Schizophrenia affects around 1% of the population and approximately 30% of patients have treatment-resistant schizophrenia for which clozapine is the only licensed medication. In addition to debilitating psychiatric symptoms it is associated with an unemployment rate of 80–90% and a life expectancy reduced by 10–20 years.Reference Owen, Sawa and Mortensen1 Approximately 5% of those diagnosed with schizophrenia die by suicide.Reference Hor and Taylor2 Although clozapine reduces overall mortality in severe schizophrenia,Reference Tiihonen, Lonnqvist, Wahlbeck, Klaukka, Niskanen and Tanskanen3 it is associated with a number of often poorly understood side-effects with a growing body of evidence linking clozapine with elevated rates of pneumonia-related admission and mortality.Reference Copeland, Mortensen, Zeber, Pugh, Restrepo and Dalack4–Reference Kuo, Yang, Liao, Chen, Lee and Shau9 Mortality from respiratory infections/sepsis despite intensive monitoring is a common reason for discontinuing treatment.Reference Mustafa, Burke, Abukmeil, Scanlon and Cox10, Reference Taylor, Douglas-Hall, Olofinjana, Whiskey and Thomas11 Various mechanisms for the increase in pneumonia have been suggested, including aspiration, sialorrhoea and impairment of swallowing function with oesophageal dilatation and hypomotility as well as agranulocytosisReference Abdelmawla and Ahmed12 and smoking.Reference Bello, Menendez, Torres, Reyes, Zalacain and Capelastegui13 The possibility of a link between antibody deficiency and clozapine use was first raised during a study of population screening for antibody deficiency using calculated globulin screening in Wales.Reference Jolles, Borrell, Zouwail, Heaps, Sharp and Moody14 Calculated globulin is derived from the difference between total serum protein and albumin concentrations, part of the liver function test profile. Immunoglobulins form a significant proportion of the globulin fraction and therefore low calculated globulin may indicate antibody deficiency.Reference Jolles, Borrell, Zouwail, Heaps, Sharp and Moody14, Reference Jolles15 Antibody deficiency is not a listed clozapine side-effect and is not a part of current clozapine monitoring. To further assess a possible association between antibody deficiency and clozapine use we undertook a cross-sectional case–control study to compare the immunoglobulin levels and specific antibody levels in patients taking either clozapine or alternative antipsychotics.
Method
Participants
Adults (>18 years) receiving either clozapine or non-clozapine antipsychotics were recruited during routine clinic visits to ten community mental health team out-patient clinics in Cardiff & Vale and Cwm Taf Health Boards by specialist research officers between November 2013 and December 2016. All potential participants were given the same participant information leaflet to read that outlined the purpose of the study and indicated that they would be asked some questions about their health and have a single (4 mL) blood sample taken. Following consent, participants completed the short lifestyle, drug history and infection questionnaire followed by blood sampling. Where required, drug histories were confirmed with the patient's general practice records. Formal psychiatric diagnoses and antipsychotic medication use were confirmed using the medical notes, in line with other studies. Patients' admission rates were confirmed by electronic review for all patients in the post-exclusion group over the 12-month period prior to recruitment and assessed for an infective trigger.
Measurements
Immunoglobulin levels (immunoglobulin (Ig)G, IgA and IgM) were assayed by nephelometry (Siemens BN2 Nephelometer; Siemens), serum electrophoresis (Sebia Capillarys 2; Sebia, Norcross, GA, USA) and, where appropriate, serum immunofixation (Sebia Hydrasys; Sebia, Norcross, GA, USA). Where immunoglobulin was detectable, the lower limit of assay sensitivity (IgG, 1.34 g/L; IgA, 0.24 g/L; and IgM, 0.17 g/L) was used for data analysis. Specific antibody titres against haemophilus influenzae, tetanus and pneumococcal capsular polysaccharide were determined by enzyme-linked immunosorbent assay (The Binding Site, Birmingham, UK). All testing was performed in the United Kingdom Accreditation Service accredited Immunology Laboratory at the University Hospital of Wales. Laboratory adult reference ranges for immunoglobulin levels used were, IgG, 6.00–16.00 g/L; IgA, 0.80–4.00 g/L; IgM 0.50–2.00 g/L.
Statistical analysis
Statistical analysis of the laboratory and clinical data was performed using Microsoft Excel and Graphpad Prism (version 6.07). Unadjusted odds ratio (ORs) and 95% confidence intervals were calculated using Fisher's exact test for the number of individuals with an immunoglobulin value below the lower limit of normal (IgG <6.00 g/L, IgA <0.80 g/L and IgM <0.50 g/L). As the distribution of all three immunoglobulin classes in the study population differed from a Gaussian distribution (Fig. 1), non-parametric Mann–Whitney U-testing was performed. Specific antibody titres, antibiotics and admission frequencies were similarly evaluated. No validated protective values are currently available for pneumococcal-specific IgA or IgM as a relevant threshold, and odds ratios were not calculated.
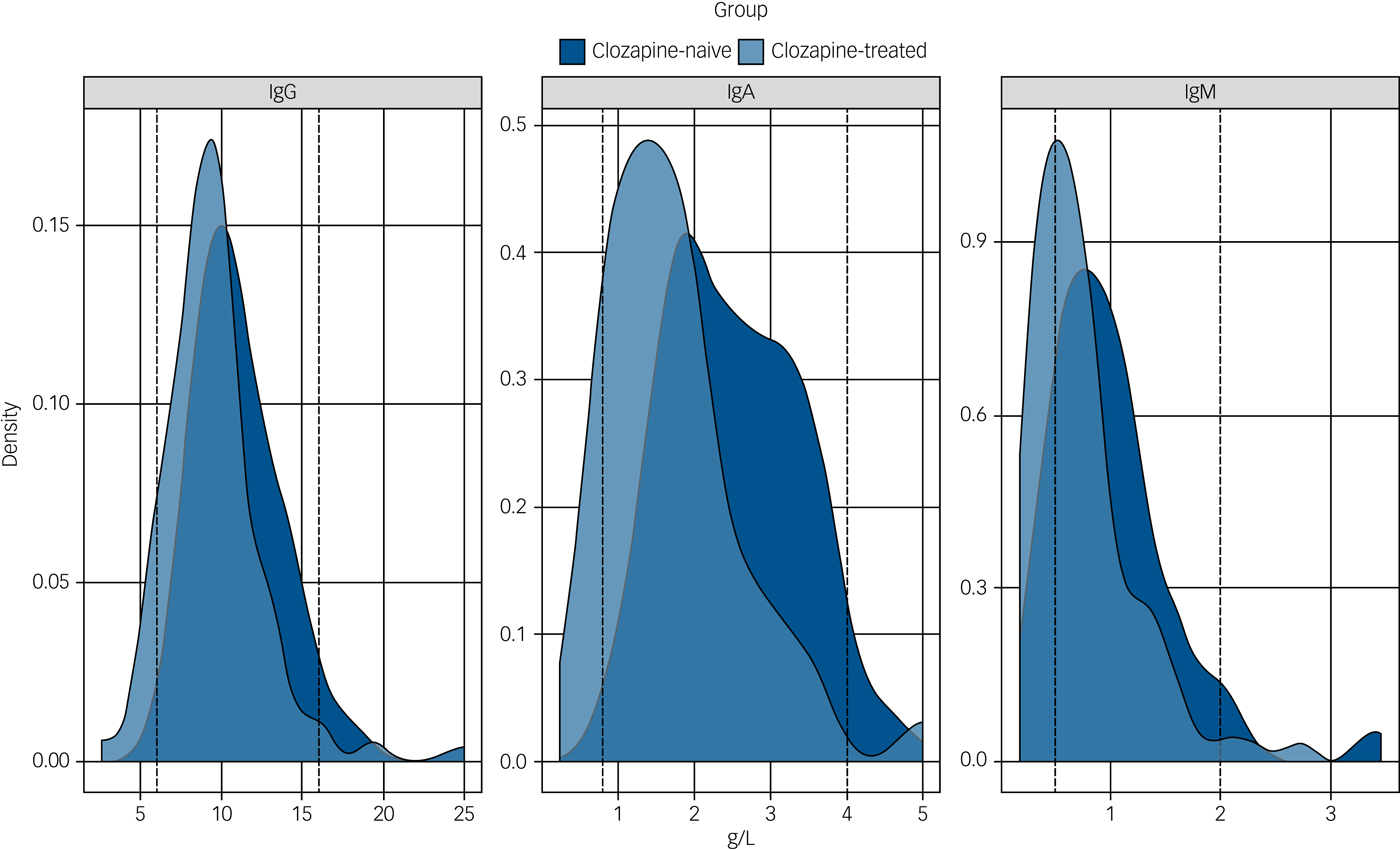
Fig. 1 Immunoglobulin levels in the clozapine-treated and clozapine-naive groups.
The distribution of the serum levels of immunoglobulin (Ig)G, IgA and IgM for the clozapine-treated (light blue, n = 94) and clozapine-naive (dark blue, n = 98) groups are shown with the laboratory 5th and 95th percentile of the reference ranges for a healthy adult population represented as vertical dotted lines. The differences between the median IgG, IgA and IgM levels for the clozapine-treated versus clozapine-naive groups are all significant (P < 0.0001).
To adjust for factors, other than clozapine treatment (age, gender, diabetes, asthma/chronic obstructive pulmonary disease (COPD), second antipsychotic, diagnosis of schizophrenia and duration of clozapine therapy) that might influence immunoglobulin levels, linear regression analysis was performed in R (version 3.4.0). These models included a term corresponding to a global difference in levels between the two groups and, by way of an interaction with treatment duration, a term corresponding to a reduction in immunoglobulin levels per year on clozapine or other antipsychotic.
The study received ethical approval from the North West Regional REC committee (REC Reference 13/EM/0209, IRAS ID 104402) and sponsorship from Cardiff & Vale University Health Board.
Results
Study participants
A total of 291 patients taking clozapine and 280 who were clozapine naive were approached and 123 taking clozapine and 113 who were clozapine naive consented to the study (Table 1). Recruitment was stopped as per protocol when more than 100 patients in each group had been achieved. There were small differences in gender with more men in the clozapine-treated group (53% versus 50%) and a lower mean age in the clozapine group (45 versus 50 years). These differences may reflect the male predominance in schizophrenia and is unlikely to be relevant as there are no gender or age differences in the adult reference range for serum immunoglobulins.Reference Milford, Joanna, Rowbottom and Wild16
Table 1 Study participants and excluded participants
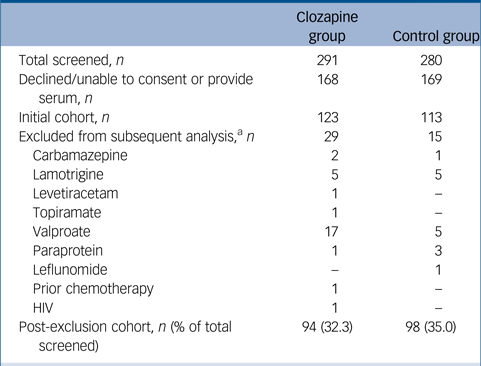
a. Included in initial cohort analysis for total and specific antibody levels and excluded from subsequent analysis after identification of possible causes of secondary hypogammaglobulinaemia (European Society of Immunodeficiencies criteria).
In the post-exclusion cohort, the levels of smoking, diabetes, COPD/asthma, and alcohol intake were similar between the groups (Table 2). As expected almost all (94%, n = 88) of the patients taking clozapine were diagnosed with schizophrenia or schizoaffective disorder whereas these diagnoses accounted for 64% (n = 63) of those taking other antipsychotic medication in the control group. This reflects the more restricted prescribing indications for clozapine compared with other antipsychotics.
Table 2 Participant characteristics of the post-exclusion cohort

Exclusion of possible confounding medications and diagnoses
Review of medical records in relation to the European Society of Immunodeficiencies differential diagnosis of hypogammaglobulinaemia17 highlighted a number of patients with possible drug or infection-related contributors; including anti-epileptic or mood stabilising medications, previous chemotherapy, presence of paraprotein, or HIV infection who were excluded from the analysis (Table 1).
Median concentrations of serum immunoglobulins for both pre- and post-exclusion patient cohorts receiving clozapine are shown in Table 3. Patients receiving clozapine showed significantly reduced median concentrations of all three immunoglobulin classes (IgG, IgA and IgM) relative to the clozapine-naive control group. The relative shift in distributions is highlighted in Fig. 1 (post-exclusion groups represented).
Table 3 Immunoglobulin concentrations and odds ratio for the clozapine-treated and clozapine-naive groupsa

a. Values represent median serum immunoglobulin concentrations in g/L. Reference range for 5th to 95th centiles in healthy adults: IgG, 6.0–16 g/L; IgA, 0.8–4.0 g/L; IgM, 0.5–2.0 g/L.
b. Mann–Whitney test.
c. Fisher's exact test.
The odds ratios for individual patients having an immunoglobulin measured below the lower limit of the reference range are also shown in Table 3. In the post-exclusion groups, 1% (n = 1) of controls and 8.5% (n = 8) of clozapine-treated group had IgG levels <6.00 g/L; 13.8% (n = 13) of clozapine and no controls had IgA levels <0.80 g/L; and 34% (n = 32) of clozapine and 15.3% (n = 15) of controls had IgM levels <0.50 g/L. Furthermore, three patients in the clozapine group had evidence of reduction of all three immunoglobulin classes; two patients had severely reduced IgG below 4.00 g/L. The lowest IgG in the clozapine group was 2.59 g/L, compared with 5.92 g/L in the control group. These differences were statistically highly significant (P < 0.017 or below) despite reduction in sample size, with exclusion of three clozapine-treated individuals with IgG values of 3.19, 4.93 and 5.42 g/L (taking concurrent anti-epileptic medications). One patient with an IgG of 5.79 g/L was similarly excluded from the control group. All subsequent analysis of results has been undertaken on the cohort following exclusion of possible confounding medications and diagnoses.
Specific antibody levels are low in both clozapine-treated and clozapine-naive groups
Large percentages of individuals in both the clozapine-treated and clozapine-naive groups have specific IgG antibody levels below the protective levels for haemophilus influenzae B (51.1% (n = 48) and 54.1% (n = 53) less than 1 mcg/mL, respectively),Reference McMillan, Douglas, Archbold, McCrum and Evans24 pneumococcus (52.1% (n = 49) and 54.1% (n = 53) less than 50 mg/L, respectively)Reference Chua, Lagos, Charalambous, Workman, Chee and Grimbacher18 and tetanus (10.6% (n = 10) and 13.3% (n = 13) less than 0.1 IU/mL, respectively). These were not significantly different using non-parametric testing. In contrast, levels in the clozapine-treated group for pneumococcal-specific IgA were reduced by 28 IU/mL over the clozapine-naive group (30.8 U/mL versus 58.8 U/mL P < 0.001) and IgM was reduced by 26 IU/mL (59.8 U/mL versus 85.8 U/mL P < 0.001) perhaps reflecting the observed greater relative reductions in total IgA and IgM levels (supplementary Table 1 available at https://doi.org/10.1192/bjp.2018.152). It should be noted that although values for protective levels of specific antibodies of haemophilus influenza B (>1 mcg/mL) and tetanus (>0.1 IU/mL) are reasonably well-validated there remains ongoing debate regarding the protective levels of pneumococcal antibodies.
Longer duration of clozapine use is associated with increased risk of hypogammaglobulinaemia
Information on duration of antipsychotic use was available for 88 of the 94 individuals in the clozapine group and 97 of 98 of those in the clozapine-naive group (post-exclusion cohort) and shows a decline in serum IgG levels only in those receiving clozapine (Fig. 2).
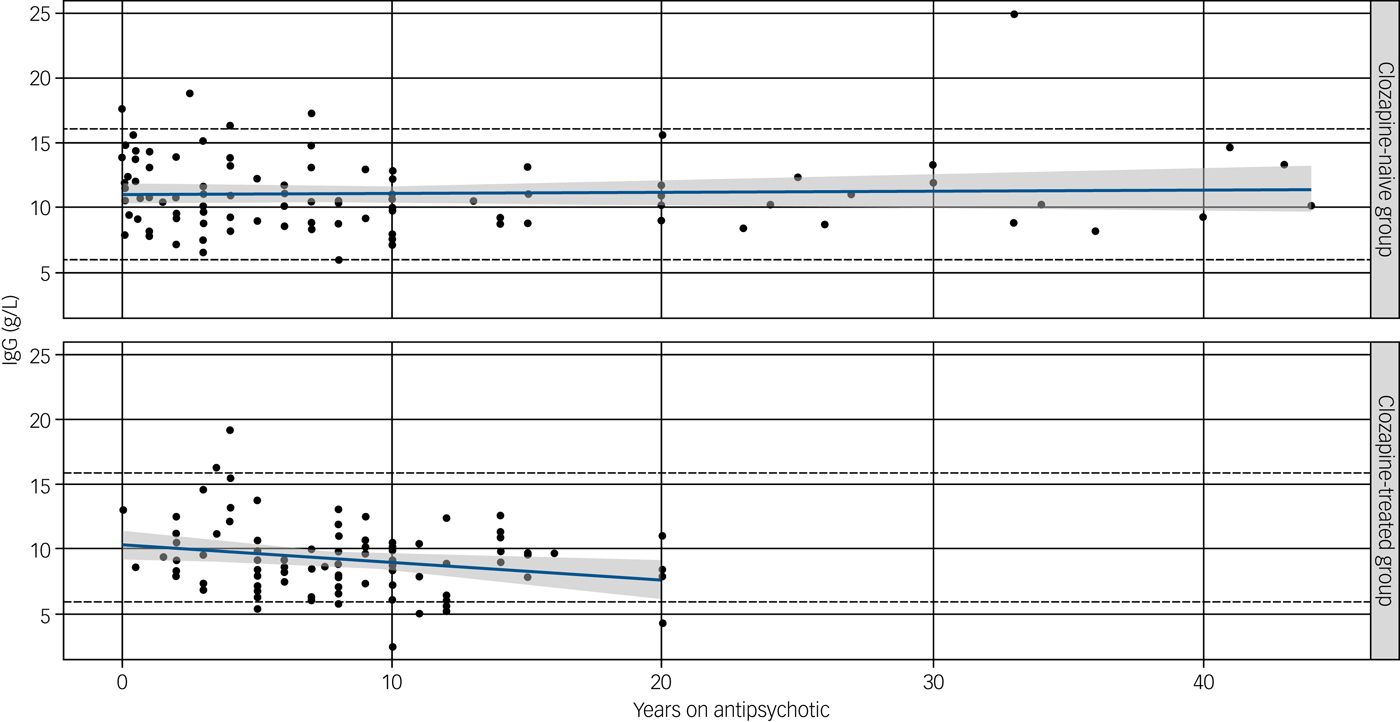
Fig. 2 Effect of duration of antipsychotic (upper) or clozapine (lower) treatment on immunoglobulin (Ig)G levels.
A significant negative correlation of duration of clozapine use (years) was observed with an annual reduction in IgG levels of 0.15 g/L (P = 0.03). No correlation was seen with serum IgG level and non-clozapine antipsychotic medications (P = 0.14) despite a longer duration on therapy. Straight lines show predicted IgG at different durations of treatment (in years), based on fitted linear models. Shaded regions display pointwise 95% confidence intervals.
To adjust for possible confounding variables that differed between groups (age, gender, diabetes, asthma/COPD, use of a second antipsychotic and diagnosis of schizophrenia) we performed linear regression analysis. Self-reported variables including current cigarette and alcohol consumption were similar between groups, although information on daily consumption was variably completed and considered vulnerable to reporting bias and were therefore not included. Medically documented diagnoses of asthma/COPD or diabetes were included as variables, as these conditions might theoretically be associated with use of immunosuppressive medication such as glucocorticoids not documented in available electronic general practitioner or psychiatric medical records.
Despite adjustment, there remained statistically significant immediate decreases in IgA (0.57 g/L, P = 0.01) and IgM (0.32 g/L, P = 0.02) and a significant association between decline in IgG and clozapine duration (P = 0.03), with a fall of serum IgG by 0.15 g/L per year (supplementary Table 2). Serum IgA and IgM did not show a significant correlation with duration of antipsychotic use, potentially as a result of an earlier effect and that the lower limit of the reference range for IgA (0.8 g/L) and IgM (0.5 g/L) is closer to the sensitivity of nephelometric testing at <0.24 g/L for IgA and <0.17 g/L for IgM. Serum IgM decreased with age in both groups (not shown).
Using this model, we were also able to estimate the impact of clozapine duration on serum IgG levels following clozapine or non-clozapine antipsychotic medication use for an average 40-year-old male, shown in supplementary Table 3 over a period of 25 years.
Antibiotic usage and admission rates for infection
Accepting the inherent shortcomings of self-reported data and the small number of reported events for the secondary outcomes of antibiotic usage and hospital admissions over the previous 12 months, the clozapine-treated group had higher antibiotic use, with a greater percentage of patients reporting more than five courses of antibiotics per year 5.3% (n = 5) versus 1% (n = 1) in the control group (Table 2). It was possible to validate hospital admissions by checking electronic medical records, thus reducing recall bias and allowing assessment of the admission trigger. There was a trend towards greater rate of confirmed infection-related admissions per patient year in the clozapine-treated group over the control group (8 versus 6, respectively; equating to 0.08 versus 0.06 per patient year); however, this was not statistically significant. On an individual patient basis, these admissions clustered in four patients taking clozapine and occurred in six control patients.
Discussion
Clozapine is a dibenzo-diazepine atypical antipsychotic, and since 1990 the only licensed therapy in the UK for the 30% of patients with treatment-resistant schizophrenia. It shows superior efficacy in patients with schizophrenia and is effective in approximately 60% of those patients who were previously treatment refractive with a significant reduction in suicide risk.Reference Owen, Sawa and Mortensen1, Reference Meltzer, Alphs, Green, Altamura, Anand and Bertoldi19 The National Institute for Health and Care Excellence guideline recommends that adults with schizophrenia that have not responded adequately to treatment with at least two antipsychotic drugs should be offered clozapine; however, evidence shows it to be underprescribed.Reference Owen, Sawa and Mortensen1, 20
In this study, we found that taking clozapine is associated with a significant and substantial reduction in serum immunoglobulins. Even following exclusion of other recognised potential causes of antibody deficiency, clozapine therapy was associated with IgG <6.0 g/L in 8.5%, IgA <0.8 g/L in 13.8% and IgM <0.5 g/L in 34% of the 94 patients. The magnitude of the reduction in immunoglobulins is considerable when compared with the percentages of patients with secondary antibody deficiency following long-term combination therapy with rituximab (a monoclonal antibody targeting B cells) and methotrexate for rheumatoid arthritis based on pooled data from the rituximab clinical trials programme: IgG <6 g/L in 3.5%, IgA <0.8 g/L in 1.1% and IgM <0.5 g/L in 22.4%.Reference van Vollenhoven, Emery, Bingham, Keystone, Fleischmann and Furst21 Our linear regression model suggests a lower initial IgG concentration (‘lower reserve’) is associated with an earlier approach to the lower limit of the reference range, which is in keeping with known risk factors for secondary antibody deficiency following immunosuppressive therapies such as steroids and rituximab.Reference Christou, Giardino, Worth and Ladomenou22 In general, the greater the fall in IgG level below the lower limit of the reference range, the greater the probability of infections. Early diagnosis of antibody deficiency enables prevention of chronic infection, sepsis, end organ damage and death.Reference Resnick, Moshier, Godbold and Cunningham-Rundles23
Strengths and limitations of this study
Limitations of this work include the lack of prospective data, duration of follow-up and study size. In addition, there are shortcomings inherent in self-reported data and research officers administered the questionnaire and thus were not masked to participant medications. The width of confidence intervals presented alongside odds ratios reflect the relatively small sample size and low numbers of patients with values below the threshold for IgG, IgA or IgM used in both groups. Despite this, a higher proportion of patients needing more than five antibiotic courses in 12 months was seen in the clozapine recipients within this study, in keeping with previously reported increased antibiotic use.
Despite these limitations, all odds ratios were above one for total immunoglobulin classes and were highly statistically significant. These findings remained despite rigorous exclusion of patients with known possible causes of antibody deficiency.17 This is a strength and limitation of this study. Our aim was to reduce potential bias, however, the evidence for associations between medications such as carbamazepine or valproate and hypogammaglobulinaemia are largely based upon case reports. Exclusion of these patients may therefore result in an underestimate of the true effect size, particularly if drug interactions can contribute to immunoglobulin deficiency. Given a potential effect of smoking on IgG levels,Reference McMillan, Douglas, Archbold, McCrum and Evans24 we assessed self-reported current smoking status. This represents a subjective measure of smoking frequency, however, prevalence was high, similar between groups (61% versus 56%), and in keeping with that reported elsewhere.Reference Kelly and McCreadie25 It is notable that the significant association between duration of antipsychotic treatment and reduction in IgG was not observed in the clozapine-naive group, despite a longer duration of exposure to other antipsychotic agents and was not accounted for by other factors such as age, gender, diabetes, diagnosis of asthma/COPD, use of second antipsychotic agent, or psychiatric diagnosis in linear regression modelling.
A number of serious adverse effects including seizures, intestinal obstruction, diabetes, thromboembolism, cardiomyopathy and sudden cardiac death have been described with clozapine. It has been postulated that some of these side-effects may relate to its immunomodulatory effects,Reference Roge, Moller, Andersen, Correll and Nielsen26 including agranulocytosis (cumulative incidence 0.8%).Reference Alvir, Lieberman, Safferman, Schwimmer and Schaaf27 An association of clozapine with selective IgM deficiency has previously been suggestedReference Lozano, Marin, Santacruz and Pascual28 identifying isolated IgM deficiency in six (18%) clozapine users and two controls (3%). Differences in study size, treatment duration, and the differential effect on immunoglobulin classes (IgM > IgA > IgG) might have contributed to the identification of the greater risks posed by reductions in all three immunoglobulin classes reported here.
An additional strength of our study was detailed immunological assessment of vaccine-specific responses, which are integral components of patient evaluation and risk stratification during assessment of humoral immunodeficiency.Reference Jolles, Chapel and Litzman29 The majority of patients in both the clozapine and control groups had baseline specific antibody levels below the protective range for pneumococcus and haemophilus, common respiratory tract pathogens. This is in contrast to the majority of patients still having immunoglobulin levels within the reference range (albeit significantly lower than controls for all three classes) and likely reflects similarities between the groups in terms of the combination of prior vaccination history and natural exposure to these pathogens. This suggests that the population as a whole might benefit from improved access to vaccination, particularly given the numerous additional risk factors (smoking, diabetes, alcohol intake, illicit drug use, poor diet and physical inactivity) they may encounter.
The clozapine-treated group differed significantly from controls with lower levels of IgA and IgM pneumococcal antibodies. IgA is involved in defence against infection at mucosal surfaces such as the upper and lower respiratory tract and gut. The reasons why IgA and IgM pneumococcal antibodies were lower in the clozapine-treated group is not entirely clear but may reflect the greater relative and more immediate reductions in total IgA and IgM compared with IgG relative to the healthy adult and control group range.
Implications
This work, if confirmed, has potential implications for monitoring and risk mitigation strategies surrounding use of this important medication. This is particularly relevant given recent changes to the United States Clozapine Risk Evaluation and Mitigation Strategy programme (www.clozapinerems.com) that includes lowering the absolute neutrophil count threshold at which interruption of clozapine treatment is required. The impact of these changes will allow greater flexibility in patient-specific decisions about continuing or resuming treatment in patients who develop moderate to severe neutropenia, and so increase access to clozapine in patients across the USA.
Further studies are urgently required to validate these findings of immunoglobulin deficiency in a wider population of patients receiving clozapine, and to address questions relating to causality. These should be sufficiently powered to assess the impact of clozapine dose and duration, and explore additional risk factors such as concomitant medication, dual antipsychotic use, initial pre-clozapine immunoglobulin levels, smoking levels, durability of vaccine responses and any impact of schizophrenia itself. If confirmed there are also potential implications for practice, as current monitoring schemes do not include immunoglobulin testing. Given routine neutrophil monitoring and the centralised-nature of registries, integration of immunoglobulin and specific antibody level testing would be relatively straightforward. This would allow identification, risk stratification and monitoring of changes over time with individualised intervention where needed.
The association between duration of clozapine use and reduction in IgG suggests that long-term monitoring may be needed. In addition, the results highlight the wider issue of protecting this vulnerable patient group from infection, irrespective of clozapine treatment, by optimising vaccination status. This could be improved by formally defining this population as a risk group (as is the case for pneumococcal vaccination for patients with chronic lung disease, chronic heart disease, splenectomy and diabetes) to ensure equitable and appropriate access to protection via vaccination.
Funding
Health and Care Research Wales, CSL Behring and The Binding Site.
Acknowledgements
This portfolio observational study was supported by Health and Care Research Wales research officers and support for laboratory consumables was provided by The Binding Site and CSL Behring. The huge support of staff at all of the community mental health teams is very gratefully acknowledged. Thank you also to Dr Jenny Hughes for careful reading of the manuscript.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.152.








eLetters
No eLetters have been published for this article.