Numerous studies have documented that attention-deficit hyperactivity disorder (ADHD) increases the risk for nicotine use across the life cycle. Milberger et al Reference Milberger, Biederman, Faraone, Chen and Jones1,Reference Milberger, Biederman, Faraone, Chen and Jones2 found significant associations between ADHD and cigarette smoking in both referred and non-referred paediatric samples. Those studies documented that ADHD increased the risk for cigarette smoking in early adolescence twofold and was associated with a 2-year earlier age at onset than controls. Likewise, Tercyak et al Reference Tercyak, Lerman and Audrain3 found that adolescents with ADHD were three times more likely to smoke than adolescents without ADHD. Pomerleau et al Reference Pomerleau, Downey, Stelson and Pomerleau4 documented that adult ADHD is associated with, and an increased risk for, tobacco addiction and, among smokers, increased difficulty quitting. Glass & Flory Reference Glass and Flory5 summarised several possible mechanisms for this relationship, including the self-medication hypothesis and other social, cognitive and psychopathological factors.
The extant literature also supports an association between ADHD and other substance use disorders (alcohol or drug misuse or dependence) that establishes itself by mid to late adolescence. Milberger et al Reference Milberger, Biederman, Faraone, Wilens and Chu6 showed that although non-nicotine substance use disorders were not associated with ADHD in early to mid-adolescence, they were significantly and robustly associated with ADHD by late adolescence. Using two 10-year follow-up studies of ADHD, Wilens and colleagues Reference Wilens, Martelon, Joshi, Bateman, Fried and Petty7 found that ADHD was a significant risk factor for the development of substance use disorders and cigarette smoking in both genders. These findings bear remarkable congruence to results from retrospective studies of adults with and without ADHD that show that ADHD is associated with an increased risk for drug and alcohol misuse and dependence substance use disorders that tend to emerge in late adolescence and young adulthood. Reference Biederman, Faraone, Monuteaux, Spencer, Wilens and Bober8,Reference Mannuzza, Klein, Bessler, Malloy and LaPadula9 Taken together these findings suggest a temporal relationship between the earlier onset of cigarette smoking in early adolescence and the later onset of drug and alcohol misuse and dependence in later adolescent and young adult years, raising the possibility that cigarette smoking may act as a gateway for the subsequent development of substance use disorders.
The gateway hypothesis was formulated to model how adolescents initiate and progress in the use of various drugs. Reference Kandel10 The hypothesis states that substance use follows developmental pathways, where cigarettes or alcohol open the door to marijuana use, and marijuana thereupon becomes a gateway to more dangerous and addictive drugs such as cocaine or heroin. Throughout this paper, cigarette smoking as a ‘gateway’ refers to the increased risk for substance use disorders subsequent to cigarette smoking. Several studies have produced convincing evidence that individuals who smoke cigarettes have an increased likelihood of engaging in other drug use. For example, Torabi et al Reference Torabi, Bailey and Majd–Jabbari11 found that individuals who consistently smoked a pack of cigarettes a day were 10 to 30 times more likely than non-smokers to subsequently use drugs and Lai et al Reference Lai, Lai, Page and McCoy12 reported that individuals who smoked cigarettes were significantly more likely to have progressed to marijuana, cocaine, crack and heroin use.
We previously reported Reference Biederman, Monuteaux, Mick, Wilens, Fontanella and Poetzl13 in a sample of adolescent girls with and without ADHD, that cigarette smoking significantly increased the risk for subsequent use of alcohol and drugs and that this association was stronger for girls with ADHD relative to controls. These findings suggest a developmental progression for addictive behaviours in ADHD that starts with cigarette smoking in early adolescence and progresses into alcohol and drug misuse or dependence by late adolescence and young adult years. This idea is consistent with the hypothesis that cigarette smoking acts as a gateway. The main goal of this study was therefore to reassess whether cigarette smoking is a gateway for subsequent alcohol and drug misuse and dependence in youth with ADHD who have grown up. Towards this end, we evaluated the risk for substance use disorders in a large longitudinal sample of boys and girls ascertained from psychiatric and paediatric sources with and without ADHD followed up for 10 years into the young adult years stratified by smoking status. We tested the hypothesis that cigarette smoking would increase the risk for subsequent alcohol and drug use disorders by the young adult years and that the magnitude of this association will be stronger in youth with ADHD. To the best of our knowledge this is the first study to assess cigarette smoking as a gateway to substance use disorders in youth with ADHD who have grown up. This follow-up sample is particularly important considering the high prevalence of substance use in young adults.
Method
Participants
Detailed study methodology has been previously described. Reference Biederman, Faraone, Mick, Williamson, Wilens and Spencer14–Reference Biederman, Petty, Monuteaux, Fried, Byrne and Mirto17 Briefly, participants were derived from two longitudinal case–control family genetic study of male and female youth with and without ADHD. The studies consisted of 280 ADHD probands (140 male, 140 female) and 242 controls without ADHD (120 male, 122 female), aged 6–17 at the time of ascertainment. We also included the 317 siblings (179 male, 138 female) of the ADHD probands and the 260 siblings (137 male, 123 female) of the control probands. Male probands and their siblings were assessed at baseline, 1-, 4- and 10-year follow-ups, whereas female probands and their siblings were assessed at baseline, 5- and 11-year follow-ups. Potential participants were excluded if they had been adopted, or if their nuclear family was not available for study. Probands were excluded if they had major sensorimotor disorders (paralysis, deafness, blindness), psychosis, autism, inadequate command of the English language, or a full scale IQ Reference Wechsler18 less than 80. All of the ADHD probands (ADHD group) met full DSM-III-R 19 diagnostic criteria for ADHD according to clinical assessment at the time of the clinical referral; at the time of recruitment for this study they all had active symptoms of the disorder. After a detailed description of the study procedures, written informed consent was obtained from all participants. For participants younger than 18, assent was also provided. This study was approved by the institutional review board of the Massachusetts General Hospital.
Psychiatric assessments of participants younger than 18 years relied on the epidemiologic version of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children – Epidemiological version (Kiddie-SADS-E). Reference Orvaschel20,Reference Orvaschel21 Participants 18 years of age and older were assessed with the Structured Clinical Interview for DSM (SCID) Reference Spitzer, Williams, Gibbon and First22,Reference First, Spitzer, Gibbon and Williams23 (supplemented with modules from the Kiddie-SADS-E to assess childhood diagnoses). We interviewed the mothers for all participants and directly interviewed participants older than 12 years. We combined data from direct and indirect interviews by considering a diagnostic criterion positive if it was endorsed in either interview. All assessment personnel were masked to proband diagnosis (ADHD or control) and ascertainment site (psychiatric or paediatric). Parents (522 mothers, 509 mothers) were assessed at baseline with the SCID. Socioeconomic status was measured using the five-point Hollingshead scale. Reference Hollingshead24
The interviewers had undergraduate degrees in psychology and were extensively trained. Based on 500 assessments from interviews of children and adults, the median kappa coefficient for diagnoses was 0.98. Kappa coefficients for individual diagnoses included: ADHD (0.88), conduct disorder (1.0), major depression (1.0), mania (0.95), separation anxiety (1.0), agoraphobia (1.0), panic (0.95) and substance use disorder (1.0). We considered a disorder positive if DSM diagnostic criteria were unequivocally met.
A committee of board-certified child and adult psychiatrists who were masked to the participant's ADHD status, referral source and all other data resolved diagnostic uncertainties. Diagnoses presented for review were considered positive only when the committee determined that diagnostic criteria were met to a clinically meaningful degree. We estimated the reliability of the diagnostic review process by computing kappa coefficients of agreement for clinician reviewers. For these diagnoses, the median reliability between individual clinicians and the review committee assigned diagnoses was 0.87. Kappa coefficients for individual diagnoses included: ADHD (1.0), conduct disorder (1.0), major depression (1.0), mania (0.78), separation anxiety (0.89), agoraphobia (0.80), panic (0.77) and substance use disorder (1.0).
Statistical analyses
Cigarette smoking was defined as ever having smoked more than three times a week or any regular habit of smoking (e.g. only at parties) for an age at onset before age 18 years. For onset at age 18 years or after, cigarette smoking was defined as smoking every day. For the primary analysis, participants were included if they were reassessed at the 10- or 11-year follow-up. Participants were excluded if they reported an age at onset of any psychoactive substance use disorder at the same age or prior to the age at onset of smoking (n = 122; 47 with ADHD, 75 without ADHD). This yielded a total of 651 participants. Participants were grouped into four groups according to their ADHD diagnosis at baseline and their lifetime smoking status. We compared the resulting four groups on demographic features. We tested the risk of developing psychoactive substance use disorders using Cox proportional hazard models. The reported age at onset was used as the failure time for each substance use disorder.
The secondary analysis used smoking status at the 4- or 5-year follow-up to predict psychoactive substance use disorders (alcohol or drug misuse or dependence) at the 10- or 11-year follow-up, so we did not have to rely on retrospectively reported ages at onset. The secondary analysis was limited to participants who were at least 12 years of age and had no history of psychoactive substance use disorders at the 4- or 5-year follow-up and were reassessed at the 10- or 11-year follow-up (n = 539). A total of 112 participants were excluded because they had a substance use disorder at the 4- or 5-year follow-up. Participants were grouped according to their ADHD diagnosis at baseline and their smoking status at the 4- or 5-year follow-up. The same statistical tests were used for the secondary analysis as described for the primary analysis.
For both analyses, we repeated each model controlling for conduct disorder, bipolar disorder or parental substance use disorder (the same disorder as the outcome) to determine whether our findings were independent of these factors. We also tested for any significant interaction effects of smoking status and comorbid disruptive behaviour disorder, mood disorder, or multiple (⩾2) anxiety disorders, proband status or gender. To account for the non-independence of siblings, we used the Huber Reference Huber25 correction to produce robust variances for all statistical tests. All tests were two-tailed with alpha set at 0.05.
Results
The analysis was restricted to participants that did not report an age at onset for alcohol misuse, alcohol dependence, drug misuse or drug dependence at the same age or before the onset of smoking (n = 213 with ADHD, n = 438 without ADHD). Among these 651 participants, the overall rate of smoking was 28% (34% and 25%, for those in the ADHD group and the control group respectively, χ2 (1) = 6.99, P = 0.008). The average age at smoking onset was significantly earlier in the ADHD group than in the control group (13.9 (s.d. = 2.7) v. 15.4 (s.d. = 2.7) years of age, t (146) = –3.64, P<0.001). Of 181 participants that met criteria for smoking, 89% (n = 161) had an onset before age 18.
TABLE 1 Demographic features in non-smokers and smokers with and without attention-deficit hyperactivity disorder (ADHD)
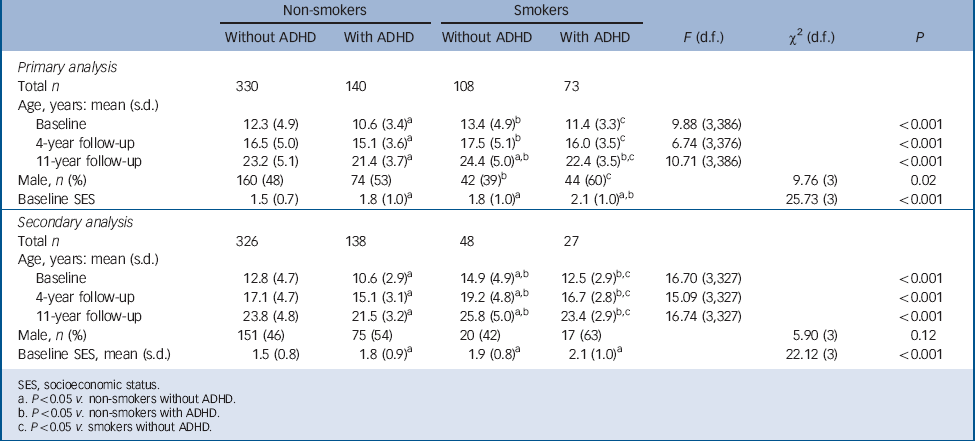
| Non-smokers | Smokers | ||||||
|---|---|---|---|---|---|---|---|
| Without ADHD | With ADHD | Without ADHD | With ADHD | F (d.f.) | χ2 (d.f.) | P | |
| Primary analysis | |||||||
| Total n | 330 | 140 | 108 | 73 | |||
| Age, years: mean (s.d.) | |||||||
| Baseline | 12.3 (4.9) | 10.6 (3.4)Footnote a | 13.4 (4.9)Footnote b | 11.4 (3.3)Footnote c | 9.88 (3,386) | <0.001 | |
| 4-year follow-up | 16.5 (5.0) | 15.1 (3.6)Footnote a | 17.5 (5.1)Footnote b | 16.0 (3.5)Footnote c | 6.74 (3,376) | <0.001 | |
| 11-year follow-up | 23.2 (5.1) | 21.4 (3.7)Footnote a | 24.4 (5.0)Footnote a , Footnote b | 22.4 (3.5)Footnote b , Footnote c | 10.71 (3,386) | <0.001 | |
| Male, n (%) | 160 (48) | 74 (53) | 42 (39)Footnote b | 44 (60)Footnote c | 9.76 (3) | 0.02 | |
| Baseline SES | 1.5 (0.7) | 1.8 (1.0)Footnote a | 1.8 (1.0)Footnote a | 2.1 (1.0)Footnote a , Footnote b | 25.73 (3) | <0.001 | |
| Secondary analysis | |||||||
| Total n | 326 | 138 | 48 | 27 | |||
| Age, years: mean (s.d.) | |||||||
| Baseline | 12.8 (4.7) | 10.6 (2.9)Footnote a | 14.9 (4.9)Footnote a , Footnote b | 12.5 (2.9)Footnote b , Footnote c | 16.70 (3,327) | <0.001 | |
| 4-year follow-up | 17.1 (4.7) | 15.1 (3.1)Footnote a | 19.2 (4.8)Footnote a , Footnote b | 16.7 (2.8)Footnote b , Footnote c | 15.09 (3,327) | <0.001 | |
| 11-year follow-up | 23.8 (4.8) | 21.5 (3.2)Footnote a | 25.8 (5.0)Footnote a , Footnote b | 23.4 (2.9)Footnote b , Footnote c | 16.74 (3,327) | <0.001 | |
| Male, n (%) | 151 (46) | 75 (54) | 20 (42) | 17 (63) | 5.90 (3) | 0.12 | |
| Baseline SES, mean (s.d.) | 1.5 (0.8) | 1.8 (0.9)Footnote a | 1.9 (0.8)Footnote a | 2.1 (1.0)Footnote a | 22.12 (3) | <0.001 | |
SES, socioeconomic status.
a P<0.05 v. non-smokers without ADHD.
b P<0.05 v. non-smokers with ADHD.
c P<0.05 v. smokers without ADHD.
Sociodemographic characteristics
As presented in Table 1, for our primary analysis the non-smoking participants with ADHD were the youngest, followed by the non-smokers without ADHD; and the smokers with ADHD, and the smokers without ADHD were the oldest. The non-smoking participants without ADHD had a significantly higher socioeconomic status compared with the other three groups, and the non-smokers with ADHD had a higher socioeconomic status than the smokers with ADHD. In addition, smokers without ADHD had fewer males compared with both ADHD groups. Therefore, socioeconomic status, gender and age at the 11-year follow-up were added as covariates to all subsequent statistical models. For our secondary analysis the demographic characteristics were largely the same, with the exception of no significance difference in gender distribution (Table 1). Therefore, only socioeconomic status and age were added as covariates for the secondary analysis.
Primary analyses: risk of developing substance use disorders
Alcohol misuse and dependence
As shown in Fig. 1, although smokers with and without ADHD had a significantly higher risk of developing alcohol misuse compared with non-smokers with and without ADHD, this risk was highest among smokers with ADHD (smokers with ADHD v. non-smokers without ADHD: hazard ratio (HR) = 2.6, 95% CI 1.8–3.8, z = 4.97, P<0.001; smokers with ADHD v. non-smokers with ADHD: HR = 2.3, 95% CI 1.5–3.5, z = 3.81, P<0.001; smokers without ADHD v. non-smokers without ADHD: HR = 1.8 95% CI 1.3–2.6, z = 3.34, P = 0.001; smokers without ADHD v. non-smokers with ADHD: HR = 1.6, 95% CI 1.04–2.4, z = 2.14, P = 0.03). At the mean age of 23 years, the estimated rate of alcohol misuse was 62% for smokers with ADHD and 47% for smokers without ADHD v. 43% for non-smokers with ADHD and 31% for non-smokers without ADHD.
Likewise, although smokers without ADHD had a significantly higher risk for developing alcohol dependence compared with non-smokers without ADHD (HR = 2.6 95% CI 1.4–4.8, z = 3.15, P = 0.002), this risk was highest among smokers with ADHD who had a significantly higher risk of developing alcohol dependence compared with the other three groups (non-smokers without ADHD: HR = 6.1, 95% CI 3.3–11.2, z = 5.84, P<0.001; non-smokers with ADHD: HR = 3.8, 95% CI 1.9–7.5, z = 3.75, P<0.001; smokers without ADHD: HR = 2.3, 95% CI 1.3–4.2, z = 2.70, P = 0.007). Estimated rates of alcohol dependence at age 23 were 7% for non-smokers without ADHD, 10% for non-smokers with ADHD, 18% for smokers without ADHD and 35% for smokers with ADHD.
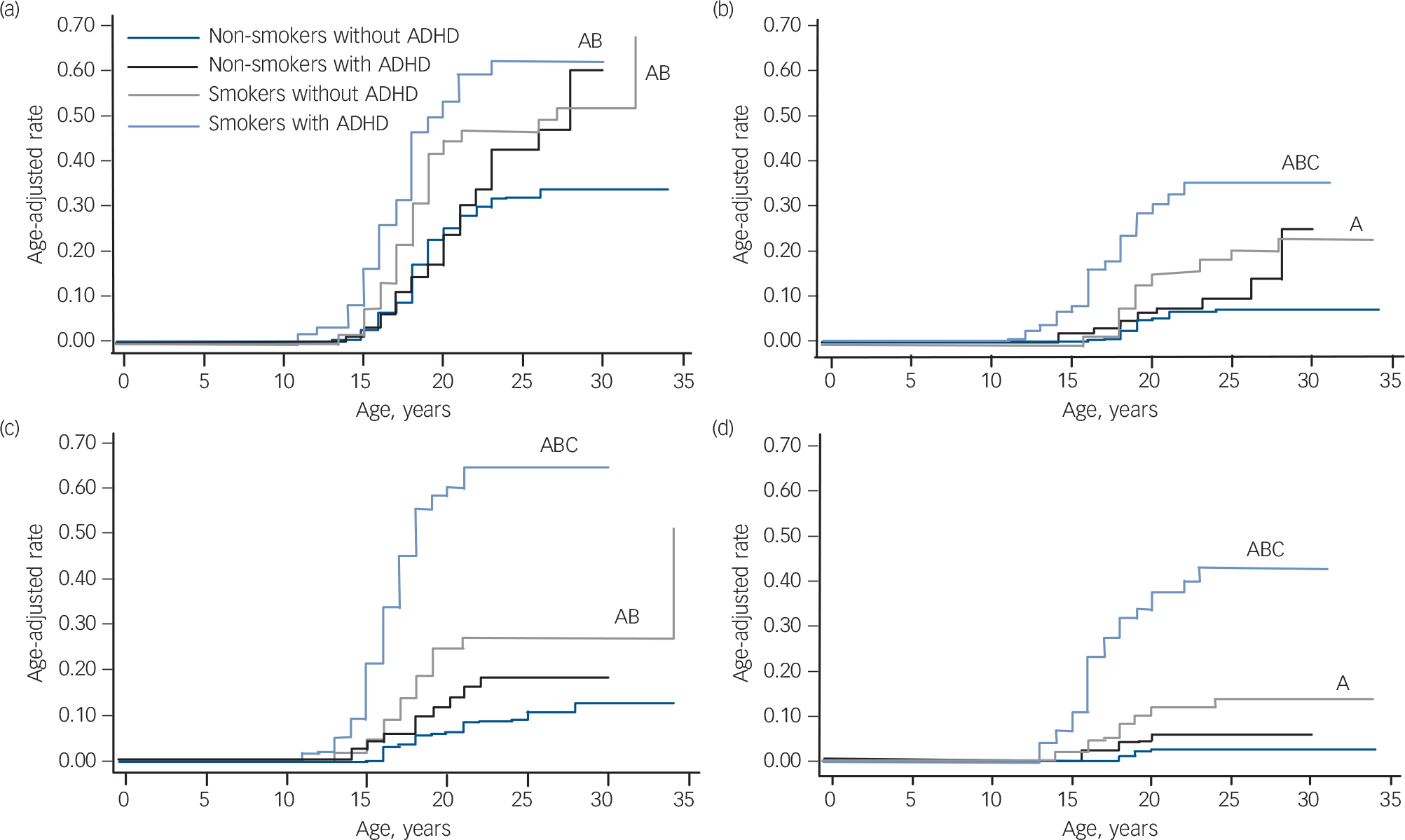
FIG. 1 Risk for psychoactive substance use disorder in smokers and non-smokers with and without attention-deficit hyperactivity disorder (ADHD): primary analysis. (a) Risk for alcohol misuse, (b) risk for alcohol dependence, (c) risk for drug misuse, (d) risk for drug dependence.
A, P<0.05 v. non-smokers without ADHD; B, P<0.05 v. non-smokers with ADHD; C, P<0.05 v. smokers with ADHD.
There were no significant interaction effects of ADHD and smoking status predicting alcohol use disorders. As Table 2 shows, independent of ADHD, smoking significantly increased the risk for alcohol misuse and dependence and, independent of smoking, ADHD significantly increased the risk for alcohol dependence.
Drug misuse and dependence
Although smokers with and without ADHD had a significantly higher risk of developing drug misuse compared with the non-smoking groups (non-smokers without ADHD: HR = 3.1, 95% CI 1.8–5.4, z = 4.20, P<0.001; non-smokers with ADHD: HR = 1.8, 95% CI 1.03–3.3, z = 2.05, P = 0.04), this risk was highest among smokers with ADHD compared with all other groups (non-smokers without ADHD: HR = 9.6, 95% CI 5.8–15.9, z = 8.76, P<0.001; non-smokers with ADHD: HR = 5.6, 95% CI 3.3–9.3, z = 6.59, P<0.001; smokers without ADHD: HR = 3.1, 95% CI 1.8–5.1, z = 4.30, P<0.001). Estimated rates of drug misuse at age 23 were 9% for non-smokers without ADHD, 18% for non-smokers with ADHD, 27% for smokers without ADHD and 65% for smokers with ADHD.
Similar results were observed for drug addiction. Smokers with and without ADHD had a significantly higher risk for drug dependence compared with the other three groups (v. non-smokers without ADHD: HR = 16.5, 95% CI 7.0–39.1, z = 6.37, P<0.001; v. non-smokers with ADHD: HR = 7.7, 95% CI 3.3–17.8, z = 4.71, P<0.001; v. smokers without ADHD: HR = 4.1, 95% CI 2.0–8.3, z = 3.91, P<0.001), this risk was highest among smokers with ADHD. Smokers with ADHD had an estimated drug dependence rate of 43%, compared with 3% for non-smokers without ADHD, 6% for non-smokers with ADHD, and 11% for smokers without ADHD.
Confounders and interaction effects
All findings remained significant after controlling for the same parental substance use disorder. There were no significant interaction effects of smoking status and comorbid disruptive behaviour disorder, mood disorders, multiple (⩾2) anxiety disorders, proband status or gender when predicting any of the psychoactive substance use disorders (all P>0.05). However, the alcohol dependence finding between the smokers with and without ADHD lost significance after controlling for conduct disorder or bipolar disorder (both P>0.05).
Secondary analyses: risk of developing substance use disorders
To get a clearer longitudinal perspective, we repeated the analysis by restricting the sample to participants that were at least 12 years of age and did not meet criteria for alcohol misuse, alcohol dependence, drug misuse or drug dependence at the 4-year follow-up (n = 165 with ADHD, n = 374 without ADHD). Despite more limited power, results from these secondary analyses were generally consistent with the previous analysis, with the strongest findings being for cigarette smoking increasing the risk for drug dependence (Fig. 2). Smokers with ADHD had a significantly higher risk for drug dependence compared with the other three groups (v. non-smokers without ADHD: HR = 5.6, 95% CI 2.5–12.7, z = 4.12, P<0.001; v. non-smokers with ADHD: HR = 5.4, 95% CI 2.2–13.5, z = 3.65, P<0.001; v. smokers without ADHD: HR = 3.1, 95% CI 1.1–8.7, z = 2.15, P = 0.03). Smokers with ADHD had an estimated drug dependence rate of 34%, compared with 5% for non-smokers without ADHD, 8% for non-smokers with ADHD, and 10% for smokers without ADHD. All findings remained significant after controlling for the same parental substance use disorder. However, after controlling for conduct disorder and bipolar disorder, the smokers with ADHD remained at higher risk for drug dependence compared with non-smokers with ADHD, but lost significance compared with smokers without ADHD (both P>0.30) and non-smokers without ADHD (conduct disorder only, P = 0.30). There were no significant interaction effects of smoking status and comorbid disruptive behaviour disorder, mood disorder or multiple (⩾2) anxiety disorders, proband status or gender when predicting any of the psychoactive substance use disorders (all P>0.05). Using the binary predictor approach, smoking significantly increased the risk of alcohol dependence, drug misuse and drug dependence independently of ADHD (Table 2). Attention-deficit hyperactivity disorder significantly increased the risk for alcohol misuse and dependence independently of smoking.
TABLE 2 Independent effects of smoking and attention-deficit hyperactivity disorder (ADHD) on the risk for psychoactive substance use disordersFootnote a
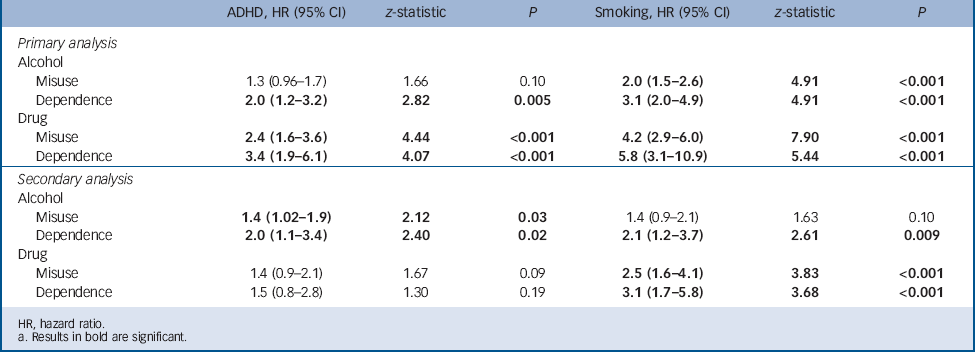
| ADHD, HR (95% CI) | z-statistic | P | Smoking, HR (95% CI) | z-statistic | P | |
|---|---|---|---|---|---|---|
| Primary analysis | ||||||
| Alcohol | ||||||
| Misuse | 1.3 (0.96–1.7) | 1.66 | 0.10 | 2.0 (1.5–2.6) | 4.91 | <0.001 |
| Dependence | 2.0 (1.2–3.2) | 2.82 | 0.005 | 3.1 (2.0–4.9) | 4.91 | <0.001 |
| Drug | ||||||
| Misuse | 2.4 (1.6–3.6) | 4.44 | <0.001 | 4.2 (2.9–6.0) | 7.90 | <0.001 |
| Dependence | 3.4 (1.9–6.1) | 4.07 | <0.001 | 5.8 (3.1–10.9) | 5.44 | <0.001 |
| Secondary analysis | ||||||
| Alcohol | ||||||
| Misuse | 1.4 (1.02–1.9) | 2.12 | 0.03 | 1.4 (0.9–2.1) | 1.63 | 0.10 |
| Dependence | 2.0 (1.1–3.4) | 2.40 | 0.02 | 2.1 (1.2–3.7) | 2.61 | 0.009 |
| Drug | ||||||
| Misuse | 1.4 (0.9–2.1) | 1.67 | 0.09 | 2.5 (1.6–4.1) | 3.83 | <0.001 |
| Dependence | 1.5 (0.8–2.8) | 1.30 | 0.19 | 3.1 (1.7–5.8) | 3.68 | <0.001 |
HR, hazard ratio.
a Results in bold are significant.
Discussion
Whether tobacco is a gateway for subsequent alcohol and drug misuse and dependence in youth with ADHD has major clinical, scientific and public health implications. If confirmed, such findings could help clinicians identify youth with ADHD at high risk for addictive behaviours. If cigarette smoking occurs first in the developmental trajectory of substance use disorders, it would suggest that the prevention of smoking could interrupt the pathway towards alcohol and drug use disorders, which would reduce their associated morbidity, mortality and cost to society. Furthermore, considering that the average age at onset of ADHD is in the pre-school years and the average age at onset of cigarette smoking is in early adolescence, there is an approximate 10-year window for the development of disorder-specific preventive and early intervention strategies to avert tobacco addiction outcomes in youth with ADHD. Scientifically, information about developmental trajectories of substance use disorders may yield new insights into the neurobiology of addictions.
Main findings
In this large, prospective, controlled study of youth with and without ADHD of both genders followed up into young adulthood, we found that smoking greatly and significantly increased the risk for the subsequent development of alcohol and drug misuse and dependence. Because the risk for substance use disorders afforded by smoking was additive with the risk afforded by ADHD, smokers with ADHD are at especially high risk for substance use disorders. These findings have important public health significance within and without the context of ADHD for the development of preventive and early intervention approaches aimed at mitigating smoking initiation within and without the context of ADHD.
These findings confirm and extend previous results documented in a sample of adolescent girls with and without ADHD Reference Biederman, Monuteaux, Mick, Wilens, Fontanella and Poetzl13 and provide further support for the hypothesis that cigarette smoking is a gateway for the subsequent development of drug misuse and dependence. These findings indicate that cigarette smoking may help identify a subgroup of youth with ADHD at the highest risk to progress into addictions and support efforts aimed at diminishing the risk for smoking initiation in children with ADHD. Results from our primary analysis indicate that youth with ADHD who smoke cigarettes have a fivefold higher risk for developing alcohol dependence and a ninefold higher risk for developing drug dependence compared with youth without ADHD who do not smoke cigarettes.
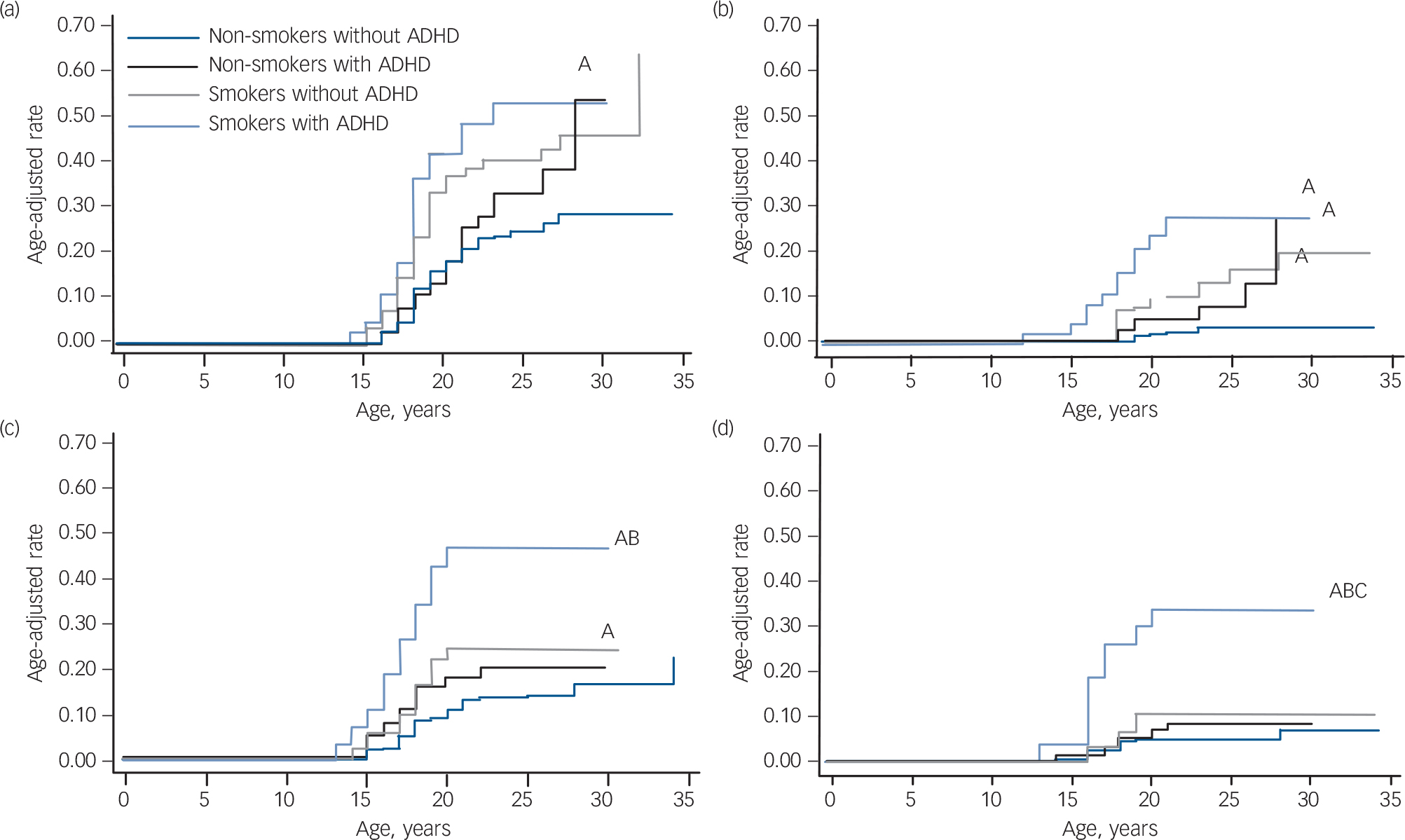
FIG. 2 Risk for psychoactive substance use disorder in smokers and non-smokers with and without attention-deficit hyperactivity disorder (ADHD): secondary analysis. (a) Risk for alcohol misuse, (b) risk for alcohol dependence, (c) risk for drug misuse, (d) risk for drug dependence.
A, P<0.05 v. non-smokers without ADHD; B, P<0.05 v. non-smokers with ADHD; C, P<0.05 v. smokers with ADHD.
Our findings indicate that cigarette smoking is not the only pathway to substance use disorders. A sizeable minority (16%) of the original group had used drugs before or at the same age that they started smoking. Additionally, some participants developed a substance use disorder and never reported cigarette smoking. Therefore, cigarette smoking should not be viewed as a sufficient or necessary explanation for substance misuse problems, but rather as one of several possible risk factors for subsequent substance use disorders.
Our finding that cigarette smoking is associated with subsequent substance use disorders is consistent with the gateway effect of cigarettes previously documented in the literature. For example, Merrill and colleagues, Reference Merrill, Kleber, Shwartz, Liu and Lewis26 analysing data from the 1995 Youth Risk Behavior Survey, found that among high-school seniors, cigarette use before age 13 was associated with a significant threefold increased risk for marijuana use. A study analysing the data from the National Household Survey on Drug Misuse also found significant associations between cigarette use and the subsequent use of cocaine, heroin, crack and marijuana. Reference Lai, Lai, Page and McCoy12 In another study of this data-set, Wagner & Anthony Reference Wagner and Anthony27 found that youth who had used tobacco or alcohol were more likely to later experience an opportunity to use marijuana and more likely to actually use it given that an opportunity had occurred. Other studies also found that regular smoking was a risk factor for the use of alcohol and other drugs. Reference Torabi, Bailey and Majd–Jabbari11,Reference Hanna, Yi, Dufour and Whitmore28
Our findings show that smokers with ADHD carry two independent and additive risks for subsequent substance use disorders. This finding is consistent with mounting evidence that ADHD is a significant risk factor for cigarette smoking Reference Tercyak, Lerman and Audrain3–Reference Glass and Flory5 and for alcohol and drug misuse and dependence. Reference Wilens, Martelon, Joshi, Bateman, Fried and Petty7,Reference Molina and Pelham29–Reference Wilens, Biederman, Mick, Faraone and Spencer31 Although ADHD also increased the risk of substance use disorders (independent of the risk afforded by smoking), having ADHD did not potentiate the gateway effect of smoking (i.e. we found no interactions between ADHD and smoking in predicting subsequent substance use disorders). This suggests that treatment programmes aimed at preventing substance use disorders in youth with ADHD should incorporate interventions for ADHD symptoms, interventions for smoking behaviour among smokers, and preventive interventions for non-smokers.
The reasons why cigarette smoking functions as a gateway for the subsequent use of alcohol and drugs are not entirely clear. Several explanations can be considered. Nicotine modulates dopaminergic activity in the mesolimbic system, 32–Reference Brody, Mandelkern, Jarvik, Lee, Smith and Huang36 which could provide positive reinforcement for other addictive behaviours. Reference Lindsay and Rainey37 This nicotinic effect could be magnified and be particularly strong in the context of ADHD by the pre-existing dopaminergic abnormalities associated with ADHD, leading to the increased magnitude of effect seen in participants with ADHD in our results. More work is needed to substantiate these intriguing hypotheses.
Implications
Irrespective of the reasons why cigarette smoking functions as a gateway for the subsequent use of alcohol and drugs, it is important to note that cigarette smoking, alcohol and drugs are major sources of morbidity and mortality in our society and across the world. In the year 2000, at least 435 000 US residents died directly as a consequence of tobacco, 85 000 as a consequence of alcohol consumption and 17 000 as a consequence of drug use. Reference Mokdad, Marks, Stroup and Gerberding38 Because the majority of smokers begin smoking in adolescence, typically by age 12, cigarette smoking Reference Sims39,Reference Collins, Epstein, Parzynski, Zimmerman, Moolchan and Heishman40 can be targeted for preventive and early intervention efforts. Preventive measures are particular important given the considerable evidence that earlier age of first use of alcohol and drugs is associated with poorer outcomes. Reference Kandel10
Considering that ADHD significantly increases the risks for both cigarette smoking Reference Tercyak, Lerman and Audrain3,Reference Molina and Pelham29 and alcohol and drug misuse and dependence, Reference Milberger, Biederman, Faraone, Wilens and Chu6–Reference Mannuzza, Klein, Bessler, Malloy and LaPadula9 if smoking could be prevented in youth with ADHD, a large amount of morbidity and mortality associated with tobacco, alcohol and drugs could be prevented. In a clinical trial examining the effects of bupropion for the prevention of smoking in youth with ADHD, we found that, although treatment with bupropion was ineffective in achieving this goal, adolescents treated with stimulants had a significantly decreased risk for smoking when compared with those that did not receive stimulant treatment. Reference Monuteaux, Spencer, Faraone, Wilson and Biederman41 Similar findings had been reported by Whalen et al Reference Whalen, Jamner, Henker, Gehricke and King42 in a community sample of adolescents with ADHD, who found that participants receiving pharmacotherapy for ADHD smoked significantly less than those who went untreated. Similar results were also documented in naturalistic samples. These studies showed that treatment with stimulants decreased the elevated risk for smoking in youth with ADHD to population rates. Reference Biederman, Monuteaux, Spencer, Wilens, Macpherson and Faraone43,Reference Biederman, Monuteaux, Spencer, Wilens and Faraone44 Most recently, Gehricke et al Reference Gehricke, Hong, Wigal, Chan and Doan45 found that medication for ADHD reduced cotinine levels and withdrawal in smokers with ADHD. If confirmed, these findings would have a large public health impact.
Limitations
Although our results are consistent with the gateway hypothesis, they could also be accounted for by the alternate idea that genetic risk, environmental exposures or a combination of these factors predispose youth to using substances in general, including both nicotine and other drugs. In this model, the use of one substance (such as nicotine) does not causally affect the subsequent use of other substances (such as alcohol). For example, family and twin studies suggest that there is some shared heritability among smoking and substance use disorders. Reference Bierut, Dinwiddie, Begleiter, Crowe, Hesselbrock and Nurnberger46,Reference Merikangas, Stolar, Stevens, Goulet, Preisig and Fenton47 The relative ease of access and availability to cigarettes would explain the observed temporal sequence of nicotine use followed by the use of other substances. Nevertheless, at the very least, our results provide valuable clinical information by identifying a strong risk factor (i.e. smoking) for future alcohol and drug use, misuse and dependence, especially in youth with ADHD.
Our results should be considered in the light of some methodological limitations. This was a secondary analysis of two longitudinal family-genetic studies of ADHD. Studies specifically designed to test the gateway hypothesis of cigarette smoking should seek to replicate our findings. Our sample was predominantly White and the probands were referred for ADHD. As such, our findings may not generalise to community samples and ethnic minorities. Future studies should attempt to replicate these findings in community samples. Another constraint on our generalisability is the limited range of socioeconomic status in our sample. Although all five of the Hollingshead categories were represented in our sample, very few participants were in the lowest categories. Also, the size of our smoking sample was relatively small. Although the results were mostly significant nonetheless, replication in a larger sample would be beneficial.
Also, we depended on the retrospectively reported ages at onset for smoking and substance use to establish the temporal sequence of smoking as an exposure. To the degree that these ages were incorrectly recalled, our results may suffer from misclassification and thus a reduction in precision. However, although the exact ages may not have been recalled accurately, the relative ordering of the ages of smoking and substance use initiation are likely to be correct, so that any misclassification of our exposure and outcome variables should be minimal. Also, there may be additional, unmeasured confounders that may account for our findings. Although it is unlikely that an unmeasured confounder could account for the magnitude of effect found here, future studies should include other predictors of adolescent substance use. Given that 16% of our original sample developed cigarette smoking at the same age or after substance use disorders, other studies should explore other possible developmental pathways. Although we did not find significant interaction effects of ADHD and cigarette smoking, the very high rates of drug misuse and dependence seen in the failure curves of the smokers with ADHD highlight the possibility that the risk conferred by cigarette smoking is much greater in the context of ADHD. Future studies should explore this possible interaction effect.
Despite these considerations, our results provide further support for cigarette smoking being a risk factor for the subsequent development of alcohol and drug misuse and dependence and that this risk adds to the risk imparted by ADHD. Thus, smokers with ADHD are at especially high risk for substance use disorders and they should be a focus for preventive interventions. These findings have important public health significance within and without the context of ADHD for the development of preventive and early intervention approaches aimed at mitigating smoking initiation within and without the context of ADHD.
Funding
This work was supported, in part, by USPHS (NIMH) grant R01 MH-41314-01A2 (JB) and the Pediatric Psychopharmacology Council Fund.







eLetters
No eLetters have been published for this article.