresponding baseline assessment for the outcome under investigation. Analyses of lengths of hospital admissions used untransformed data, but employed bootstrapping to generate valid 95% confidence intervals for the treatment effects. All analyses were carried out using Stata version 9. 43 We allowed for the presence of missing outcome data under the assumption that the data are missing completely at random conditional on the covariates included in the regression models (i.e. missing at random, using the terminology of Little & Rubin). Reference Little and Rubin44 The sensitivity of the results to departures from this assumption was checked for the main secondary outcomes (symptom severity measures as provided by the PANSS) through the use of inverse probability weights. Reference Everitt and Pickles45,Reference Heyting, Tolboom and Essers46 Here, the probability of having a non-missing PANSS score was modelled and predicted separately using logistic regression for those not offered an intervention treatment and those offered either CBT or family intervention. This was carried out separately for 12- and 24-month outcomes. The variables used in the logistic regression models were relevant baseline PANSS score, pathway, treatment centre, in-patient status, gender and clinical outcome (number of months in partial or full remission over the first 12 months and also over the second 12 months). An inverse probability weight for each individual participant providing the relevant outcome measurement was then calculated as the reciprocal of the modelled probability of providing a non-missing outcome.
In a separate series of exploratory analyses (i.e. that were not part of the original analysis plan), the treatment effects on selected outcome variables were again estimated for participants in both pathways, using the same models as above, but after first excluding those without a carer (thus estimating the treatment effects for those with carers, irrespective of whether they actually entered the carer pathway of the trial).
Results
Demographic and clinical characteristics of participants
A total of 301 patients and 83 carers participated in the study. The socio-demographic and clinical characteristics of the patients (online Tables DS1 and DS2) and of the carers (online Table DS3) did not differ between groups.
Therapy provision
One hundred and thirty-three people were allocated to CBT. They received a mean of 14.3 sessions (s.d.=7.8), each session lasting on average 1 hour. Those in the no carer pathway (n=106) received a mean of 14.4 sessions (s.d.=7.8); those in the carer pathway (n=27), a mean of 13.9 sessions (s.d.=8.0); the group with carers allocated to the no carer pathway (n=18) received a mean of 15.1 sessions (s.d.=8.8). Twenty-eight families were allocated to the family intervention. They received a mean of 13.9 sessions (s.d=7.5) or 14.1 h of therapy (s.d.=7.9). Figure 1 shows therapy sessions in each pathway, including the numbers of participants receiving a partial (6–11 sessions) or full (12 or more sessions) ‘dose’ of therapy.
Provision of non-intervention therapy and medication
We used the CSRI to examine non-intervention counselling or psychological therapy delivered to the treatment and control (treatment as usual) groups in the 6 months preceding baseline, and in the two 12-month periods following induction. The provision of psychological therapy in addition to the trial interventions was rare, was more often than not non-significant in group comparisons, and favoured treatment as usual where it occurred. At baseline there were no differences in either pathway between treatment and control groups in the mean number of therapist contacts for other interventions. At 12 months following induction, there were no differences in the no carer pathway, but there were small but statistically significant differences in the carer pathway: those allocated to treatment as usual were more likely than those allocated to CBT or to family intervention to have an additional non-intervention therapy (Fishers exact χ2=5.6, d.f.=1, P=0.041). Three people in treatment as usual received a non-intervention therapy, compared with none in the family intervention or CBT groups. Between 12 and 24 months following induction, there was no longer a difference between the groups in the carer pathway (with one person in treatment as usual, one in CBT and none in family intervention receiving non-intervention therapy); nor was there a significant difference between CBT and treatment as usual groups in the no carer pathway (eight participants in treatment as usual and two in CBT had been receiving a non-intervention therapy).
Antipsychotic medication data were extracted from medical records and dosages were converted into chlorpromazine equivalents grouped into low (0–200 mg), medium (200–400 mg) and high (⩾400 mg). Changes in all medication from baseline to 12 months and from 12 months to 24 months were recorded as no change, increasing or decreasing doses. Additionally, clozapine was recorded as commenced, stopped or unchanged. There were no differences between the groups in baseline medication or in changes in medication over the course of the trial.
Primary outcomes
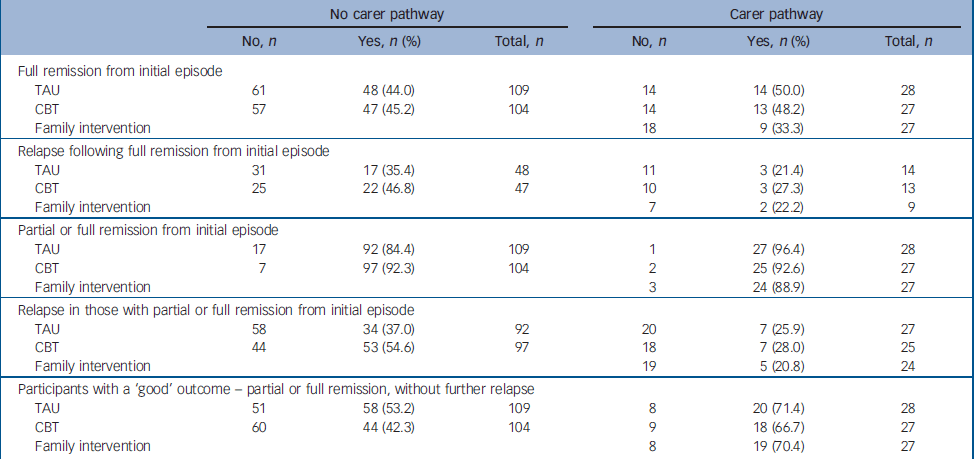
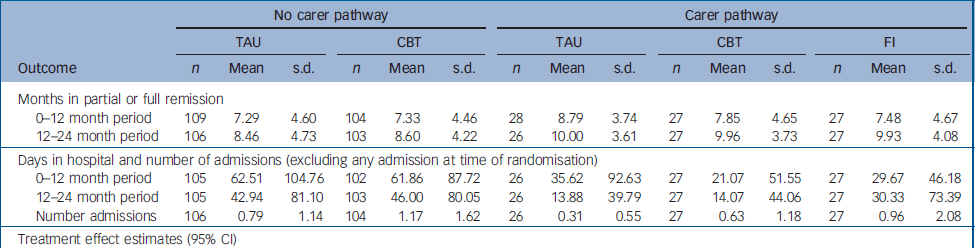
Primary outcome data were available on 96% of the total sample. There were no differences between the groups, in either pathway, in the primary outcomes of patterns of remission and relapse, and total days in hospital at 12 months or at 24 months (Tables 1 and 2). It will be seen from Table 1 that the proportion of participants who made a full remission from the index episode was disappointingly low (less than 50%). This makes the planned intention-to-treat analysis of subsequent relapse problematic. Most participants did, however, make a partial remission. Survival curves (not shown) for full or partial remission failed to reveal any interesting treatment effects (median remission times from the initial episode being about 3 months in the no carer pathway, and 2 months in the carer pathway). Whether we examine full remission and the possibility of relapse from full remission, or the less stringent criterion of full or partial remission and subsequent relapse, it is clear from Table 1 that there are no signs of any treatment effects in either pathway. In order to carry out a formal analysis of treatment effects, we used months in partial or full remission as our indicator of the primary outcome. Summary statistics and estimated treatment effects are given in Table 2. Again, there is no evidence of any treatment effects. The results for the number of hospital admissions (in addition to any admission at the time of randomisation) and days spent in hospital (within the first 12 months and between 12 and 24 months) again fail to reveal any significant treatment effects.
Table 1 Primary outcomes: patterns of remission and relapse for patients with and without carers
| No carer pathway | Carer pathway | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No, n | Yes, n (%) | Total, n | No, n | Yes, n (%) | Total, n | |||||
| Full remission from initial episode | ||||||||||
| TAU | 61 | 48 (44.0) | 109 | 14 | 14 (50.0) | 28 | ||||
| CBT | 57 | 47 (45.2) | 104 | 14 | 13 (48.2) | 27 | ||||
| Family intervention | 18 | 9 (33.3) | 27 | |||||||
| Relapse following full remission from initial episode | ||||||||||
| TAU | 31 | 17 (35.4) | 48 | 11 | 3 (21.4) | 14 | ||||
| CBT | 25 | 22 (46.8) | 47 | 10 | 3 (27.3) | 13 | ||||
| Family intervention | 7 | 2 (22.2) | 9 | |||||||
| Partial or full remission from initial episode | ||||||||||
| TAU | 17 | 92 (84.4) | 109 | 1 | 27 (96.4) | 28 | ||||
| CBT | 7 | 97 (92.3) | 104 | 2 | 25 (92.6) | 27 | ||||
| Family intervention | 3 | 24 (88.9) | 27 | |||||||
| Relapse in those with partial or full remission from initial episode | ||||||||||
| TAU | 58 | 34 (37.0) | 92 | 20 | 7 (25.9) | 27 | ||||
| CBT | 44 | 53 (54.6) | 97 | 18 | 7 (28.0) | 25 | ||||
| Family intervention | 19 | 5 (20.8) | 24 | |||||||
| Participants with a ‘good’ outcome – partial or full remission, without further relapse | ||||||||||
| TAU | 51 | 58 (53.2) | 109 | 8 | 20 (71.4) | 28 | ||||
| CBT | 60 | 44 (42.3) | 104 | 9 | 18 (66.7) | 27 | ||||
| Family intervention | 8 | 19 (70.4) | 27 | |||||||
CBT, cognitive–behaviour therapy; TAU, treatment as usual
Table 2 Primary outcomes: remission and total number of days in hospital for patients with and without carers
| No carer pathway | Carer pathway | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAU | CBT | TAU | CBT | FI | ||||||||||||||||||||||||||
| Outcome | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | n | Mean | s.d. | |||||||||||||||
| Months in partial or full remission | ||||||||||||||||||||||||||||||
| 0–12 month period | 109 | 7.29 | 4.60 | 104 | 7.33 | 4.46 | 28 | 8.79 | 3.74 | 27 | 7.85 | 4.65 | 27 | 7.48 | 4.67 | |||||||||||||||
| 12–24 month period | 106 | 8.46 | 4.73 | 103 | 8.60 | 4.22 | 26 | 10.00 | 3.61 | 27 | 9.96 | 3.73 | 27 | 9.93 | 4.08 | |||||||||||||||
| Days in hospital and number of admissions (excluding any admission at time of randomisation) | ||||||||||||||||||||||||||||||
| 0–12 month period | 105 | 62.51 | 104.76 | 102 | 61.86 | 87.72 | 26 | 35.62 | 92.63 | 27 | 21.07 | 51.55 | 27 | 29.67 | 46.18 | |||||||||||||||
| 12–24 month period | 105 | 42.94 | 81.10 | 103 | 46.00 | 80.05 | 26 | 13.88 | 39.79 | 27 | 14.07 | 44.06 | 27 | 30.33 | 73.39 | |||||||||||||||
| Number admissions | 106 | 0.79 | 1.14 | 104 | 1.17 | 1.62 | 26 | 0.31 | 0.55 | 27 | 0.63 | 1.18 | 27 | 0.96 | 2.08 | |||||||||||||||
| Treatment effect estimates (95% CI) | ||||||||||||||||||||||||||||||

| 0–12 months | 12–24 months | |||||
|---|---|---|---|---|---|---|
| CBT | FI | CBT | FI | |||
| Months in remission | –0.15 (–1.22 to 0.91) | –0.89 (–3.01 to 1.23) | 0.09 (–0.95 to 1.14) | –0.06 (–2.13 to 2.01) | ||
| Days in hospital a | –4.23 (–24.40 to 16.26) | 3.55 (–23.75 to 33.37) | 2.27 (–15.94 to 20.10) | 19.13 (–11.10 to 51.68) | ||
CBT, cognitive–behavioural therapy; FI, family intervention; TAU, treatment as usual
a. Confidence interval from 1000 bootstrap samples, using the non-parametric (percentile) method
Secondary outcomes
Secondary outcome data were available for 82% of the total sample at 12 months and for 80% at 24 months.
Psychotic and emotional symptoms
The mean scores at baseline, 12 and 24 months on the PANSS total, positive, negative and general symptoms for each group in the no carer and carer pathway are presented in online Table DS4. It also presents the same data for the PSYRATS, hallucination frequency (A1) and distress (A8), and delusional conviction (B1) and distress (B4). All these scores declined over the course of the trial in all groups. Scores on the BDI and BAI are given in online Table DS5. In Table 3, we show the treatment effect estimates for the difference in mean scores between CBT and treatment as usual and family intervention and treatment as usual on the PANSS, PSYRATS, BDI and BAI at 12 and 24 months. Almost none of the results were significant. The only exception was a significant difference between treatment as usual and those who received CBT in improvements in depression at 24 months, favouring CBT. A weighted analysis of the 24-month PANSS outcomes (to allow for missing data) produced results very similar to those given in Table 3.
Table 3 Treatment effect estimates: difference in means (95% CI) between treatment condition (cognitive–behavioural therapy or family intervention) and treatment as usual

| 12 months | 24 months | |||||
|---|---|---|---|---|---|---|
| CBT | FI | CBT | FI | |||
| PANSS | ||||||
| Total | –1.53 (–5.28 to 2.22) | –4.26 (–11.68 to 3.17) | –0.03 (–3.72 to 3.66) | –3.03 (–10.59 to 4.53) | ||
| Positive | –0.74 (–2.20 to 0.73) | –1.22 (–4.09 to 1.65) | 0.78 (–0.83 to 2.38) | 2.06 (–1.15 to 5.27) | ||
| Negative | –0.55 (–1.80 to 0.71) | –1.51 (–4.02 to 1.00) | –0.01 (–1.25 to 1.23) | –0.65 (–3.17 to 1.87) | ||
| General | –0.30 (–2.19 to 1.60) | –1.43 (–5.16 to 2.31) | –0.77 (–2.59 to 1.04) | –2.43 (–6.14 to 1.28) | ||
| PSYRATS | ||||||
| A1 | –0.10 (–0.65 to 0.46) | –0.22 (–1.18 to 0.75) | –0.07 (–0.60 to 0.45) | –0.35 (–1.28 to 0.58) | ||
| A8 | –0.10 (–0.72 to 0.51) | –0.37 (–1.47 to 0.73) | 0.09 (–0.52 to 0.70) | 1.03 (–0.06 to 2.13) | ||
| B1 | –0.02 (–0.41 to 0.38) | 0.35 (–0.41 to 1.12) | 0.11 (–0.27 to 0.50) | 0.54 (–0.23 to 1.32) | ||
| B4 | –0.13 (–0.64 to 0.39) | 0.11 (–0.84 to 1.06) | –0.03 (–0.60 to 0.54) | –0.25 (–1.35 to 0.85) | ||
| BAI | 1.93 (–1.24 to 5.10) | –1.27 (–7.48 to 4.93) | 0.59 (–2.92 to 4.11) | –3.13 (–10.45 to 4.19) | ||
| BDI | –0.28 (–3.38 to 2.81) | 0.98 (–5.01 to 6.97) | –3.07 (–6.04 to −0.11) * | –0.36 (–6.35 to 5.63) | ||
| Time budget | ||||||
| Total | 5.25 (–0.89 to 11.38) | –2.38 (–14.05 to 9.29) | 2.55 (–2.81 to 7.91) | 5.97 (–4.61 to 16.55) | ||
| Zero/one | –1.86 (–4.09 to 0.36) | 0.98 (–3.28 to 5.23) | –1.87 (–4.02 to 0.29) | –3.23 (–7.51 to 1.06) | ||
| SOFAS | 2.77 (–1.02 to 6.55) | 1.90 (–5.52 to 9.32) | 2.42 (–1.42 to 6.26) | 2.13 (–5.78 to 10.04) | ||
| EuroQol | –4.68 (–10.93 to 1.57) | –5.91 (–18.80 to 6.99) | ||||
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CBT, cognitive–behavioural therapy; EuroQol, Quality of Life measure; FI, family intervention; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scale (A1, hallucination frequency; A8, hallucination distress; B1, delusional conviction; B4, delusional distress); SOFAS, Social and Occupational Functioning Assessment Scale; TAU, treatment as usual; zero/one, mean number of time periods rated 0 or 1 on time budget
* P<0.05
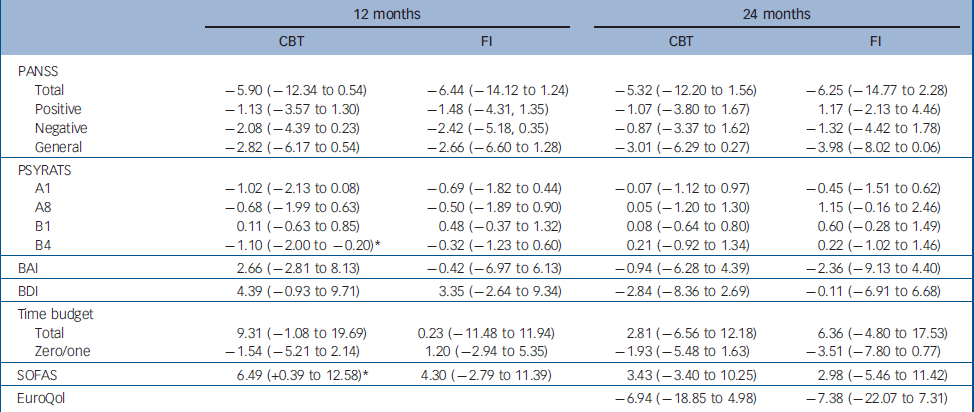
In a separate, exploratory, series of analyses, we examined the data for people with carers, irrespective of whether they had been recruited to the no carer or carer pathways. Selected change scores are summarised in online Table DS6. It will be seen that similar change scores were shown for CBT for people with carers, whether they were in the no carer or the carer pathway, and for family intervention in the carer pathway, apparently favouring both treatments over treatment as usual. These change scores were generally greater than those for CBT in the no carer pathway. The estimated treatment effects for people with carers of CBT and of family intervention, both compared with treatment as usual, are shown in Table 4. There was a statistically significant reduction in delusional distress at 12 months for those with carers who received CBT; all other results for psychotic and emotional symptoms were non-significant, although a number of other variables indicate a consistent treatment effect that might be common to both CBT and family intervention. Both CBT and family intervention treatments were then combined in a further exploratory analysis: the estimated treatment effects on PANSS scores of receiving a psychological treatment, whether CBT or family intervention, for people with carers are shown in Table 5. At 12 months, treatment resulted in significantly better PANSS total scores and PANSS negative symptom scores. At 24 months there were significant benefits on PANSS general symptom scores.
Table 4 Treatment effect estimates for patients with carers: difference in means (95% CI) between treatment condition (cognitive–behavioural therapy or family intervention) and treatment as usual
| 12 months | 24 months | |||||
|---|---|---|---|---|---|---|
| CBT | FI | CBT | FI | |||
| PANSS | ||||||
| Total | –5.90 (–12.34 to 0.54) | –6.44 (–14.12 to 1.24) | –5.32 (–12.20 to 1.56) | –6.25 (–14.77 to 2.28) | ||
| Positive | –1.13 (–3.57 to 1.30) | –1.48 (–4.31, 1.35) | –1.07 (–3.80 to 1.67) | 1.17 (–2.13 to 4.46) | ||
| Negative | –2.08 (–4.39 to 0.23) | –2.42 (–5.18, 0.35) | –0.87 (–3.37 to 1.62) | –1.32 (–4.42 to 1.78) | ||
| General | –2.82 (–6.17 to 0.54) | –2.66 (–6.60 to 1.28) | –3.01 (–6.29 to 0.27) | –3.98 (–8.02 to 0.06) | ||
| PSYRATS | ||||||
| A1 | –1.02 (–2.13 to 0.08) | –0.69 (–1.82 to 0.44) | –0.07 (–1.12 to 0.97) | –0.45 (–1.51 to 0.62) | ||
| A8 | –0.68 (–1.99 to 0.63) | –0.50 (–1.89 to 0.90) | 0.05 (–1.20 to 1.30) | 1.15 (–0.16 to 2.46) | ||
| B1 | 0.11 (–0.63 to 0.85) | 0.48 (–0.37 to 1.32) | 0.08 (–0.64 to 0.80) | 0.60 (–0.28 to 1.49) | ||
| B4 | –1.10 (–2.00 to −0.20)* | –0.32 (–1.23 to 0.60) | 0.21 (–0.92 to 1.34) | 0.22 (–1.02 to 1.46) | ||
| BAI | 2.66 (–2.81 to 8.13) | –0.42 (–6.97 to 6.13) | –0.94 (–6.28 to 4.39) | –2.36 (–9.13 to 4.40) | ||
| BDI | 4.39 (–0.93 to 9.71) | 3.35 (–2.64 to 9.34) | –2.84 (–8.36 to 2.69) | –0.11 (–6.91 to 6.68) | ||
| Time budget | ||||||
| Total | 9.31 (–1.08 to 19.69) | 0.23 (–11.48 to 11.94) | 2.81 (–6.56 to 12.18) | 6.36 (–4.80 to 17.53) | ||
| Zero/one | –1.54 (–5.21 to 2.14) | 1.20 (–2.94 to 5.35) | –1.93 (–5.48 to 1.63) | –3.51 (–7.80 to 0.77) | ||
| SOFAS | 6.49 (+0.39 to 12.58)* | 4.30 (–2.79 to 11.39) | 3.43 (–3.40 to 10.25) | 2.98 (–5.46 to 11.42) | ||
| EuroQol | –6.94 (–18.85 to 4.98) | –7.38 (–22.07 to 7.31) | ||||
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CBT, cognitive–behavioural therapy; EuroQol, Quality of Life measure; FI, family intervention; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scale (A1, hallucination frequency; A8, hallucination distress; B1, delusional conviction; B4, delusional distress); SOFAS, Social and Occupational Functioning Assessment Scale; TAU, treatment as usual; zero/one, mean number of time periods rated 0 or 1 on time budget
Table 5 Treatment effect estimates for patients with carers: difference in means (95% CI) between treatment (family intervention and cognitive–behavioural therapy not distinguished) and treatment as usual

| 12 months | 24 months | |
|---|---|---|
| PANSS | ||
| Total | –6.09 (–11.89 to −0.29) * | –5.62 (–11.86 to 0.62) |
| Positive | –1.26 (–3.44 to 0.92) | –0.28 (–2.76 to 2.20) |
| Negative | –2.20 (–4.29 to −0.11) * | –1.02 (–3.29 to 1.25) |
| General | –2.76 (–5.76 to 0.24) | –3.34 (–6.30 to −0.37) * |
| PSYRATS | ||
| A1 | –0.14 (–1.13 to 0.85) | –0.41 (–1.35 to 0.53) |
| A8 | –0.13 (–1.32 to 1.07) | 1.13 (–0.02 to 2.27) |
| B1 | 0.42 (–0.33 to 1.17) | 0.56 (–0.23 to 1.36) |
| B4 | 0.32 (–0.62 to 1.27) | 0.21 (–0.73 to 1.15) |
| BAI | 1.59 (–3.39 to 6.58) | –1.38 (–6.25 to 3.48) |
| BDI | 3.98 (–0.76 to 8.72) | –1.94 (–6.97 to 3.10) |
| Time budget total | 5.80 (–3.72 to 15.33) | 4.09 (–4.26 to 12.49) |
| SOFAS | 5.68 (+0.20 to 11.15) * | 3.28 (–2.88 to 9.43) |
| EuroQol | –7.09 (–17.80 to 3.63) |
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CBT, cognitive–behavioural therapy; EuroQol, Quality of Life measure; FI, family intervention; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scale (A1, hallucination frequency; A8, hallucination distress; B1, delusional conviction; B4, delusional distress); SOFAS, Social and Occupational Functioning Assessment Scale; TAU, treatment as usual; TB, time budget; zero/one, mean number of time periods rated 0 or 1 on time budget
* P<0.05
Social functioning
The mean scores on our measures of social functioning and quality of life (time budget total, time doing nothing, SoFAS and EuroQol Reference Brazier, Jones and Kind47 ) are presented in online Table DS5. Table 3 presents the estimated treatment effects on these measures: there were no significant effects. As for the symptoms, we also separately examined the effects of CBT in the group with carers allocated to the no carer pathway and the effects of CBT and of family intervention in the carer pathway (Table 4). This showed a significant improvement in social functioning (SoFAS scores) at 12 months, in those with carers who received CBT. There is also a significant effect on social functioning (SoFAS scores) of receiving a psychological treatment (whether CBT or family intervention (Table 5).
Treatment mediators
There were no significant changes in the predicted direction of the treatment mediators, with the exception of greater reductions in levels of criticism (EE) in those who were in receipt of family intervention, as hypothesised.
Adverse events
There were three patient deaths over the course of the trial, all in the treatment as usual condition. The causes were suicide, pulmonary embolism and kidney failure. Two carers also died, one in the treatment as usual and one in the family intervention condition, recorded respectively as natural causes and lung cancer. There were 22 serious suicide attempts by trial patients over the 2-year period of their participation: 10 in the no carer pathway CBT group, 8 in the no carer pathway treatment as usual group (including the completed suicide), 3 in the carer pathway CBT group, 1 in the carer family intervention group and none in the carer treatment as usual group. Violent incidents were recorded for 45 patients: 22 out of 106 patients in the no carer pathway CBT group, 17 out of 112 patients in the no carer pathway treatment as usual group, and 2 in each of the carer pathway randomisation cells.
Discussion
This trial found no benefits of psychological interventions, whether CBT or family intervention, for the primary outcomes of relapse and days in hospital. There were limited benefits for CBT on the secondary outcomes of improvements in depression, symptoms and social functioning. For these secondary outcomes, there is a clear risk of type 1 errors, arising from multiple significance testing. We must therefore conclude that generic CBT for psychosis is not indicated for relapse reduction in unselected recently relapsed patients.
Methodology
Cognitive–behavioural therapy
The trial, especially with respect to CBT, was methodologically robust. Randomisation was independent and successful; rates of follow-up were excellent for primary outcomes and very good for secondary outcomes; raters were successfully masked; the assessment of relapse used a clear definition with a protocol ensuring a valid and reliable assessment by clinicians shown to have remained masked to allocation; interview assessments for secondary outcomes were checked for reliability; and the therapy was conducted by therapists both well-trained and carefully supervised, whose competence was monitored throughout and confirmed by independent assessors. The analysis of CBT, by combining the effects in the no carer and carer pathways, was adequately powered, as specified in advance in the trial protocol.
Family intervention
The analysis of family intervention was in contrast underpowered, as was, to a lesser extent, the exploratory analysis of treatment in those with carers. The primary outcome analysis in the carer pathway was further compromised by the unexpectedly low rates of relapse for all groups. This contrasted markedly with the rates in the no carer pathway and made demonstration of a further effect on our primary outcomes more challenging. We do not think this group differed substantially in terms of time in contact from other studies of carers. Although the minimum contact requirement was 10 h per week, in practice this was higher, with a total mean contact time of 39 h per week, Reference Kuipers, Bebbington, Dunn, Fowler, Freeman, Watson, Hardy and Garety48 and, as shown in online Table DS3, the means for the randomised groups range from 34–44 h a week. We do know that the carers in this study had predominantly low ratings of EE. Reference Kuipers, Bebbington, Dunn, Fowler, Freeman, Watson, Hardy and Garety48 This may reflect secular changes in carers' knowledge and attitudes, or the largely suburban and rural residence of patients who had carers. This suggests that for unselected groups of carers in 21st-century Britain, family intervention may not improve outcomes further than a good standard of treatment as usual. Where criticism was present, it was reduced in those given family intervention. However, our study has too little power for robust conclusions about family intervention.
General outcomes
The outcomes for this sample of recently relapsed people with psychosis were disappointing, regardless of treatment allocation. Particularly in the no carer pathway, full remission from the index relapse was relatively infrequent, with high levels of persisting symptoms. Despite some improvement, the group remained very symptomatic at 24 months. The failure of psychological interventions substantially to affect outcome should not lead us to disregard the poor outcome of standard care, including medication, delivered to this group.
People with carers
People with carers fared rather better, both in general and in response to treatment. Those in the carer pathway allocated to CBT made improvements in delusional distress and social functioning. Furthermore, our exploratory analyses of treatment in the total group of those with carers revealed consistent indications of a positive benefit on general and negative symptoms and social functioning, whether from CBT or family intervention. These results suggest that having a carer may improve the response to a psychological intervention. This has not been noted elsewhere in the literature. Other studies of CBT in psychosis have not distinguished participants according to whether they have carers. All studies of family intervention, of course, involve patients with carers. We did not anticipate this finding, and cannot explain it, since the effect of having a carer may be confounded by other variables. In the present study, those with carers were more likely to live in less urbanised areas and were more likely to be White, and the carer effect on treatment might be related to these or other unknown factors; however, our data, being limited in range, do not permit further investigation. The only other published example of a study of psychological intervention to have employed separate pathways for individuals living with families and those who were not Reference Hogarty, Kornblith, Greenwald, DiBarry, Cooley, Ulrich, Carter and Flesher49,Reference Hogarty, Greenwald, Ulrich, Kornblith, DiBarry, Cooley, Carter and Flesher50 did not find consistent effects related to carers. The more recent literature on the effects of social environments, both proximal and distal, Reference Garety, Bebbington, Fowler, Freeman and Kuipers51 gives credence to the positive effect of social support and the detrimental effect of social adversity, and suggests this might be particularly relevant to the development and maintenance of positive symptoms of psychosis. Reference Bebbington, Bhugra, Brugha, Singleton, Farrell, Jenkins, Lewis and Meltzer52,Reference Morgan, Kirkbride, Leff, Craig, Hutchinson, McKenzie, Morgan, Dazzan, Doody, Jones, Murray and Fearon53 The effect of carers both on symptoms and on response to psychological interventions warrants further investigation.
The sample
We consider that there are two main aspects of the study that might explain our largely negative findings for CBT: factors associated with the sample and the nature of the therapy. First, the sample recruited into this study was drawn from consecutive series of acutely ill patients who had experienced a recent relapse. This strategy was deliberate and was intended to address the public health question of the benefit of treatment for an unselected population, following the recommendation of the NICE guidelines for schizophrenia. 16 Clearly, this population differs from patients in a stable phase of illness with distressing persistent symptoms studied in many earlier studies of CBT and for whom moderately positive effects have consistently been demonstrated. Reference Zimmermann, Favrod, Trieu and Pomini8,Reference Pfammatter, Junghan and Brenner9 Our sample was very mixed. Many had clearly relapsed in response to ceasing medication and they thus represent a medication-sensitive, if not a medication-adherent, group. Inevitably, some had a rapid response to hospitalisation and medication; by the time they had started therapy, this group reported few problems. Some had low distress levels, despite persisting symptoms, and some had very limited interest in having psychological therapy. This last group might include some who have an avoidant (‘sealing over’) recovery style and are particularly fragile. Reference Tait, Birchwood and Trower54
The therapy
Second, was the therapy right? There is no doubt that it was competently delivered. However, therapists reported that it was sometimes difficult, in the absence of symptoms or of distress, to maintain a clear focus on the positive psychotic symptoms for which generic CBT for psychosis is best established. Instead, therapists covered a wide range of self-reported problems and symptoms, adopting a general approach to emotional distress. Nevertheless, the CBT as delivered did have a particular focus on relapse interventions, as shown recently in a content analysis of therapy tapes. Reference Rollinson, Haig, Warner, Garety, Kuipers, Freeman, Bebbington, Dunn and Fowler55 The therapy did not influence the predicted mediators of change, such as specific core beliefs or reasoning. Reference Fowler, Freeman, Smith, Kuipers, Bebbington, Bashforth, Coker, Hodgekins, Gracie, Dunn and Garety35,Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler, Kuipers and Dudley36 It should be noted that the failure of treatment in the context of a failure to change hypothesised mediators leaves the hypothesis of proposed mediation unrefuted. Thus, it proved difficult to deliver a therapy sufficiently targeted on, or effective with, the key factors influencing psychotic symptom maintenance or recurrence. Rather, the indications were that such benefits as occurred were not specific to psychotic symptoms, being more general effects on depression, emotional well-being and functioning. We conclude that generic CBT for psychosis should continue to be offered for distressing and persistent positive symptoms, rather than be applied to relapse prevention. Future development of CBT should be directed at targeting and improving the key cognitive and emotional processes identified in theoretical models of distressing symptoms and relapse. Reference Gumley, O'Grady, McNay, Reilly, Power and Norrie13,Reference Garety, Bebbington, Fowler, Freeman and Kuipers51,Reference Birchwood and Trower56 The recent successful trial of CBT for command hallucinations, which aims to reduce distress by changing appraisals of these particular experiences, is a good example of this approach. Reference Trower, Birchwood, Meaden, Byrne, Nelson and Ross14
Acknowledgements
The study was supported by a Wellcome Trust Programme Grant (062452). We thank all the patient and carer participants; the staff of the Camden and Islington Mental Health and Social Care NHS Trust, North East London Mental Healthcare Trust, Norfolk and Waveney Mental Health Partnership NHS Trust, South London and Maudsley NHS Foundation Trust; lead therapists (Suzanne Jolley, Juliana Onwumere, Rebecca Rollinson, Ben Smith, Craig Steel); research workers (Hannah Bashforth, Susannah Colbert, Ellen Craig, Amber Elliot, Jane Evans, Dite Felekki, Laura Fialko, Sarah Fish, Miriam Fornells-Ambrojo, Alison Gracie, Amy Hardy, Joanne Hodgekins, Louise Isham, Rosie Moore, Kathryn Ruffell, Philip Watson); trial advisory group members (Max Birchwood, John Geddes, Tony Johnson, Jan Scott, Mike Took); external assessors of therapy quality (Catherine Gamble, Andrew Gumley, Mike Jackson, David Kingdon, Mike Startup); and Julian Leff for family intervention supervision. All authors contributed substantially to the conception, design, and interpretation of data. P.A.G. took the main responsibility for drafting the article and G.D. for the analysis of data. All authors contributed to revising the article for important intellectual content and for final approval of the version to be published.









eLetters
No eLetters have been published for this article.