The neural abnormalities found in schizophrenia implicate disturbances in several cognitive domains. However, the manifestation of these dysfunctional neural systems varies (Reference Heinrichs and AwadHeinrichs & Awad, 1993; Reference Goldstein and ShermanskyGoldstein & Shermansky, 1995; Reference Palmer, Heaton and PaulsenPalmer et al, 1997; Reference Hill, Ragland and GurHill et al, 2002). For example, Weickert et al (Reference Weickert, Goldberg and Gold2000) found subgroups of in-patients with chronic schizophrenia with different cognitive profiles based on change in IQ from premorbid values. As cognitive impairment is a significant determinant of social and occupational function (Reference Addington and AddingtonAddington & Addington, 2000; Reference Gold, Goldberg and McNaryGold et al, 2002), it is important to ascertain whether such cognitive heterogeneity is representative of schizophrenia in general and specifically if it is present at illness onset. This will contribute to an understanding of the extent and timing of cognitive impairment and inform early rehabilitation strategies. Therefore, we examined cognitive heterogeneity in individuals presenting to mental health services with first-episode schizophrenia.
METHOD
Study participants
Ninety-three patients and 50 healthy volunteers, recruited as part of the West London Study of First Episode Schizophrenia (Reference Hutton, Puri and DuncanHutton et al, 1998), participated in this study. The patients had received no more than 12 weeks of antipsychotic medication and subsequently fulfilled DSM–IV criteria for schizophrenia (American Psychiatric Association, 1994). The controls were recruited from the same catchment area as the patients and had no personal or family history of mental illness or personal medical illness that might impair cognitive function. Approval for the study was obtained from the local ethics committees of Merton, Sutton and Wandsworth, Kingston and Richmond, and Ealing, Hammersmith and Hounslow Health Authorities, London, UK. All participants gave informed written consent.
Clinical assessments
Patients were assessed with the Scales for the Assessment of Positive and Negative Symptoms (SAPS; Reference AndreasenAndreasen, 1983; SANS; Reference AndreasenAndreasen, 1981). Scores for the three symptom-derived syndromes of schizophrenia (positive, negative and disorganisation; Reference Liddle and BarnesLiddle & Barnes, 1990) were calculated for each patient (Table 1). The date at onset of positive psychotic symptoms was established using a modified questionnaire (Reference Lieberman, Jody and GeislerLieberman et al, 1993; Reference Barnes, Hutton and ChapmanBarnes et al, 2000).
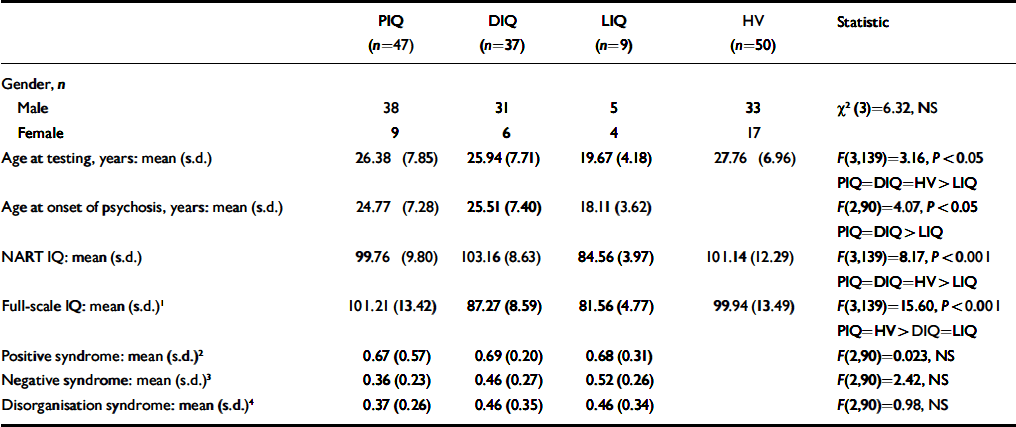
Table 1 Demographic and psychopathological syndrome scores in groups of patients with schizophrenia classified as: preserved normal IQ (PIQ); low current IQ which had deteriorated from normal premorbid values (DIQ); or low premorbid IQ (LIQ) compared with a control group of healthy volunteers (HV)
| PIQ (n=47) | DIQ (n=37) | LIQ (n=9) | HV (n=50) | Statistic | |
|---|---|---|---|---|---|
| Gender, n | |||||
| Male | 38 | 31 | 5 | 33 | χ2 (3)=6.32, NS |
| Female | 9 | 6 | 4 | 17 | |
| Age at testing, years: mean (s.d.) | 26.38 (7.85) | 25.94 (7.71) | 19.67 (4.18) | 27.76 (6.96) | F(3,139)=3.16, P<0.05 |
| PIQ=DIQ=HV>LIQ | |||||
| Age at onset of psychosis, years: mean (s.d.) | 24.77 (7.28) | 25.51 (7.40) | 18.11 (3.62) | F(2,90)=4.07, P<0.05 | |
| PIQ=DIQ>LIQ | |||||
| NART IQ: mean (s.d.) | 99.76 (9.80) | 103.16 (8.63) | 84.56 (3.97) | 101.14 (12.29) | F(3,139)=8.17, P<0.001 |
| PIQ=DIQ=HV>LIQ | |||||
| Full-scale IQ: mean (s.d.)1 | 101.21 (13.42) | 87.27 (8.59) | 81.56 (4.77) | 99.94 (13.49) | F(3,139)=15.60, P<0.001 |
| PIQ=HV>DIQ=LIQ | |||||
| Positive syndrome: mean (s.d.)2 | 0.67 (0.57) | 0.69 (0.20) | 0.68 (0.31) | F(2,90)=0.023, NS | |
| Negative syndrome: mean (s.d.)3 | 0.36 (0.23) | 0.46 (0.27) | 0.52 (0.26) | F(2,90)=2.42, NS | |
| Disorganisation syndrome: mean (s.d.)4 | 0.37 (0.26) | 0.46 (0.35) | 0.46 (0.34) | F(2,90)=0.98, NS |
Neuropsychological assessments
These were performed when the patients were clinically stable at a time when they were able to tolerate the testing procedures. This was within 4 weeks of the initial clinical assessment for 68 (72%), within 8 weeks for 85 (91%) and within 20 weeks for 92 (99%) (one patient was tested at 41 weeks). Premorbid IQ was estimated using the Revised National Adult Reading Test (NART), which measures reading ability by scoring the pronunciation of 50 irregular words (Reference Nelson and WillisonNelson & Willison, 1991). Numerous studies have substantiated the use of the NART in healthy volunteers and in people with a variety of neuropsychiatric disorders where cognitive decline is suspected (Reference Nelson and O'ConnellNelson & O'Connell, 1978; O'Carroll, Reference O'Carroll1987, Reference O'Carroll1995; Crawford et al, Reference Crawford, Parker and Stewart1989, Reference Crawford, Deary and Starr2001). Studies have found the NART is also a valid measure of premorbid IQ in schizophrenia (Reference Crawford, Besson and BremnerCrawford et al, 1992; Reference O'Carroll, Walker and DunanO'Carroll et al, 1992) and that it is stable over time in this population (Reference Smith, Roberts and BrewerSmith et al, 1998; Reference Morrison, Sharkey and AllardyceMorrison et al, 2000). Current IQ was calculated from four sub-tests of the Wechsler Adult Intelligence Scale – Revised (WAIS–R) or WAIS–III, which have been shown to provide a reliable measure of full-scale IQ in schizophrenia (Reference Missar, Gold and GoldbergMissar et al, 1994; Reference Blyler, Gold and IannoneBlyler et al, 2000). During the course of the study, the short forms of the tests were changed in order to save testing time without compromising accuracy (Reference KaufmanKaufman, 1990) and to accommodate the introduction of the WAIS–III. Subsidiary analyses showed that subgroup allocation of patients and controls based on current IQ was not a reflection of the short-form tests used.
Memory and executive function tests were taken from the Cambridge Automated Neuropsychological Test Battery (CANTAB; Reference Sahakian and OwenSahakian & Owen, 1992). These were: (a) spatial short-term memory (spatial span; Reference Owen, Downes and SahakianOwen et al, 1990); (b) episodic memory (pattern recognition memory; Reference Sahakian, Morris and EvendenSahakian et al, 1988); (c) spatial working memory (Reference Owen, Downes and SahakianOwen et al, 1990), which measures the ability to remember the location of previously retrieved ‘tokens’ while searching up to eight ‘boxes’ for a new token (an error occurs when a participant returns to open a box in which a token has already been found); (d) spatial working memory strategy, measured as the number of times search trials begin with the same box; (e) planning (Reference Owen, Downes and SahakianOwen et al, 1990), a task based on the Tower of London (Reference ShalliceShallice, 1982), which measures the ability to plan and execute a sequence of moves for problems requiring a minimum of 2, 3, 4 or 5 moves; and (f) attentional set-shifting (Reference Owen, Roberts and PolkeyOwen et al, 1991), which has some similarity to the Wisconsin Card Sorting Test, and in which participants are required to learn a series of visual discriminations in which one of two stimulus dimensions (shape or line) is ‘correct’; they are also required to deduce and reverse rules governing correct responding.
Analysis
Data were analysed using the Statistical Package for the Social Sciences version 10 (SPSS, 1999). For ordinal data, group comparisons were performed using the t-test or analysis of variance (ANOVA). Post hoc comparisons were performed using least squares difference and correlations using Pearson's test. Nominal data were analysed using the χ2-test.
RESULTS
Differences between the groups with respect to age and to gender were not significant (age: t(141)=1.72, P=0.09; gender: χ2(1)=3.18, P=0.08). At the time of testing, 11 patients were drug naïve, 39 were receiving conventional antipsychotic drugs and 43 were receiving atypical antipsychotic drugs.
Premorbid and current IQ
The range of NART IQ was 73–122 for controls and 75–122 for patients. For full-scale IQ, these values were 70–132 and 72–147, respectively. Paired t-tests between NART and full-scale IQ showed that these scores differed for patients (t (92)=5.31, P<0.001) but not for controls (t (49)=0.89, P=0.38). Examination of the distribution of the scores showed that there was a downward shift of full-scale IQ compared with NART IQ in the patients, indicating a decline in IQ from premorbid levels.
IQ subgroups
To determine whether the decline in IQ pertained to all or a subgroup of patients, we followed the methodology of Weickert et al (Reference Weickert, Goldberg and Gold2000) to derive the following groups: (a) normal and preserved IQ (PIQ), defined as a NART IQ of 90 or more and a NART-full-scale IQ difference within 10 points; (b) deteriorated IQ (DIQ), defined as a NART IQ of 90 or more and a NART-full-scale IQ difference of more than 10 points; (c) premorbid low IQ (LIQ), defined as a NART IQ and full-scale IQ of less than 90. For five patients, the NART IQ was lower than the full-scale IQ by more than 10 points and these people were placed in the PIQ category. Table 1 shows the NART IQ and full-scale IQ values of the subgroups and relevant statistical comparisons with the control group. Post hoc comparisons revealed that the NART IQ values of the PIQ and DIQ groups were similar to those of the control group whereas the NART IQ of the LIQ group was significantly lower than that of all other groups (least squares difference: all P<0.001). The full-scale IQ values of the control and PIQ groups were similar. The full-scale IQ of the DIQ group had fallen to the same level as the LIQ group and both were significantly lower than the PIQ group and controls (least squares difference: both P<0.001). To check the validity of the NART as a measure of premorbid IQ in those with schizophrenia, the control group was subjected to the same subgroup analysis. For five controls, NART IQ was lower than full-scale IQ by greater than 10 points and these were placed in the PIQ category. The allocation of participants to these categories was different for patient and control groups (χ2(2)=8.90, P=0.012) indicating that more patients than controls fell into the DIQ category (controls: declined 16%, preserved 74%, low 10% v. patients: declined 40%, preserved 50%, low 10%).
Comparisons of patients within each group revealed no significant differences in syndrome scores, gender ratio (see Table 1), medication status (χ2(4)=4.29, NS) or duration of untreated psychosis (F (2,92)=1.06, NS). The mean ages of the PIQ and DIQ groups were similar to that of the controls. The LIQ group was significantly younger than the other groups with respect to age at testing and age at onset of psychosis (Table 1; least squares difference: all P<0.05).
Age and IQ
To further investigate the impact of age on the IQ, Pearson correlation coefficients were calculated between NART full-scale IQ and age at testing in patients and controls (see Fig. 1). There were no significant correlations with age in controls (full-scale IQ: r=–0.22, P=0.13; NART: r=0.10, P=0.51). For patients, age correlated significantly with NART IQ (r=0.37, P<0.001) but not full-scale IQ (r=0.16, P=0.12). The significant relationship for the patients between age and NART also held for age at onset of psychosis (r=0.38, P<0.001), indicating that patients with a lower IQ had a younger age at onset. This correlation remained significant when the group with low premorbid IQ was excluded (r=0.29, P<0.01).
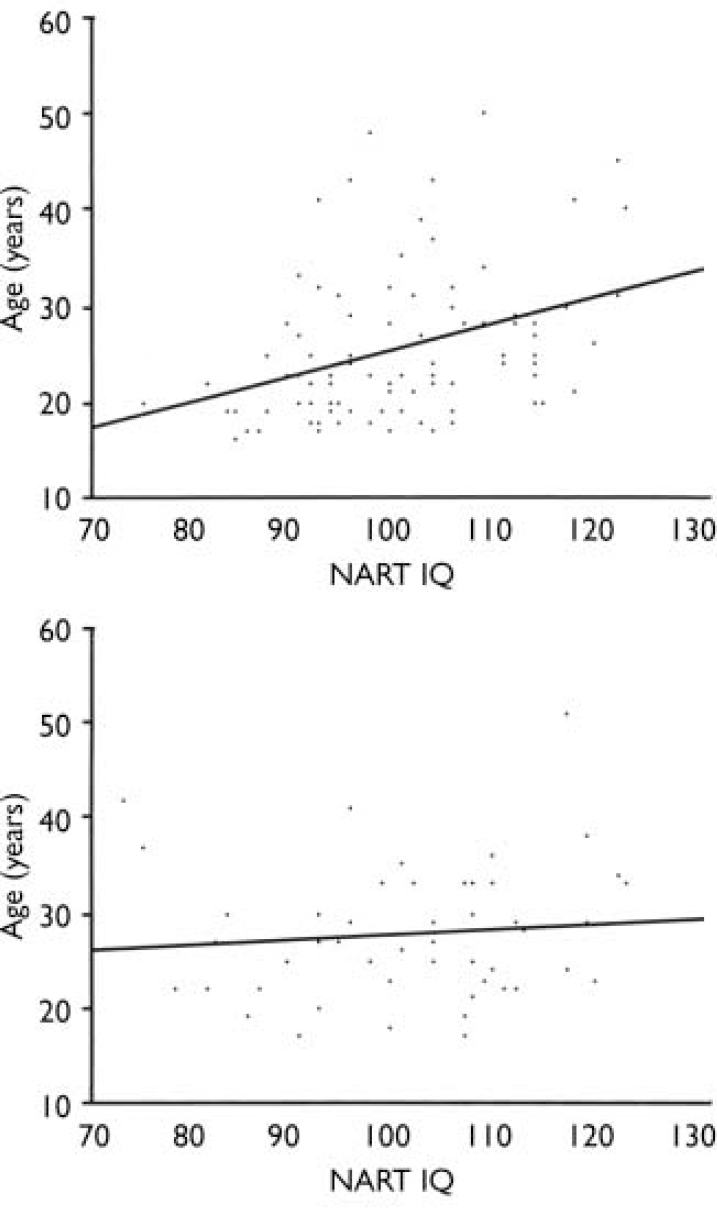
Fig. 1 Scatterplots of age and premorbid IQ (National Adult Reading Test; NART) in patients with first-episode schizophrenia (upper panel) and healthy controls (lower panel).
IQ, memory and executive dysfunction
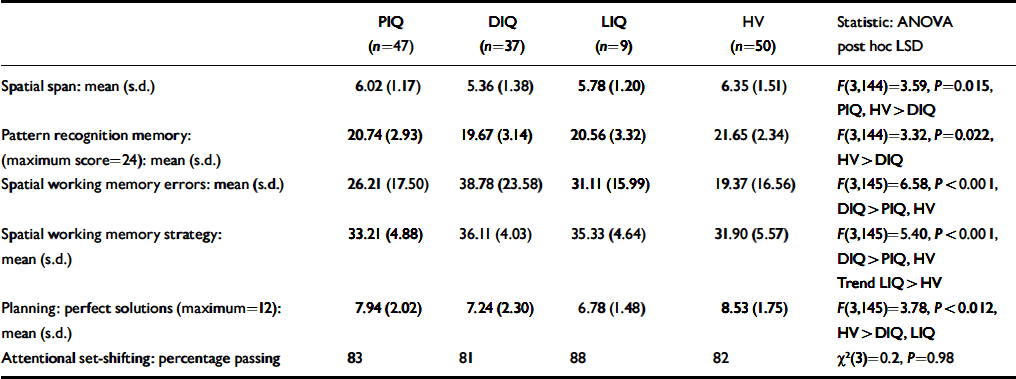
The three patient IQ subgroups and the control group were compared for tests of memory and executive function (Table 2). There were significant overall group differences for all measures except attentional set-shifting. Post hoc analysis of the significant group effects revealed that the PIQ group was no different from controls on any measure and the DIQ group was impaired on all measures relative to controls (least squares difference range: P<0.001 to 0.005). The LIQ group was intermediate between the other two groups and significantly impaired relative to controls for planning (least squares difference: P=0.02), with a trend difference for spatial working memory strategy (least squares difference: P=0.07).
Table 2. Memory and executive function in groups of patients with schizophrenia according to whether they demonstrated preserved normal IQ (PIQ), low current IQ which had deteriorated from normal premorbid values (DIQ) and low premorbid IQ (LIQ), compared with a control group of healthy volunteers (HV)1
| PIQ (n=47) | DIQ (n=37) | LIQ (n=9) | HV (n=50) | Statistic: ANOVA post hoc LSD | |
|---|---|---|---|---|---|
| Spatial span: mean (s.d.) | 6.02 (1.17) | 5.36 (1.38) | 5.78 (1.20) | 6.35 (1.51) | F(3,144)=3.59, P=0.015, PIQ, HV > DIQ |
| Pattern recognition memory: (maximum score=24): mean (s.d.) | 20.74 (2.93) | 19.67 (3.14) | 20.56 (3.32) | 21.65 (2.34) | F(3,144)=3.32, P=0.022, HV > DIQ |
| Spatial working memory errors: mean (s.d.) | 26.21 (17.50) | 38.78 (23.58) | 31.11 (15.99) | 19.37 (16.56) | F(3,145)=6.58, P <0.001, DIQ > PIQ, HV |
| Spatial working memory strategy: mean (s.d.) | 33.21 (4.88) | 36.11 (4.03) | 35.33 (4.64) | 31.90 (5.57) | F(3,145)=5.40, P <0.001, DIQ > PIQ, HV Trend LIQ > HV |
| Planning: perfect solutions (maximum=12): mean (s.d.) | 7.94 (2.02) | 7.24 (2.30) | 6.78 (1.48) | 8.53 (1.75) | F(3,145)=3.78, P <0.012, HV > DIQ, LIQ |
| Attentional set-shifting: percentage passing | 83 | 81 | 88 | 82 | χ2(3)=0.2, P=0.98 |
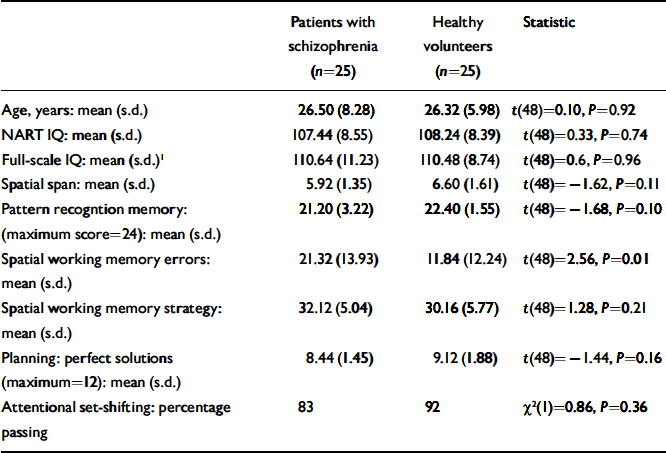
These results indicate that a substantial subgroup of patients can have neuropsychological function within the normal range at illness onset whereas another has undergone a general intellectual decline. To examine whether specific cognitive impairments are present independently of IQ, we compared cognitive performance in patients and controls with a high/average IQ, defined as a current IQ of 100 or more, closely matched for age, premorbid and current IQ (Table 3). The patients showed a specific impairment in spatial working memory errors.
Table 3. Neurocognitive function in people with first-episode schizophrenia and healthy volunteers with high current IQ
| Patients with schizophrenia (n=25) | Healthy volunteers (n=25) | Statistic | |
|---|---|---|---|
| Age, years: mean (s.d.) | 26.50 (8.28) | 26.32 (5.98) | t(48)=0.10, P=0.92 |
| NART IQ: mean (s.d.) | 107.44 (8.55) | 108.24 (8.39) | t(48)=0.33, P=0.74 |
| Full-scale IQ: mean (s.d.)1 | 110.64 (11.23) | 110.48 (8.74) | t(48)=0.6, P=0.96 |
| Spatial span: mean (s.d.) | 5.92 (1.35) | 6.60 (1.61) | t(48)=–1.62, P=0.11 |
| Pattern recogntion memory: (maximum score=24): mean (s.d.) | 21.20 (3.22) | 22.40 (1.55) | t(48)=–1.68, P=0.10 |
| Spatial working memory errors: mean (s.d.) | 21.32 (13.93) | 11.84 (12.24) | t(48)=2.56, P=0.01 |
| Spatial working memory strategy: mean (s.d.) | 32.12 (5.04) | 30.16 (5.77) | t(48)=1.28, P=0.21 |
| Planning: perfect solutions (maximum=12): mean (s.d.) | 8.44 (1.45) | 9.12 (1.88) | t(48)=–1.44, P=0.16 |
| Attentional set-shifting: percentage passing | 83 | 92 | χ2(1)=0.86, P=0.36 |
DISCUSSION
Cognitive heterogeneity in schizophrenia
We found that the majority (50%) of patients with schizophrenia showed a preservation of IQ, which remained in the normal range or above. A second large group (40%) showed a decline in IQ from normal levels and the rest demonstrated preserved but low IQ. These findings support those of Weickert et al (Reference Weickert, Goldberg and Gold2000), who investigated in-patients with a mean illness duration of 11 years at a tertiary referral centre. In contrast, we studied patients following their first psychotic episode. Furthermore, whereas those studied by Weickert et al (Reference Weickert, Goldberg and Gold2000) were mainly receiving conventional antipsychotics, more than half of our group were either receiving atypical antipsychotics or were medication naïve. The similarity of the findings despite the stark differences between the two patient groups suggests that these cognitive groups are representative of schizophrenia in general.
Intellectual decline in schizophrenia
We confirmed that it is possible for people with a low current IQ to have had a normal IQ prior to the onset of schizophrenia (e.g. Reference Nelson, Pantelis and CarruthersNelson et al, 1990; Reference Goldberg, Hyde and KleinmanGoldberg et al, 1993; Reference Weickert, Goldberg and GoldWeickert et al, 2000; Reference Kremen, Seidman and FaraoneKremen et al, 2001). Our patients who demonstrated a decline in IQ also showed considerable impairment on tests of memory and executive function. The difference between this subgroup and those with no evidence of reduced IQ could not be explained by more severe psychotic symptoms or by the type of antipsychotic medication. Almost half (46%) of this subgroup had become psychotic during the 12 months preceding presentation and the duration of untreated psychosis did not differ between this group and those with normal preserved IQ. This suggests that deterioration in IQ from normal levels is not attributable to a longer course of illness. Thus, in a substantial number of our patients, global deterioration in intellectual function appears to have occurred as an early manifestation of the illness.
The finding at first presentation of deterioration in IQ from normal premorbid levels suggests that individuals can be on a normal cognitive trajectory during development. Studies of army conscripts, in which direct measures of premorbid IQ were obtained, found that low IQ is a prominent feature in adults prior to the onset of psychosis (Reference David, Malmberg and BrandtDavid et al, 1997; Reference Davidson, Reichenberg and RabinowitzDavidson et al, 1999) and that intellectual underperformance is greatest in those nearest the onset of psychosis (Reference Rabinowitz, Reichenberg and WeiserRabinowitz et al, 2000; Reference Gunnell, Harrison and RasmussenGunnell et al, 2002). This suggests that cognitive function is declining during the years immediately preceding the onset of psychosis. Fuller et al (Reference Fuller, Nopoulos and Arndt2002), in a longitudinal assessment of premorbid cognitive functioning in people who went on to develop schizophrenia, found that scholastic aptitude actually increased between the ages of 9 and 13 years, prior to a precipitous decline over the following 4 years. Kremen et al (Reference Kremen, Buka and Seidman1998) found evidence of a decline in IQ even earlier in childhood, between the ages of 4 and 7 years, in those who subsequently developed psychotic symptoms as adults. In accordance with previous studies (Reference Jones, Murray and RodgersJones et al, 1994), these researchers found that low IQ per se at the age of 7 years predicted subsequent psychosis. However, a prior decline in IQ, whether from normal or low values, was a stronger predictor. Taken together, these data suggest that individuals destined to develop schizophrenia can undergo deterioration in IQ at various stages during childhood, adolescence and early adulthood. The influences bringing about these changes at different ages are yet to be determined but they may be different for each age level, consistent with the hypothesis that there are several types of morbid process operating at different times during development (e.g. Reference KeshavanKeshavan, 1999). Whether the timing of this decline has implications for different outcomes also remains to be determined.
In our study, the proportion of patients with pre-existing low IQ may be underrepresented. Weickert et al (Reference Weickert, Goldberg and Gold2000) found that a quarter of their patients had a low premorbid IQ, compared with a tenth in our study. Although we assessed a large group with schizophrenia, they were self-selecting and it is possible that more patients with lower rather than higher IQ declined to take part in our study. Other studies find that low IQ is overrepresented as far back as childhood in those destined to develop schizophrenia (e.g. Reference Erlenmeyer-Kimling, Rock and Squires-WheelerErlenmeyer-Kimling et al, 1991; Reference AmbelasAmbelas, 1992; Reference Jones, Murray and RodgersJones et al, 1994), suggesting that low premorbid IQ is a risk factor for the development of schizophrenia or even part of the disorder itself (Reference Jones, Murray and RodgersJones et al, 1994; Reference Russell, Munro and JonesRussell et al, 1997). Our data support the view that low IQ is a risk factor for an earlier age at onset of schizophrenia. Those with a low premorbid IQ had a younger age at onset compared with the other two groups, suggesting that IQ development was curtailed by the onset of psychosis, as has been demonstrated in those with childhood-onset childhood-onset psychosis (Reference Bedwell, Keller and SmithBedwell et al, 1999). It is also possible that their low IQ contributed to an earlier age at onset, as concluded by previous investigators (e.g. Reference Erlenmeyer-Kimling and CornblattErlenmeyer-Kimling & Cornblatt, 1987; Reference Grimes and WalkerGrimes & Walker, 1994). The finding of a significant positive association between premorbid IQ and age at onset for all patients, even when those with low IQ were removed from the analysis, favours the latter explanation. A continuous relationship between IQ and risk of schizophrenia has also been shown in studies with direct premorbid measures of intellectual function (Reference Jones, Murray and RodgersJones et al, 1994; Reference David, Malmberg and BrandtDavid et al, 1997).
One limitation of this study is the use of an indirect measure of premorbid IQ. The use of the NART in schizophrenia has been criticised on the grounds that the disorder itself may cause an impairment in verbal ability and thus results in an underestimation of IQ. Against this are studies which have found that a measure of current vocabulary approximates direct measures of premorbid IQ (Reference Russell, Munro and JonesRussell et al, 2002; Reference Eberhard, Riley and LevanderEberhard et al, 2003). Another criticism of the NART is that it can overestimate IQ in the low IQ range and in schizophrenia this may give a spurious impression of IQ decline (Reference Russell, Munro and JonesRussell et al, 2002). However, this cannot explain our findings because we applied the same analysis to a matched control group and significantly fewer of these showed evidence of IQ decline. Nevertheless 16% of healthy volunteers could be classified as having intellectual decline using this methodology, suggesting that care should be taken in categorising individuals in this way without taking other indices of premorbid function into consideration (Reference Crawford, Allan and CochraneCrawford et al, 1990; Reference Russell, Munro and JonesRussell et al, 2002).
Core cognitive impairments in first-episode schizophrenia
Half of our patients with first-episode schizophrenia had no evidence of deterioration in IQ, and their performance on a range of memory and executive tests was no different from controls. Studies of people with chronic schizophrenia have also found evidence for intact cognition in a subgroup (Reference Heinrichs and AwadHeinrichs & Awad, 1993; Reference Palmer, Heaton and PaulsenPalmer et al, 1997), suggesting that cognitive impairment might not be a core feature of schizophrenia. However, Kremen et al (Reference Kremen, Seidman and Faraone2001) suggest that even in patients with high IQ, evidence of neuropsychological impairment can be found. Indeed, when we closely matched subgroups of controls and patients with high/average premorbid and current IQ, we found that the patients exhibited a particular impairment of executive function, indicated by an increased number of spatial working memory errors. Weickert et al (Reference Weickert, Goldberg and Gold2000) also found that their subgroup with preserved IQ displayed subtle impairments in executive function and this is in turn consistent with studies finding empirically derived subgroups with preserved general cognitive function and mild executive impairment (Reference Heinrichs and AwadHeinrichs & Awad, 1993; Reference Goldstein and ShermanskyGoldstein & Shermansky, 1995). Thus, there is accumulating evidence that executive dysfunction is a pervasive abnormality, intrinsic to schizophrenia, which is not accounted for by illness chronicity, antipsychotic treatment or antipsychotic type.
Working memory as a marker of cognitive impairment at first episode
In previous studies of cognitive heterogeneity the most consistent indicator of executive dysfunction has been impaired performance on the Wisconsin Card Sorting Test. This is a complex task subsuming several executive processes, including working memory, planning and response inhibition. We examined each of these processes separately and found that patients with high/average preserved IQ at first episode of schizophrenia show a specific impairment in working memory. Our spatial working memory task measures the ability to remember the location of a number of tokens previously retrieved while searching for new tokens. This suggests that a disturbance of executive function, of the type required to hold information in memory while performing other cognitive operations, is present in all patients independent of IQ. In a recent family study there was a dose–response relationship between the degree of spatial working memory impairment and increasing genetic predisposition to schizophrenia, suggesting that impaired spatial working memory may ‘constitute an effective endophenotype’ of the disorder (Reference Glahn, Therman and ManninenGlahn et al, 2003). It has recently been argued that increased antisaccade errors, which are also considered to be a promising endophenotype for schizophrenia, reflect a more fundamental deficit in spatial working memory processes (Reference Hutton, Huddy and BarnesHutton et al, 2004). Thus, in our patients presenting for the first time with schizophrenia, the spatial working memory deficit, which permeated all cognitive subgroups, may represent such a genetic marker.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ People with schizophrenia may not achieve their premorbid level of functioning after recovery from psychosis because of cognitive decline.
-
▪ Widespread cognitive impairment should not be assumed in all people with schizophrenia.
-
▪ Care should be taken in categorising individuals with schizophrenia using the National Adult Reading Test without taking other indices of premorbid function into consideration.
LIMITATIONS
-
▪ The measure of premorbid IQ was indirect.
-
▪ Patients with low IQ were probably underrepresented.
-
▪ The study focused on executive function.
Acknowledgements
We are grateful to Lesley-Jane Duncan, Ian Cuthbert, Heidi Gibbins, Fiona Ambery, Emma Webb, Sonja Paul and Caroline Dibnah for their contribution to patient assessments. We are grateful to the consultants and nurses of the West London and South West London and St George's Mental Health NHS Trusts for greatly facilitating the study. This study was funded by the Wellcome Trust.







eLetters
No eLetters have been published for this article.