Schizophrenia is a disabling illness that affects over 2 million people in the USA alone, but its aetiology remains poorly understood (Reference HarrisonHarrison, 1999; Reference Siever and DavisSiever & Davis, 2004). In past years the disorder was studied by examining pathological brain tissue samples, often derived from patients who had died after a prolonged period of illness. In recent years, with the advent of brain imaging methods such as magnetic resonance imaging (MRI), it has become possible to study patients during their first episode of psychosis, before disease effects are obscured by the confounding influences typical of cases of chronic schizophrenia. This may make it possible to test hypotheses as to which brain volumetric changes are primary to the development of schizophrenia (Reference Shenton, Dickey and FruminShenton et al, 2001). Our goal in the study reported here is to provide an update of two excellent earlier reviews (Reference Wright, Rabe-Hesketh and WoodruffWright et al, 2000; Reference Shenton, Dickey and FruminShenton et al, 2001), but with a focus specifically on patients with first-episode schizophrenia.
METHOD
Study selection criteria
Relevant studies of patients with first-episode schizophrenia were identified in multiple searches as late as November 2004. The primary search used PubMed and the keywords SCHIZOPHRENIA and FIRST-EPISODE and MAGNETIC RESONANCE IMAGING and VOLUME, in all possible combinations. This search was repeated, substituting the keyword DRUG NAIVE for FIRST-EPISODE, and using the same search words again in all possible combinations. A secondary search was then undertaken, using each primary reference as a source. The bibliography of each source was searched for additional references that were missed by the PubMed search. In addition, the bibliographies of eight key review articles were searched (Reference Wright, Rabe-Hesketh and WoodruffWright et al, 2000; Reference Konick and FriedmanKonick & Friedman, 2001; Reference Okubo, Tomoyuki and OdaOkubo et al, 2001; Reference Shenton, Dickey and FruminShenton et al, 2001; Reference Kasai, Iwanami and YamasueKasai et al, 2002; Reference TorreyTorrey, 2002; Reference Davidson and HeinrichsDavidson & Heinrichs, 2003; Reference Antonova, Sharma and MorrisAntonova et al, 2004) for papers relating specifically to patients with first-episode disorder. Finally, current journals were reviewed to find references too new to have been reviewed. The primary search found 75 relevant references, whereas the secondary searches found an additional 16 references.
Studies were included in our analysis if brain MRI volumetric data were reported for both a population of patients with schizophrenia at first episode and a population of healthy controls evaluated concurrently. We excluded seven studies that did not report exclusively on patients with first-episode illness, and five studies that did not report concurrent data from healthy controls. Studies were also excluded if data from patients with first-episode psychosis were not separated from a larger population of patients with psychosis of some other type, or if results included patients with childhood-onset schizophrenia. We specifically excluded children younger than age 13 years from our analysis because there are rapid changes in brain volume among healthy children up to about age 9 years (Reference Pfefferbaum, Mathalon and SullivanPfefferbaum et al, 1994; Reference Giedd, Blumenthal and JeffriesGiedd et al, 1999), and the young age of patients with childhood-onset illness would make it difficult to control adequately for the effects of normal brain growth. Studies were also excluded if data were reported in a format that did not enable us to calculate patient brain volume as a percentage of the control group volume. Thus, we excluded studies that used voxel-based morphometry, since our calculations are based on volume rather than on number of pixels. We also excluded studies that reported results from a non-volumetric analysis of the data, or from a non-quantitative analysis of the data.
Of the total of 91 articles that were originally identified, 26 were excluded for any of the above reasons. A total of 65 articles were evaluated (Table 1), including 52 cross-sectional and 16 longitudinal studies. Data from all 52 eligible cross-sectional studies were entered into a spreadsheet that tabulated study details, including a brief description of the study, demography of the study populations, patient medications and the statistical analyses used. For patients, additional data were entered summarising the percentage difference in structure volume relative to controls, and whether or not this difference was statistically significant according to the analysis presented in the original reference.
Table 1 Summary of cross-sectional and longitudinal studies included in review
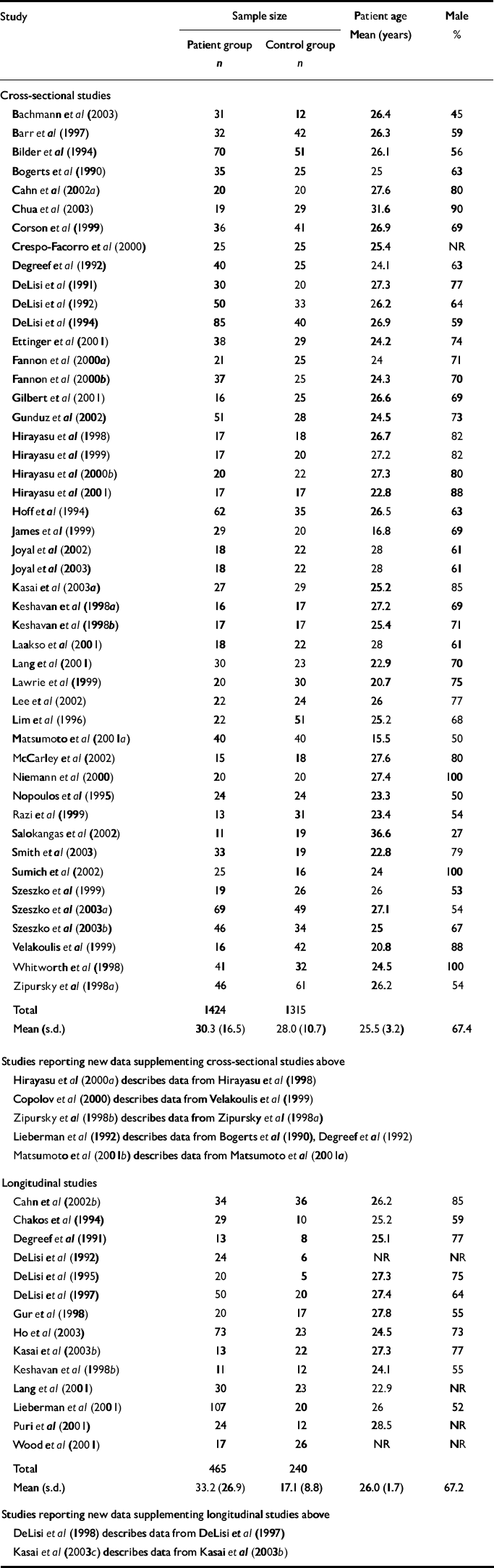
The data entry process was then repeated for all eligible longitudinal studies. If a longitudinal study reported baseline data in a format that could be analysed as a cross-sectional study, this study was entered into both the cross-sectional and the longitudinal databases. The final database contained approximately 29 084 cells.
Data analysis
We sorted the cross-sectional database by brain structure, to determine where brain volumetric changes had been sought. Brain volume changes in the first-episode group were summarised, with respect to controls, on a structure-by-structure basis (further information available from the author upon request). We then conducted a meta-analysis of all cross-sectional studies that measured whole-brain volume in the first-episode group relative to controls (Table 2). For each component study in the meta-analysis, we abstracted information about sample properties (size, mean and standard deviation) from the original paper and fitted a blocked analysis of variance model (with study as the blocking factor) to examine group differences. We additionally fitted models with the group×treatment interaction, to assess heterogeneity; interactions were non-significant in all cases, so we used the models without the interaction terms. We did similar meta-analyses of cross-sectional studies that measured differences in hippocampal volume (Table 3) and ventricular volume (Table 4). Finally, we summarised all studies that reported longitudinal volumetric changes significant at P≤0.01 (further information available from the author upon request), to address the issue of which longitudinal changes are most robust by statistical criteria.
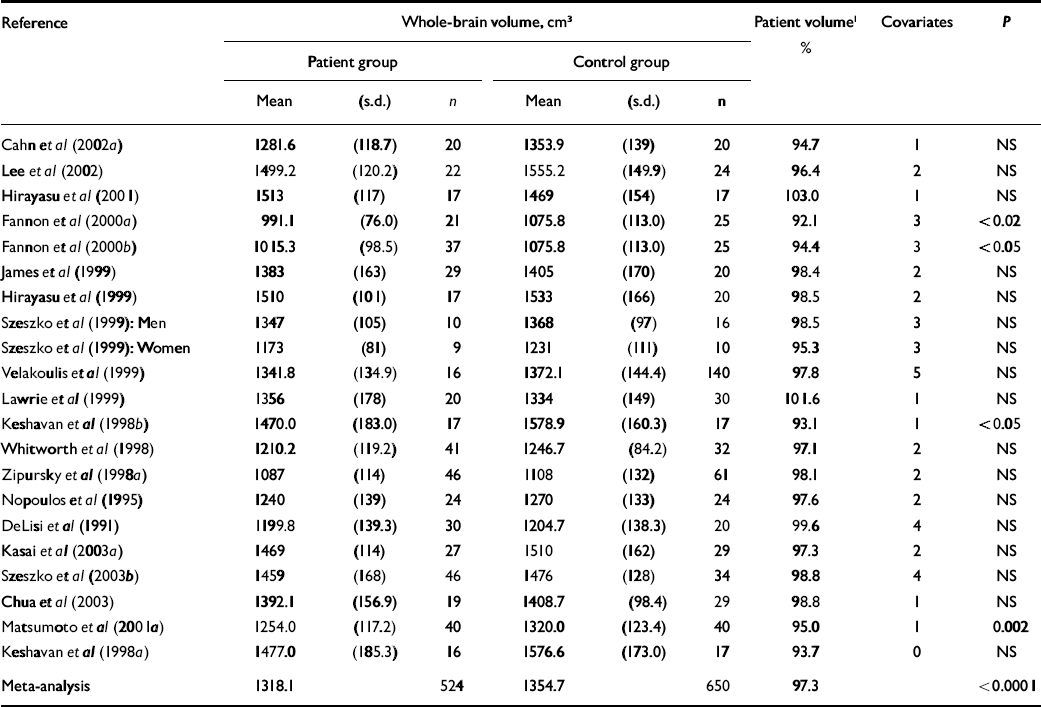
Table 2 Whole-brain volume in cross-sectional studies
| Reference | Whole-brain volume, cm3 | Patient volume1 % | Covariates | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patient group | Control group | ||||||||
| Mean | (s.d.) | n | Mean | (s.d.) | n | ||||
| Cahn et al (Reference Cahn, Hulshoff Pol and Bongers2002a ) | 1281.6 | (118.7) | 20 | 1353.9 | (139) | 20 | 94.7 | 1 | NS |
| Lee et al (Reference Lee, Shenton and Salisbury2002) | 1499.2 | (120.2) | 22 | 1555.2 | (149.9) | 24 | 96.4 | 2 | NS |
| Hirayasu et al (Reference Hirayasu, Tanaka and Shenton2001) | 1513 | (117) | 17 | 1469 | (154) | 17 | 103.0 | 1 | NS |
| Fannon et al (Reference Fannon, Tennakoon and O'Ceallaigh2000a ) | 991.1 | (76.0) | 21 | 1075.8 | (113.0) | 25 | 92.1 | 3 | <0.02 |
| Fannon et al (Reference Fannon, Chitnis and Deku2000b ) | 1015.3 | (98.5) | 37 | 1075.8 | (113.0) | 25 | 94.4 | 3 | <0.05 |
| James et al (Reference James, Crow and Renowden1999) | 1383 | (163) | 29 | 1405 | (170) | 20 | 98.4 | 2 | NS |
| Hirayasu et al (Reference Hirayasu, Shenton and Salisbury1999) | 1510 | (101) | 17 | 1533 | (166) | 20 | 98.5 | 2 | NS |
| Szeszko et al (Reference Szeszko, Bilder and Lenez1999): Men | 1347 | (105) | 10 | 1368 | (97) | 16 | 98.5 | 3 | NS |
| Szeszko et al (Reference Szeszko, Bilder and Lenez1999): Women | 1173 | (81) | 9 | 1231 | (111) | 10 | 95.3 | 3 | NS |
| Velakoulis et al (Reference Velakoulis, Pantelis and McGorry1999) | 1341.8 | (134.9) | 16 | 1372.1 | (144.4) | 140 | 97.8 | 5 | NS |
| Lawrie et al (Reference Lawrie, Whalley and Kestelman1999) | 1356 | (178) | 20 | 1334 | (149) | 30 | 101.6 | 1 | NS |
| Keshavan et al (Reference Keshavan, Rosenberg and Sweeney1998b ) | 1470.0 | (183.0) | 17 | 1578.9 | (160.3) | 17 | 93.1 | 1 | <0.05 |
| Whitworth et al (Reference Whitworth, Honeder and Kremser1998) | 1210.2 | (119.2) | 41 | 1246.7 | (84.2) | 32 | 97.1 | 2 | NS |
| Zipursky et al (Reference Zipursky, Lambe and Kapur1998a ) | 1087 | (114) | 46 | 1108 | (132) | 61 | 98.1 | 2 | NS |
| Nopoulos et al (Reference Nopoulos, Torres and Flaum1995) | 1240 | (139) | 24 | 1270 | (133) | 24 | 97.6 | 2 | NS |
| DeLisi et al (Reference DeLisi, Hoff and Schwartz1991) | 1199.8 | (139.3) | 30 | 1204.7 | (138.3) | 20 | 99.6 | 4 | NS |
| Kasai et al (Reference Kasai, Shenton and Salisbury2003a ) | 1469 | (114) | 27 | 1510 | (162) | 29 | 97.3 | 2 | NS |
| Szeszko et al (Reference Szeszko, Goldberg and Gunduz-Bruce2003b ) | 1459 | (168) | 46 | 1476 | (128) | 34 | 98.8 | 4 | NS |
| Chua et al (Reference Chua, Lam and Tai2003) | 1392.1 | (156.9) | 19 | 1408.7 | (98.4) | 29 | 98.8 | 1 | NS |
| Matsumoto et al (Reference Matsumoto, Simmons and Williams2001a ) | 1254.0 | (117.2) | 40 | 1320.0 | (123.4) | 40 | 95.0 | 1 | 0.002 |
| Keshavan et al (Reference Keshavan, Rosenberg and Sweeney1998a ) | 1477.0 | (185.3) | 16 | 1576.6 | (173.0) | 17 | 93.7 | 0 | NS |
| Meta-analysis | 1318.1 | 524 | 1354.7 | 650 | 97.3 | <0.0001 | |||
Table 3 Hippocampal volume in cross-sectional studies

| Reference1 | Hippocampal volume, mm3 | Patient volume2 % | P | |||||
|---|---|---|---|---|---|---|---|---|
| Patient group | Control group | |||||||
| Mean | (s.d.) | n | Mean | (s.d.) | n | |||
| Left | ||||||||
| Smith et al (Reference Smith, Lang and Kopala2003): Men | 3.01 | (0.42) | 26 | 3.13 | (0.36) | 10 | 96.2 | NS |
| Smith et al (Reference Smith, Lang and Kopala2003): Women | 2.68 | (0.18) | 7 | 2.92 | (0.31) | 9 | 91.8 | NS |
| Sumich et al (Reference Sumich, Chitnis and Fannon2002) | 2.70 | (0.30) | 25 | 3.08 | (0.25) | 16 | 87.7 | 0.007 |
| Laakso et al (Reference Laakso, Tiihonen and Syvalahti2001) | 1.21 | (0.24) | 18 | 1.23 | (0.18) | 22 | 98.4 | NS |
| Niemann et al (Reference Niemann, Hammers and Coenen2000) | 1.85 | (0.32) | 20 | 1.88 | (0.25) | 20 | 98.4 | NS |
| James et al (Reference James, Crow and Renowden1999) | 2.35 | (0.47) | 29 | 2.52 | (0.51) | 20 | 93.3 | NS |
| Velakoulis et al (Reference Velakoulis, Pantelis and McGorry1999) | 2.71 | (0.52) | 16 | 3.05 | (0.37) | 42 | 88.9 | 0.02 |
| Whitworth et al (Reference Whitworth, Honeder and Kremser1998) | 2.45 | (0.38) | 41 | 2.82 | (0.51) | 32 | 86.9 | <0.01 |
| Barr et al (Reference Barr, Ashtari and Bilder1997) | 2.42 | (0.47) | 32 | 2.55 | (0.44) | 42 | 94.9 | <0.001 |
| Szeszko et al (Reference Szeszko, Goldberg and Gunduz-Bruce2003b ) | 3.31 | (0.41) | 46 | 3.56 | (0.43) | 34 | 93.0 | <0.01 |
| Matsumoto et al (Reference Matsumoto, Simmons and Williams2001a ) | 2.45 | (0.49) | 40 | 2.69 | (0.50) | 40 | 91.1 | NS |
| Meta-analysis | 2.46 | 300 | 2.68 | 287 | 91.8 | >0.0001 | ||
| Right | ||||||||
| Smith et al (Reference Smith, Lang and Kopala2003): Men | 3.00 | (0.47) | 26 | 3.19 | (0.36) | 10 | 94.0 | NS |
| Smith et al (Reference Smith, Lang and Kopala2003): Women | 2.86 | (0.39) | 7 | 3.01 | (0.33) | 9 | 95.0 | NS |
| Sumich et al (Reference Sumich, Chitnis and Fannon2002) | 2.74 | (0.38) | 25 | 3.17 | (0.29) | 16 | 86.4 | 0.02 |
| Laakso et al (Reference Laakso, Tiihonen and Syvalahti2001) | 1.22 | (0.23) | 18 | 1.27 | (0.19) | 22 | 96.1 | NS |
| Niemann et al (Reference Niemann, Hammers and Coenen2000) | 2.01 | (0.27) | 20 | 2.10 | (0.34) | 20 | 95.7 | NS |
| James et al (Reference James, Crow and Renowden1999) | 2.40 | (0.45) | 29 | 2.44 | (0.43) | 20 | 98.4 | NS |
| Velakoulis et al (Reference Velakoulis, Pantelis and McGorry1999) | 2.94 | (0.39) | 16 | 3.22 | (0.40) | 42 | 91.3 | NS |
| Whitworth et al (Reference Whitworth, Honeder and Kremser1998) | 2.42 | (0.35) | 41 | 2.75 | (0.66) | 32 | 88.0 | <0.01 |
| Barr et al (Reference Barr, Ashtari and Bilder1997) | 2.21 | (0.40) | 32 | 2.71 | (0.47) | 42 | 81.5 | NS |
| Szeszko et al (Reference Szeszko, Goldberg and Gunduz-Bruce2003b ) | 3.41 | (0.43) | 46 | 3.61 | (0.43) | 34 | 94.5 | NS |
| Matsumoto et al (Reference Matsumoto, Simmons and Williams2001a ) | 2.70 | (0.42) | 40 | 2.80 | (0.51) | 40 | 96.4 | NS |
| Meta-analysis | 2.53 | 300 | 2.76 | 287 | 91.7 | <0.0001 | ||
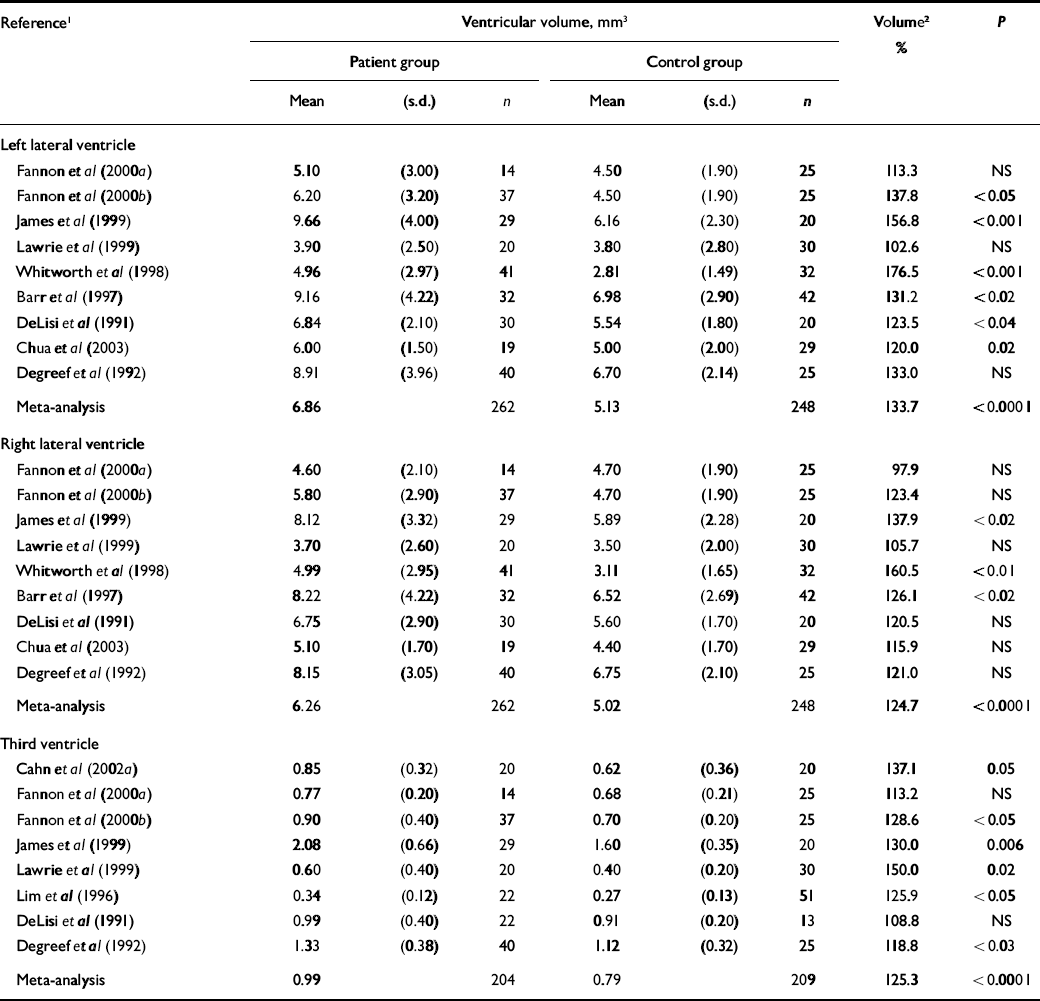
Table 4 Ventricular volume in cross-sectional studies
| Reference1 | Ventricular volume, mm3 | Volume2 % | P | |||||
|---|---|---|---|---|---|---|---|---|
| Patient group | Control group | |||||||
| Mean | (s.d.) | n | Mean | (s.d.) | n | |||
| Left lateral ventricle | ||||||||
| Fannon et al (Reference Fannon, Tennakoon and O'Ceallaigh2000a ) | 5.10 | (3.00) | 14 | 4.50 | (1.90) | 25 | 113.3 | NS |
| Fannon et al (Reference Fannon, Chitnis and Deku2000b ) | 6.20 | (3.20) | 37 | 4.50 | (1.90) | 25 | 137.8 | <0.05 |
| James et al (Reference James, Crow and Renowden1999) | 9.66 | (4.00) | 29 | 6.16 | (2.30) | 20 | 156.8 | <0.001 |
| Lawrie et al (Reference Lawrie, Whalley and Kestelman1999) | 3.90 | (2.50) | 20 | 3.80 | (2.80) | 30 | 102.6 | NS |
| Whitworth et al (Reference Whitworth, Honeder and Kremser1998) | 4.96 | (2.97) | 41 | 2.81 | (1.49) | 32 | 176.5 | <0.001 |
| Barr et al (Reference Barr, Ashtari and Bilder1997) | 9.16 | (4.22) | 32 | 6.98 | (2.90) | 42 | 131.2 | <0.02 |
| DeLisi et al (Reference DeLisi, Hoff and Schwartz1991) | 6.84 | (2.10) | 30 | 5.54 | (1.80) | 20 | 123.5 | <0.04 |
| Chua et al (Reference Chua, Lam and Tai2003) | 6.00 | (1.50) | 19 | 5.00 | (2.00) | 29 | 120.0 | 0.02 |
| Degreef et al (Reference Degreef, Ashtari and Bogerts1992) | 8.91 | (3.96) | 40 | 6.70 | (2.14) | 25 | 133.0 | NS |
| Meta-analysis | 6.86 | 262 | 5.13 | 248 | 133.7 | <0.0001 | ||
| Right lateral ventricle | ||||||||
| Fannon et al (Reference Fannon, Tennakoon and O'Ceallaigh2000a ) | 4.60 | (2.10) | 14 | 4.70 | (1.90) | 25 | 97.9 | NS |
| Fannon et al (Reference Fannon, Chitnis and Deku2000b ) | 5.80 | (2.90) | 37 | 4.70 | (1.90) | 25 | 123.4 | NS |
| James et al (Reference James, Crow and Renowden1999) | 8.12 | (3.32) | 29 | 5.89 | (2.28) | 20 | 137.9 | <0.02 |
| Lawrie et al (Reference Lawrie, Whalley and Kestelman1999) | 3.70 | (2.60) | 20 | 3.50 | (2.00) | 30 | 105.7 | NS |
| Whitworth et al (Reference Whitworth, Honeder and Kremser1998) | 4.99 | (2.95) | 41 | 3.11 | (1.65) | 32 | 160.5 | <0.01 |
| Barr et al (Reference Barr, Ashtari and Bilder1997) | 8.22 | (4.22) | 32 | 6.52 | (2.69) | 42 | 126.1 | <0.02 |
| DeLisi et al (Reference DeLisi, Hoff and Schwartz1991) | 6.75 | (2.90) | 30 | 5.60 | (1.70) | 20 | 120.5 | NS |
| Chua et al (Reference Chua, Lam and Tai2003) | 5.10 | (1.70) | 19 | 4.40 | (1.70) | 29 | 115.9 | NS |
| Degreef et al (Reference Degreef, Ashtari and Bogerts1992) | 8.15 | (3.05) | 40 | 6.75 | (2.10) | 25 | 121.0 | NS |
| Meta-analysis | 6.26 | 262 | 5.02 | 248 | 124.7 | <0.0001 | ||
| Third ventricle | ||||||||
| Cahn et al (Reference Cahn, Hulshoff Pol and Bongers2002a ) | 0.85 | (0.32) | 20 | 0.62 | (0.36) | 20 | 137.1 | 0.05 |
| Fannon et al (Reference Fannon, Tennakoon and O'Ceallaigh2000a ) | 0.77 | (0.20) | 14 | 0.68 | (0.21) | 25 | 113.2 | NS |
| Fannon et al (Reference Fannon, Chitnis and Deku2000b ) | 0.90 | (0.40) | 37 | 0.70 | (0.20) | 25 | 128.6 | <0.05 |
| James et al (Reference James, Crow and Renowden1999) | 2.08 | (0.66) | 29 | 1.60 | (0.35) | 20 | 130.0 | 0.006 |
| Lawrie et al (Reference Lawrie, Whalley and Kestelman1999) | 0.60 | (0.40) | 20 | 0.40 | (0.20) | 30 | 150.0 | 0.02 |
| Lim et al (Reference Lim, Tew and Kushner1996) | 0.34 | (0.12) | 22 | 0.27 | (0.13) | 51 | 125.9 | <0.05 |
| DeLisi et al (Reference DeLisi, Hoff and Schwartz1991) | 0.99 | (0.40) | 22 | 0.91 | (0.20) | 13 | 108.8 | NS |
| Degreef et al (Reference Degreef, Ashtari and Bogerts1992) | 1.33 | (0.38) | 40 | 1.12 | (0.32) | 25 | 118.8 | <0.03 |
| Meta-analysis | 0.99 | 204 | 0.79 | 209 | 125.3 | <0.0001 | ||
RESULTS
The primary PubMed search was able to find 75 of the 91 papers evaluated in this study, or 82% of the relevant references. This suggests that PubMed, although an effective tool, cannot be relied upon to find all relevant references.
A total of 52 studies were included in the cross-sectional analysis (Table 1), these studies involving 1424 patients with first-episode schizophrenia (30.3 patients per study, s.d.=16.5) and 1315 healthy controls (28.0 controls per study, s.d.=10.7). A total of 16 studies were included in the longitudinal analysis, these studies involving 465 patients (33.2 patients per study, s.d.=26.9) and 240 healthy controls (17.1 controls per study, s.d.=8.8). The average age of patients across all studies was 26 years. Even after excluding studies that comprised only male patients (reasoning that a study with a sample composed only of men must have made an effort to exclude women), roughly two-thirds of patients were men, suggesting that males are more common among young patients with first episodes of schizophrenia.
Relatively few brain structures have been evaluated in multiple studies (further information available from the author upon request); of 14 comparisons that showed a significant volumetric decrease in grey matter of patients’ brains, only 6 comparisons were replicated more than three times. Most volumetric studies were small, even when the focus was a structure in which measurement error could be substantial. The total number of patients evaluated per structure, averaged across all 14 central grey matter structures, was 97.6 (s.d.=82.5), but it was only 65.5 (s.d.=47.6) if amygdala, hippocampus and the amygdala– hippocampal complex were excluded. Most volumetric changes that are significant relate to grey matter, and more findings relate to central than to peripheral (cortical) grey matter. Virtually all significant volumetric differences from normal in grey matter are patient deficits in volume, compared with controls.
Cross-sectional studies that measured whole-brain volume deficits in patients with first-episode schizophrenia are summarised in Table 2. For this particular comparison there have been 21 studies, with a large number of participants (patients, n=524; controls, n=650), but only 4 studies found significance. Meta-analysis showed that the average patient brain volume was 2.7% smaller than the average control brain volume (P<0.0001). Group (patient v. control) and study differences together account for 57% of the variation in brain volume, but group differences alone were able to account for less than 1% of the variation in brain volume (P<0.0001). Thus, there was a significant variation in brain volume between studies (P<0.0001), although there was no significant study heterogeneity.
There was variation in the number (and type) of covariates used in the various studies of brain volume, suggesting that it may be problematic to pool studies in a single meta-analysis. Nevertheless, the number of statistical covariates used in analysis did not seem to be related to the level of statistical significance obtained. The 4 studies that found significance had an average of 2.0 covariates, whereas the 17 non-significant studies had an average of 2.2 covariates.
Cross-sectional studies that measured hippocampal volume deficits in patients with first-episode schizophrenia are summarised in Table 3. There have been 10 separate studies of the hippocampus, with total participant numbers of 300 patients and 287 controls. Meta-analysis shows that the volume deficit in patient hippocampus is about 8% on both right and left sides (P<0.0001). This deficit is somewhat larger than the 4% volume deficit reported in a meta-analysis of hippocampal volume in patients with chronic schizophrenia (Reference Nelson, Saykin and FlashmanNelson et al, 1998). Group and study differences together accounted for 64% of the variation in hippocampal volume, but group differences alone were able to account for only 2% of this variation (P<0.0001). Study-related variation in hippocampal volume was significant (P<0.0001), without significant study heterogeneity.
Cross-sectional studies that measured the lateral or third ventricles in patients with first-episode schizophrenia are also summarised (Table 4). There have been 11 studies of ventricular volume, with total participant numbers of 204 patients and 209 controls. Meta-analysis shows that the lateral ventricle volume surplus in patients is about 34% on the left side and 25% on the right side (both P<0.0001). Group and study differences together account for 31% of the variation in ventricular volume on the left side and 26% on the right side (both P<0.0001), with group differences accounting for 6% or less of the variation in ventricular volume (both P<0.0001). For third ventricle measurements, group and study differences together accounted for 68% of the variation in third ventricle volume (P<0.0001), with group differences accounting for 4% of the variation in ventricular volume. Study-related variation in ventricular volume was significant (all P<0.0001), without significant study heterogeneity.
We summarised robustly significant (P≤0.01) findings from longitudinal studies of brain volume change in patients (further information available from the author upon request). This compilation demonstrates that longitudinal studies are generally of recent vintage; of eight studies recorded, five were published within the past 5 years. Several longitudinal changes in the volume of the brain were robustly significant after diagnosis, including a significant decrease in volume of the whole brain after diagnosis. No significant longitudinal change was identified in white matter or cerebellum, so longitudinal changes in whole-brain volume may be limited to the grey matter.
DISCUSSION
Our synthesis of brain volumetric studies suggests that a great deal more work is needed. There are relatively few studies that specifically relate to patients with first episodes of schizophrenia (see Table 1); the existing studies have a rather small sample size and studies that reported a high degree of significance tended to have a smaller sample size than normal. Most significant volumetric findings are not well replicated, and few findings are robustly significant, in either cross-sectional or longitudinal studies. Studies of patients with first episodes tend to be smaller than the average of 33 patients per study reported in a systematic review of 180 studies of patients with (mostly) chronic schizophrenia (Reference Shenton, Dickey and FruminShenton et al, 2001). Thus, the total number of first-episode cases that have been evaluated overall is small, given the complexity of the illness.
Whole-brain volume deficits
Whole-brain volume differences between first-episode cases and controls are apparently quite subtle (Reference Harrison, Freemantle and GeddesHarrison et al, 2003). Cross-sectional studies that measured whole-brain volume reported an average volume deficit in the first-episode group of less than 3% (see Table 2), despite a large sample size. This finding agrees well with a study of brain weight at autopsy, in which 540 older patients with chronic schizophrenia were compared with 794 controls (Reference Harrison, Freemantle and GeddesHarrison et al, 2003). This study found that the brain weight of patients with chronic disorder was 2% less than that of healthy controls (P=0.04), but that disease-related differences were far less significant than brain weight differences attributable to either age or gender (both P<0.0001). No correlation was found between brain weight and the duration of psychosis, which may mean that brain atrophy is not progressive after diagnosis (Reference Harrison, Freemantle and GeddesHarrison et al, 2003).
It is critically important to determine when whole-brain volume deficits in patients with schizophrenia first become significant, as this could have bearing on the aetiology of the disorder (Reference HarrisonHarrison, 1999). If whole-brain volume becomes abnormal early in childhood, this would suggest a neurodevelopmental aetiology; alternatively, if whole-brain volume becomes abnormal shortly before the onset of symptoms – or even after symptoms have developed – this would suggest a neurodegenerative aetiology. Population-based data suggest that head size is abnormal at birth among those who later develop schizophrenia, compared with controls (Reference Ward, Friedman and WiseWard et al, 1996; Reference HarrisonHarrison, 1999). Research in the offspring of people with schizophrenia, in people at high genetic risk of this disorder or in patients in its prodromal phase might help to address this aetiological question.
When does volumetric change occur?
Some brain structures in people with first-episode schizophrenia appear to show a volumetric deficit that is significant at diagnosis and that is also progressive over the later course of illness. For example, the lateral ventricles are significantly larger than normal at diagnosis (Table 4) and ventricular volume tends to increase significantly in longitudinal studies. Volumetric deficits at diagnosis are seen in the hippocampus (Table 3), in cortical grey matter, in Heschl's gyrus, in the planum temporale and in temporal grey matter, and all of these structures also show continued volumetric loss over time (further information available from the author upon request).
Some brain tissues appear to show a volumetric deficit at diagnosis, but the deficit may not progress over time. For example, there are volumetric deficits in the thalamus at diagnosis, according to four studies, but no longitudinal change has yet been described. Similarly, volume deficits in the insula are significant at diagnosis, according to two studies, but no longitudinal change has been described. This may mean that volumetric changes in the thalamus and insula are indeed not progressive, or it may mean that there are simply too few longitudinal studies to identify a progressive volume loss that is actually present in these structures.
Imaging difficulties
There are a great many difficulties in measuring brain volumes of patients with schizophrenia by MRI. A major problem is that the volumetric loss in patients is no more than 4% per year (further information available upon request), which may be close to the limit of detection by MRI, given the precision of volumetric methods (Reference Howard, Roberts and Garcia-FinanaHoward et al, 2003; Reference MacFall, Payne and KrishnanMacFall et al, 2004). A longitudinal study of a volume phantom found that changes of up to 5% could be introduced by changes in scanner hardware or software (Reference MacFall, Payne and KrishnanMacFall et al, 2004). Such ‘machine drift’ can have an impact on volume measurement, as shown by a study of intracranial content in 113 healthy elderly participants (Reference MacFall, Payne and KrishnanMacFall et al, 2004). Although the intracranial content cannot change after the cranial sutures close (Reference Pfefferbaum, Mathalon and SullivanPfefferbaum et al, 1994; Reference Giedd, Blumenthal and JeffriesGiedd et al, 1999), error in its measurement averaged ±1.5% (Reference MacFall, Payne and KrishnanMacFall et al, 2004). This error could be corrected but, in the absence of correction, would confound any longitudinal measurement of brain volume (Reference MacFall, Payne and KrishnanMacFall et al, 2004). In studies that control for intracranial volume, imprecision or inaccuracy may not have a major impact, but poor precision or low accuracy in even a subset of volumetric studies would lead to a lack of consensus among the various studies.
Imprecision or inaccuracy in the measurement of brain volume can arise in many ways. Perhaps the most likely source of error is voxel misclassification during brain segmentation (Reference Wang and DoddrellWang & Doddrell, 2002). Voxels classified as one tissue type could, with a relatively minor change in tissue T 1 or T 2, be classified as another tissue type (Reference Steen, Ogg and ReddickSteen et al, 1997). A second major issue is the familiar partial volume problem; since several tissues can occur in a volume much smaller than a typical imaging voxel, this would introduce error into the volume estimate of any tissue type (Reference Tofts, Barker and FilippiTofts et al, 1997; Reference Ballester, Zisserman and BradyBallester et al, 2000; Reference Wang and DoddrellWang & Doddrell, 2002), and could potentially change the proportional allocation of tissue to tissue type. A third problem is the inconspicuousness of tissue edges; this type of error is really another type of partial volume error that would primarily affect the estimate of grey matter volume, since this often has poorly defined edges with cerebrospinal fluid. Error in the measurement of grey matter volume would change the estimate of total brain volume, so controlling for brain volume would not necessarily eliminate ‘machine drift’ in a longitudinal study. A fourth issue is head tilt, or angulation of the imaging slab over the brain, since different volumes of brain may be interrogated in different imaging examinations. This problem can only be overcome by striving for full brain coverage during an examination. Finally, non-systematic non-systematic errors (mistakes) can be made during the complex analytic process that is required for MRI volumetry (Reference Haller, Banerjee and ChristensenHaller et al, 1997). In short, because error can be substantial and because brain volumetric changes from normal in patients with first-episode schizophrenia appear to be quite small, some of the differences reported between patients and controls (Tables 2, 3, 4) are probably artefactual.
Clinical difficulties
A great many clinical difficulties complicate a volumetric search for the causes of schizophrenia. An enormous problem is that patients are typically treated with antipsychotic medications as soon as possible after diagnosis. Different patients may receive different medications at different dosages, and such treatment heterogeneity is almost impossible to eliminate. This makes it essential to determine whether there are acute effects of medication on total brain volume (Reference DeLisi, Hoff and SchwartzDeLisi et al, 1991; Reference Chakos, Lieberman and BilderChakos et al, 1994; Reference Gur, Cowell and TuretskyGur et al, 1998). If brain volumetric changes in response to medication are rapid, then the length of time between first medication and imaging evaluation could be a major confounder. Antipsychotic medication has been postulated to have an effect on basal ganglia volume in as little as 6 months (Reference Chakos, Lieberman and BilderChakos et al, 1994), and it is possible that brain volumetric change in response to medication occurs even more rapidly. A further difficulty inherent to studying first-episode cases is that some patients may have been symptomatic, but undiagnosed, for a long time. If progressive brain volume changes are rapid in the period surrounding diagnosis, then the duration of undiagnosed illness would be a serious confounder. However, since no consistent relationship has been found between duration of illness and brain volume loss (Reference Harrison, Freemantle and GeddesHarrison et al, 2003), this may be less likely.
Recruiting patients with schizophrenia can be time-consuming, difficult and expensive, since many are unable or unwilling to comply with study requirements. Another problem is that brain structure may be weakly correlated with brain function, so that substantial variation in brain volume could be found in the absence of any variation in brain function (Reference UttalUttal, 2001). These two problems together probably account for why so many studies of brain volume appear to be underpowered (Table 2). Many studies lack a sample size sufficient to test hypotheses that relate to what may be an inherently weak relationship, especially given the limitations of the methods (Reference Haller, Banerjee and ChristensenHaller et al, 1997; Reference Howard, Roberts and Garcia-FinanaHoward et al, 2003; Reference MacFall, Payne and KrishnanMacFall et al, 2004). To complicate the picture further, there may be genetic heterogeneity within the diagnosis, such that patients in a single study might actually have different diseases that converge in causing psychotic symptoms.
Concluding remarks
The most robust volumetric findings in patients with schizophrenia are those of grey matter volume loss (Table 3) and ventricular volume increase (Table 4), and these findings are probably linked. In monozygotic twins discordant for schizophrenia, there is a correlation between reduced left temporal grey matter volume and increased volume of cerebrospinal fluid in the left temporal horn, suggesting that loss of grey matter leads to an increase in ventricular volume (Reference Suddath, Casanova and GoldbergSuddath et al, 1989). Many more longitudinal studies of brain volume change in patients with first psychotic episodes are needed to determine which tissues are prone to the atrophy that manifests as ventricular volume increase.
This review confirms that grey matter deficits are present in patients with first-episode psychosis (Reference Hulshoff-Pol, Schnack and MandlHulshoff-Pol et al, 2001), whereas white matter changes have seldom been described (Reference Sanfilipo, Lafargue and RusinekSanfilipo et al, 2000; Reference Hulshoff-Pol, Brans and van HarenHulshoff-Pol et al, 2004). Yet it is still not known whether changes in grey matter volume at first episode are associated with disease progression itself or with the many correlates of disease, including antipsychotic medication, alcoholism, drug misuse, malnutrition or even social deprivation. Both alcoholism (Reference JoyceJoyce, 1996) and malnutrition (Reference Swayze, Anderson and ArndtSwayze et al, 1996) are associated with acutely reversible changes in brain volume. Such volumetric changes are postulated to result from changes in brain water content, secondary to systemic hydration or serum protein content (Reference JoyceJoyce, 1996; Reference Swayze, Anderson and ArndtSwayze et al, 1996). Similar hydration mechanisms could be important in schizophrenia, since many patients suffer from malnutrition, dehydration and exposure (Reference Shenton, Dickey and FruminShenton et al, 2001), so it is important to control for such environmental effects in studies.
It remains to be determined whether schizophrenia is a neurodegenerative process that begins at about the time of symptom onset and manifests as progressive volumetric loss thereafter, or whether it is better characterised as a neurodevelopmental process that results in abnormal brain volume beginning at an early age (Reference Maynard, Sikich and LiebermanMaynard et al, 2001).
Acknowledgements
R.G.S. is supported by the National Alliance for Research on Schizophrenia and Depression as a Hofmann Trust Investigator. Our research was also supported by MH61603 (J.A.L.), the University of North Carolina at Chapel Hill Schizophrenia Research Center, the National Institute of Mental Health Silvio Conte Center for the Neuroscience of Mental Disorders (MH164065) and the Foundation of Hope.







eLetters
No eLetters have been published for this article.