Children with attention-deficit hyperactivity disorder (ADHD) are impaired in a well-validated test of response inhibition known as the ‘stop’ signal task. Reference Oosterlaan, Logan and Sergeant1,Reference Lijffijt, Kenemans, Verbaten and van Engeland2 In an extension of the stop task, the ‘change’ task, the participant must inhibit a prepotent response tendency and quickly shift to another response, i.e. response re-engagement. Reference Oosterlaan and Sergeant3 Thus, the executive function demands of the change task are greater than the stop task, and the stop signal reaction time (SSRT) and change response reaction time (CRRT) indices are considered sensitive measures of inhibitory control and response re-engagement respectively. Reference Oosterlaan and Sergeant3 It has been proposed that abnormalities in a prefrontal-striatal-posterior parietal ‘attention system’ of the brain Reference Berger and Posner4 account for this and other core symptoms of ADHD. However, in a recent large scale, longitudinal analysis detailing cortical thickness in ADHD, Shaw et al Reference Shaw, Eckstrand, Sharp, Blumenthal, Lerch, Greenstein, Clasen, Evans, Giedd and Rapoport5 provided evidence that the pattern of brain maturation in ADHD was delayed, rather than abnormal. In the present study we used voxel-based structural magnetic resonance imaging (MRI) analyses to test a predicted link between SSRT and CRRT and fronto-striatal volumes in children with ADHD. Since symptoms of impulse control improve with age in ADHD Reference Biederman, Mick and Faraone6 and the stop signal reaction task is known to be sensitive to developmental changes in performance, Reference Carver, Livesey and Charles7 we also expected that delayed maturation in ADHD would be reflected by faster reaction times and larger regional brain volumes in older children.
Method
Participants
The study was approved by the local hospital institutional review board. Participants were 51 male Hong Kong Chinese children (22 with ADHD and 29 typically developing) aged between 6 and 12 years who had been recruited for a voxel-based group comparison MRI study published previously. Reference McAlonan, Cheung, Cheung, Chua, Murphy, Suckling, Tai, Yip, Leung and Ho8 All the children attended local mainstream schools and had a verbal IQ above 80 (estimated on the verbal subset of the Weschler Intelligence Scale for Children, (WISC–III)) Reference Wechsler9 (Table 1). Diagnostic status was confirmed for all participants using the parental Chinese Diagnostic Interview Schedule for Children for DSM–IV. Reference Ho, Leung, Lee, Tang, Hung, Kwong, Lucas, Lieh-Mak and Shaffer10 No child had a significant medical condition affecting brain function (e.g. epilepsy) or history of head injury. All but three (newly diagnosed) children with ADHD were considered by their case doctors to be responsive to methylphenidate.
Table 1 Group statistics
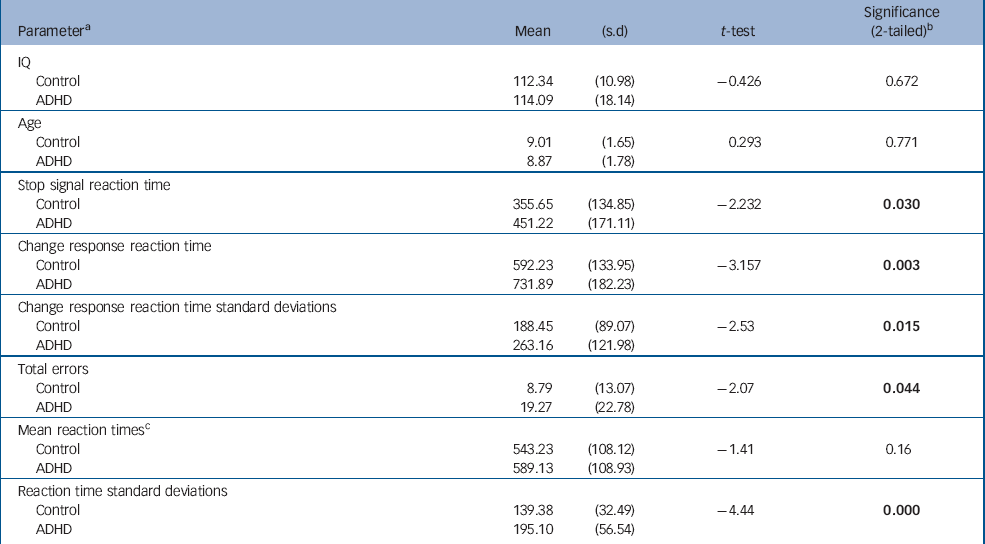
| Parameter a | Mean | (s.d) | t-test | Significance (2-tailed) b |
|---|---|---|---|---|
| IQ | ||||
| Control | 112.34 | (10.98) | -0.426 | 0.672 |
| ADHD | 114.09 | (18.14) | ||
| Age | ||||
| Control | 9.01 | (1.65) | 0.293 | 0.771 |
| ADHD | 8.87 | (1.78) | ||
| Stop signal reaction time | ||||
| Control | 355.65 | (134.85) | -2.232 | 0.030 |
| ADHD | 451.22 | (171.11) | ||
| Change response reaction time | ||||
| Control | 592.23 | (133.95) | -3.157 | 0.003 |
| ADHD | 731.89 | (182.23) | ||
| Change response reaction time standard deviations | ||||
| Control | 188.45 | (89.07) | -2.53 | 0.015 |
| ADHD | 263.16 | (121.98) | ||
| Total errors | ||||
| Control | 8.79 | (13.07) | -2.07 | 0.044 |
| ADHD | 19.27 | (22.78) | ||
| Mean reaction times c | ||||
| Control | 543.23 | (108.12) | -1.41 | 0.16 |
| ADHD | 589.13 | (108.93) | ||
| Reaction time standard deviations | ||||
| Control | 139.38 | (32.49) | -4.44 | 0.000 |
| ADHD | 195.10 | (56.54) |
ADHD, attention-deficit hyperactivity disorder
a. Control, n=29; ADHD, n=22
b. Significant group differences are shown in bold
c. Mean reaction times, reaction times on go trials
Cognitive testing
Children taking methylphenidate were asked to stop taking their medication 48 h prior to testing. Participants were tested on an extended version of the stop task – the change task. Reference Oosterlaan and Sergeant3 In the change task, the stop signal prompts both response inhibition and re-engagement which can be measured by two performance indices (i.e. SSRT and CRRT). In brief, 75% of trials in the task were ‘go’ trials; participants had to locate an aircraft presented on the left or right of a computer screen and use their left hand to press the corresponding left or right response button with middle or index fingers respectively. Twenty-five per cent of trials were stop trials presented pseudo-randomly. In response to an auditory signal in stop trials, participants had to inhibit their response and immediately press a third button with their right thumb (the change response). A lengthened SSRT is thought to reflect impaired inhibitory control and is derived from the ‘inhibition function’, generated by plotting the probability of inhibition against the range of stop signal intervals and correcting for non-responses, as previously reported. Reference Tannock, Schachar, Carr, Chajczyk and Logan11 The rationale behind this correction is that non-responses may occur on stop trials, thereby increasing the probability of inhibition. The CRRT is the time taken to shift to a new response using the right thumb. A lengthened reaction time suggests inefficient response re-engagement, i.e. difficulty shifting to a new response.
We used independent t-tests in SPSS (version 15.0 for Windows) to examine group differences in SSRT and CRRT. In addition, although the groups were balanced for age, we also examined the relationship of age to reaction time indices for each group separately in bivariate correlation analyses. Between-group differences in age-related performance were investigated by transforming Pearson r's into Fisher z-scores to test the significance of the difference between correlations. Reference Pallant12
MRI acquisition and analyses
Three millimetre slice thickness, dual-echo fast spin echo data-sets aligned to the anterior–posterior commissural (AC–PC) line were acquired across the whole brain on a GE signa 1.5 T system (General Electric, Milwaukee, Wisconsin, USA). Pre-processing of the images followed methods previously described in a group comparison study which included the majority of these children. Reference Biederman, Mick and Faraone6 That is, images were segmented by setting voxels representing extracerebral tissue to zero and probability of each intracerebral voxel belonging to grey matter, white matter, cerebrospinal fluid or dura/vasculature tissue classes was calculated. Knowing the voxel size (2.2 mm3), the volume of any tissue class could be estimated at each voxel and summed across all intracerebral voxels to yield global tissue class volumes. The segmented grey matter images were mapped onto standard space by minimising the sum of square intensity difference of each proton density image to a group-specific template Reference Biederman, Mick and Faraone6 and smoothed with a 4.4 mm kernel. Simple linear regression of reaction time indices with grey matter volume at each intracerebral voxel was carried out using BAMM software (Brain Analysis Morphological Mapping version 2.5, Cambridge University) for each group separately. Regions of significant correlation were identified in two stages by permutation test. Initially, a voxel-wise probabilistic threshold was applied to generate three-dimensional clusters characterised by their mass, or the sum of suprathreshold voxel statistics it comprised. Clusters were then subject to a non-parametric analysis by randomly generating 10 permutated maps to sample the null hypothesis that significant correlations occur by chance. The statistical thresholds were corrected for multiple comparisons by controlling the ‘family wise error rate’ expected such that the number of false positive tests for each map was less than 1 false positive cluster. We also examined the bivariate correlations of age with the brain volumes derived from the voxel-based analysis in each group using SPSS 15.0 for Windows.
Results
Change task performance
Children with ADHD made more errors than children in the control group. Despite having similar reaction times to controls on go trials, their reaction times were more variable, as indicated by their significantly greater reaction time standard deviations. We also confirmed a significantly slower SSRT and CRRT in this sample of children with ADHD compared with typically developing controls (Table 1). Older children with ADHD had faster reaction times than younger children (SSRT: age r=–0.45, P=0.04; CRRT: age r=–0.56, P=0.007). As shown in Fig. 1, children with ADHD had similar reaction times to controls at a later age, and tended to ‘catch-up’ with controls by 12 years. Age did not strongly correlate with SSRT in the control group (r=–0.33, P=0.11); the correlation of age with CRRT in the control group just failed to reach significance (r=–0.39, P=0.054). However, the group difference between the correlation coefficients for SSRT or CRRT was not significant (z obs=0.45 and z obs=0.80 respectively).
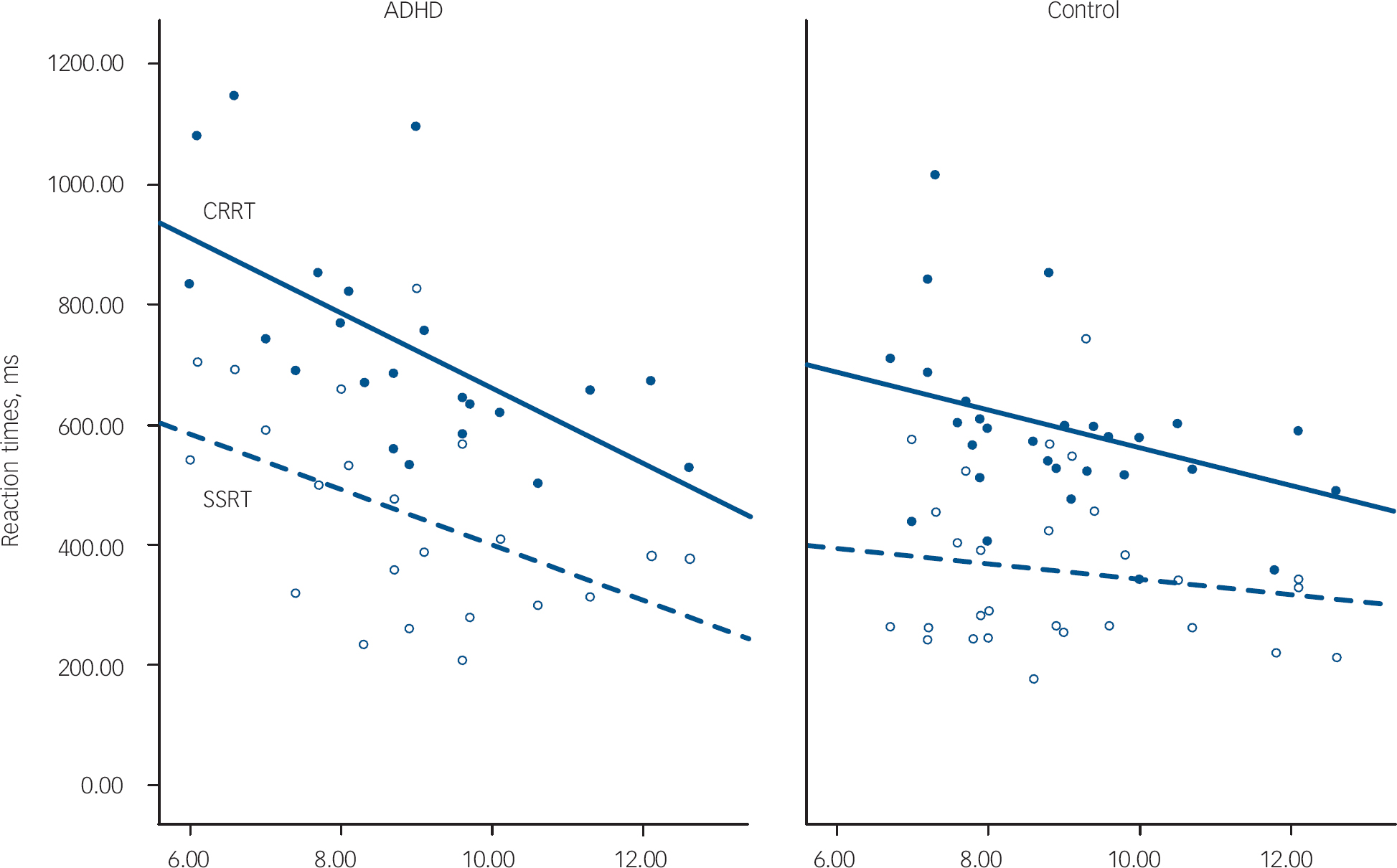
Fig. 1 Age-related changes to reaction times. ADHD, attention-deficit hyperactivity disorder; CRRT, change response reaction time in closed circles and solid line; SSRT, stop signal reaction time in open circles and dashed line.
MRI reaction time correlates
In the ADHD group, two scans with movement artifact were excluded. In the control group, four children refused a scan; one scan with movement artifact was excluded. Therefore, scans from 20 children with ADHD and 24 controls entered analyses.
Grey matter correlates of SSRT
Attention-deficit hyperactivity disorder
There was a significant negative correlation between SSRT and the volume of grey matter clusters in the anterior cingulate, right lentiform nucleus and the left medial temporal lobe (involving amygdala, hippocampus and parahippocampal regions) in children with ADHD (false positive clusters<1, cluster test significance P=0.001; Fig. 1 and Table 2). Thus, better/faster inhibition was associated with greater grey matter volumes in these regions in those with ADHD. Grey matter regions correlated with SSRT showed significant positive intercorrelations (Table 2). Eighty-nine per cent of the variance in SSRT was jointly explained by these volumes (r=–0.94, P<0.001, R2=0.887). Age was significantly positively correlated with regional grey matter volumes, but not total grey matter volumes. Thus older children, who had larger regional brain volumes in temporal–pallidal–anterior cingulate, had faster SSRT (Fig. 2).

Fig. 2 Regional brain volume correlates of reaction time indices. (a) Stop signal reaction time correlates in attention-deficit hyperactivity disorder (ADHD); (b) change response reaction time (CRRT) correlates in ADHD; (c) CRRT correlates in controls. Blue clusters: negative correlation (false positive clusters<1, P=0.001). Left side of brain is on the right of the panel (z-coordinates shown).
Table 2 Correlates of reaction times in participants with attention-deficit hyperactivity disorder a
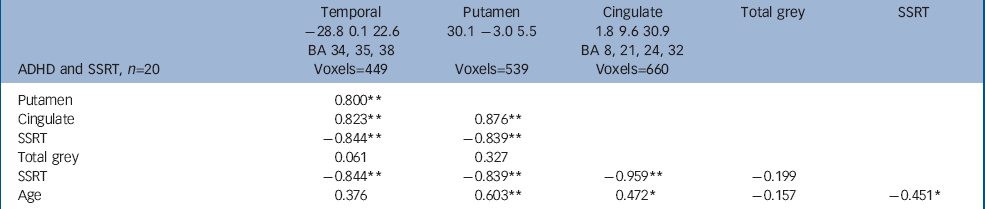
| ADHD and SSRT, n=20 | Temporal -28.8 0.1 22.6 BA 34, 35, 38 Voxels=449 | Putamen 30.1 -3.0 5.5 Voxels=539 | Cingulate 1.8 9.6 30.9 BA 8, 21, 24, 32 Voxels=660 | Total grey | SSRT |
|---|---|---|---|---|---|
| Putamen | 0.800 ** | ||||
| Cingulate | 0.823 ** | 0.876 ** | |||
| SSRT | -0.844 ** | -0.839 ** | |||
| Total grey | 0.061 | 0.327 | |||
| SSRT | -0.844 ** | -0.839 ** | -0.959 ** | -0.199 | |
| Age | 0.376 | 0.603 ** | 0.472 * | -0.157 | -0.451 * |
ADHD, attention-deficit hyperactivity disorder; BA, Brodmann area; SSRT, stop signal reaction time
a. A sample Talaraich coordinate (x, y, z) is given for the approximate centre of each cluster. Labels including BAs are for guidance; clusters are not confined to these areas, nor are they all encompassing
* P < 0.05
** P < 0.01 (2-tailed)
Controls
No regional volumes were correlated with SSRT in the control group. Age was not correlated with SSRT in the control group (r=–0.33, P=0.11).
Grey matter correlates of CRRT
Attention-deficit hyperactivity disorder
Negative correlations between regional brain volumes and CRRT in the ADHD group involved the right lentiform nucleus and left cerebellum (false positive clusters<1, cluster test significance P=0.001; Fig. 2 and Table 3). Again, larger regional volumes were linked with faster reaction times (Table 2). The volume of the cerebellar cluster and basal ganglia clusters correlated with CRRT also showed a significant positive intercorrelation (Table 3). Approximately 75% of the variance in CRRT was jointly explained by these volumes (r=–0.87, P<0.001, R2=0.75). Age was significantly correlated with these volumes, but not total grey matter volume as shown in Table 2. Thus, older children with larger regional brain volumes in basal ganglia and cerebellum had faster CRRT (Fig. 2).
Table 3 Correlates of reaction times in participants with attention-deficit hyperactivity disorder a

| ADHD and CRRT, n=20 | Cerebellum -34.1 -62.5 -22.1 Voxels=723 | Striatum 28.0 -4.9 6.0 Voxels=460 | Total grey | CRRT |
|---|---|---|---|---|
| Striatum | 0.682 ** | |||
| Total grey | 0.238 | 0.227 | ||
| CRRT | -0.815 ** | -0.798 ** | -0.131 | |
| Age | 0.498 * | 0.715 ** | -0.157 | -0.587 ** |
ADHD, attention-deficit hyperactivity disorder; BA, Brodmann area; SSRT, stop signal reaction time; CCRT, change response reaction time
a. A sample Talaraich coordinate (x, y, z) is given for the approximate centre of each cluster. Labels including BAs are for guidance; clusters are not confined to these areas, nor are they all encompassing
* P < 0.05
** P < 0.01 (2-tailed)
Controls
The volume of grey matter clusters in ventral prefrontal cortex, right medial temporal lobe and cerebellum (midline and left hemisphere) was negatively correlated with CRRT (false positive clusters<1, cluster test significance P=0.001; Fig. 2 and Table 4). Thus, larger regional volumes were linked with faster reaction times. These grey matter volumes showed significant positive intercorrelations (Table 4). Seventy-seven per cent of the variance in CRRT in controls was jointly explained by these volumes (r=–0.88, P<0.001, R2=0.77). Age did not correlate with regional brain volumes.
Table 4 Correlates of change response reaction times in controls a
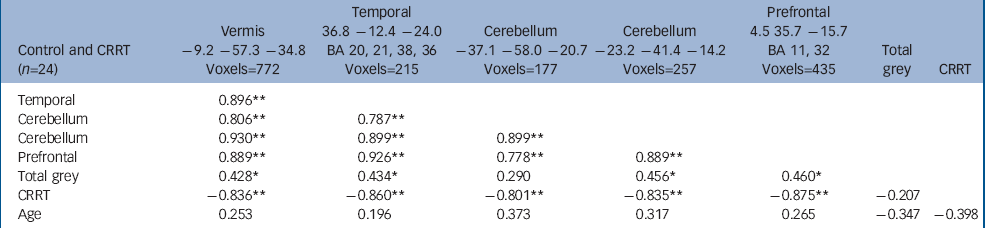
| Control and CRRT (n=24) | Vermis -9.2 -57.3 -34.8 Voxels=772 | Temporal 36.8 -12.4 -24.0 BA 20, 21, 38, 36 Voxels=215 | Cerebellum -37.1 -58.0 -20.7 Voxels=177 | Cerebellum -23.2 -41.4 -14.2 Voxels=257 | Prefrontal 4.5 35.7 -15.7 BA 11, 32 Voxels=435 | Total grey | CRRT |
|---|---|---|---|---|---|---|---|
| Temporal | 0.896 ** | ||||||
| Cerebellum | 0.806 ** | 0.787 ** | |||||
| Cerebellum | 0.930 ** | 0.899 ** | 0.899 ** | ||||
| Prefrontal | 0.889 ** | 0.926 ** | 0.778 ** | 0.889 ** | |||
| Total grey | 0.428 * | 0.434 * | 0.290 | 0.456 * | 0.460 * | ||
| CRRT | -0.836 ** | -0.860 ** | -0.801 ** | -0.835 ** | -0.875 ** | -0.207 | |
| Age | 0.253 | 0.196 | 0.373 | 0.317 | 0.265 | -0.347 | -0.398 |
BA, Brodmann area; CCRT, change response reaction time
a. A sample Talaraich coordinate (x, y, z) is given for the approximate centre of each cluster. Labels including Brodman Areas (BA) are for guidance; clusters are not confined to these areas, nor are they all encompassing
* P < 0.05
** P < 0.01 (2-tailed)
Supplementary analysis
To control for the confounding effect of age on regional brain volume we ran a partial correlation analysis on regional volumes and reaction times with age controlled. For all children, controls and those with ADHD, the highly significant correlation between regional brain volume and reaction time was preserved. The minimum Pearson r=0.53, P<0.01.
Discussion
Consistent with previous reports, Reference Oosterlaan, Logan and Sergeant1,Reference Lijffijt, Kenemans, Verbaten and van Engeland2 the ADHD group performed more poorly than age and IQ-matched typically developing controls in the change task. They had difficulty inhibiting a prepotent response (longer SSRTs) and took longer to shift to a new response (CRRT). These reaction times were very highly correlated with fronto-striatal-temporal volumes. The volumes increased with age, and older children with ADHD had faster reaction times than younger children. Specifically, more efficient inhibitory control was linked to larger regional grey matter volumes in bilateral anterior cingulate, right basal ganglia and left medial temporal circuitry in ADHD. Faster response re-engagement was linked to larger regional grey matter volumes in striatum and the left cerebellum. In the control group SSRT was not linked to regional grey matter volumes. Faster CRRT was associated with greater volumes in ventral prefrontal cortex, right medial temporal lobe and cerebellar regions. Although reaction time indices did tend to improve with age in the control group, the size of brain regions correlated with these indices did not increase with age.
Taken together, these observations suggest an important age-related improvement in reaction time indices in ADHD parallel to an age-related increase volume across a specific striatal grey matter circuitry. This is consistent with recent evidence that the brain, especially the frontal lobe, matures late in ADHD. Reference Shaw, Eckstrand, Sharp, Blumenthal, Lerch, Greenstein, Clasen, Evans, Giedd and Rapoport5 It is important to note that the present study was not designed to investigate brain volume abnormalities in ADHD, only volume-reaction time correlates. However, in our previous study of brain structure in ADHD, which incorporated many of the same children, we did find significantly lower volumes in striatal regions in children with ADHD that overlapped with the regions reported to be linked to reaction times here. Reference McAlonan, Cheung, Cheung, Chua, Murphy, Suckling, Tai, Yip, Leung and Ho8 In addition, others have reported that the dorsal anterior cingulate, right lentiform nucleus, Reference Overmeyer, Bullmore, Suckling, Simmons, Williams, Santosh and Taylor13–Reference Seidman, Valera, Makris, Monuteaux, Boriel, Kelkar, Kennedy, Caviness, Bush, Aleaedi, Faraone and Bierderman15 medial temporal lobe Reference Sowell, Thompson, Welcome, Henkenius, Toga and Peterson16 and cerebellar regions Reference Mackie, Shaw, Lenroot, Pierson, Greenstein, Nugent, Sharp, Giedd and Rapoport17 are smaller in ADHD, and the dorsal anterior cingulate has also been consistently shown to have activation deficits during functional imaging studies of the stop task. Reference Bush, Valera and Seidman18–Reference Dickstein, Bannon, Castellanos and Milham22
Associations between right-sided frontostriatal volumes and response inhibition have previously been reported in a region-of-interest study of ADHD. Reference Casey, Castellanos, Giedd, Marsh, Hamburger, Schubert, Vauss, Vaituzis, Dickstein, Sarfatti and Rapoport23 In their study, Casey et al Reference Casey, Castellanos, Giedd, Marsh, Hamburger, Schubert, Vauss, Vaituzis, Dickstein, Sarfatti and Rapoport23 investigated three different response inhibition tasks. However, only one component of the range of attention tasks examined is in some way comparable to the SSRT measure of inhibition examined here, namely the reaction time in go/no-go ‘response execution’ inhibitory trials. Interestingly, Casey's group found a significant positive correlation between left globus pallidus volume and mean reaction time in control boys, but not boys with ADHD. Reference Casey, Castellanos, Giedd, Marsh, Hamburger, Schubert, Vauss, Vaituzis, Dickstein, Sarfatti and Rapoport23 In contrast, in our whole brain grey matter voxel-wise analyses we found the SSRT was very strongly correlated with a prefrontal-temporal-right pallidal circuit in boys with ADHD and not controls. Thus task differences, as well as very different approaches to analysis, may contribute to the discrepancy between the studies.
Our findings of bilateral frontal correlations of impaired inhibitory control in ADHD deviate from those of an elegant series of studies comparing inhibitory dysfunction after right frontal lesions to dysfunction in ADHD. Reference Aron, Dowson, Sahakian and Robbins24–Reference Clark, Blackwell, Aron, Turner, Dowson, Robbins and Sahakian26 These authors emphasised right lateralised frontal involvement in this function. However, both studies implicated the right striatum. Disentangling the direct effects of a lesion from the indirect or compensatory actions of intact brain structures is a challenge which can complicate interpretation of lesion studies. The voxel-based approach adopted here has the advantage of exploring potential whole brain grey matter correlates of inhibitory control in ADHD directly, and may explain why our results do not completely coincide with lesion studies.
The choice of the change task rather than the stop signal task, meant that additional demands upon executive function, in terms of response re-engagement, could be addressed. We found the volume of the right basal ganglia linked to both inhibition and response shifting ability in ADHD. The demands of response re-engagement were also correlated with left cerebellar volume in ADHD. In the control group, this reaction time index was strongly correlated with cerebellar volumes. The implication, that the cerebellum is important for response shifting, fits growing recognition of its interaction with the prefrontal lobe to affect higher order cognitive processing. Reference Middleton and Strick27,Reference Middleton and Strick28 Indeed CRRT in the control group was also correlated with volumes in the ventral prefrontal cortex.
We expected to find prefrontal cortex volumes correlated with CRRT in the ADHD group. Therefore, since the variance in CRRT accounted for jointly by the volumes of clusters in the right basal ganglia and left cerebellum was modest at 75%, we relaxed the statistical thresholding to allow <2 false positive clusters at P<0.002. In this analysis we found significant clusters in anterior cingulate and right medial temporal lobe were also associated with time taken to shift response. When subsequently added, these volumes together explained approximately 83% of the variance in CRRT (r=–0.91, P<0.001, R2=0.83). Thus, the correlates of inhibition and choice reaction times appear to involve a similar neural system in ADHD. Interestingly, studies of other populations with neurodevelopmental difficulties agree that a selective network incorporating the cerebellum, Reference Nosarti, Rubia, Smith, Frearson, Williams, Rifkin and Murray29 right basal ganglia Reference Nosarti, Rubia, Smith, Frearson, Williams, Rifkin and Murray29,Reference Vink, Ramsey, Raemaekers and Kahn30 and cingulate Reference Nosarti, Rubia, Smith, Frearson, Williams, Rifkin and Murray29,Reference Gothelf, Hoeft, Hinard, Hallmayer, Stoecker, Antonarakis, Morris and Reiss31 appears important for inhibitory processes.
The present study implicates the medial temporal lobe in inhibitory control in ADHD. Disinhibition may theoretically arise from disruption to a motivational limbic-based system. Reference Nigg32,Reference Slusarek, Velling, Bunk and Eggers33 In this conceptualisation, motivational anomalies in children with ADHD result in inappropriate behaviour. Animal models support a key role for hippocampal areas in a ‘behavioural inhibition system’ which normally interrupts ongoing activity when an expected reward is not evident, or when a signal for punishment is detected. Reference Gray34 This model has been applied to children with ADHD Reference Quay35 who appear to have an altered sensitivity to reward contingencies. Reference Tripp and Alsop36,Reference Luman, Oosterlaan and Sergeant37 Thus, our finding that inhibitory control in children with ADHD is associated with the volume of both frontal (anterior cingulate) grey matter and the amygdala/hippocampal complex is exciting. It fits with recent evidence that the anterior cingulate in humans is essential for integrating information about reward and directing decision-making. Reference Williams, Bush, Rauch, Cosgrove and Eskandar38,Reference Bush, Luu and Posner39 Of note, unlike for the striatum and cingulate, age did not strongly correlate with temporal volumes in the ADHD group. This may point to some rather more fixed impact of temporal lobe structure. It will be interesting to manipulate reward contingencies in future studies to explore how motivational variables might have contributed to the present result.
Age-related changes in grey matter in ADHD were not global and only regional, not total, grey matter volumes showed significant age-related increase. Thus, there appeared to be a dissociation of global grey matter volumes and the striatal network implicated here in ADHD. We speculate that this may have something to do with an altered pattern of maturation in ADHD. Indeed, the regional brain volumes implicated in reaction timing in ADHD did not correlate with total grey matter volumes. In contrast, regional grey matter volumes associated with CRRT in the control group did generally correlate with total grey matter volume. We postulate that a delay in grey matter maturation in a restricted striatal network in ADHD might render this circuit ‘out-of-step’ with overall whole brain development. We identified strong positive volumetric correlations between prefrontal cortex, basal ganglia and medial temporal lobe linked to reaction time indices in ADHD. Such intercorrelations are thought to reflect connectivity Reference McAlonan, Cheung, Cheung, Suckling, Lam, Tai, Yip, Murphy and Chua40 as interconnecting systems share common developmental and maturation influences. The present anatomical pattern is consistent with known direct projections between the anterior cingulate and amygdala/hippocampus and indirect connections via the basal ganglia. Reference Barbas41 Moreover, a genetic ‘dopamine-deficit’ in this mesolimbic-cortical network, especially the D4-rich frontal lobes, has been postulated in ADHD. Reference Swanson, Flodman, Kennedy, Spence, Moyzis, Schuck, Murias, Moriarity, Barr, Smith and Posner42 Taken together, the evidence suggests that executive dysfunction in ADHD depends upon maturation of a restricted dopaminergic frontostriatal network.
Limitations
Our study has a number of limitations. We documented the relationship between functional indices, brain volume and age in children less than 12 years old, i.e. likely to be prepubertal. Cortical grey matter volumes begin to decrease from the age of 12 years, with prefrontal lobe volume reduction happening last in sequence. Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans and Rapoport43 Thus, it will be important to establish how neuropsychological function and brain morphology are affected by ADHD in an older age group. Our study was cross-sectional, therefore we can only comment on age-related findings. Clearly further longitudinal studies are needed to properly address the issue of brain maturation delay in ADHD. This would be particularly important in those few individuals with ADHD in whom regional brain volumes were markedly lower and reaction times longer than children of a similar age. It is possible that deviations from the age-dependent pattern observed here have implications for prognosis.
With the exception of three newly diagnosed children, the participants in our study were considered to be responsive to stimulant medication. Therefore we cannot say whether the results apply to children with ADHD who do not respond to medication. Moreover, we did not have detailed information about the treatment protocol followed by these boys, so we cannot be certain what effect drug treatment had on the results. Although medication does not appear to grossly alter brain structure in ADHD, Reference Castellanos, Lee, Sharp, Jeffries, Greenstein, Clasen, Blumenthal, James, Ebens, Walter, Zijdenbos, Evans, Giedd and Rapoport44 evidence from a recent positron emission tomography study suggests that the degree of inattention and impulsivity in adolescents with ADHD is linked with dopamine receptor sensitivity to medication. Reference Rosa-Neto, Lou, Cumming, Pryds, Karrebaek, Lunding and Giedd45 The interaction of brain, behaviour and medication needs closer examination and will be a focus of further studies. Lastly, our work focused solely on male children with ADHD and we do not know to what extent our observations generalise to females and adults with ADHD. Future work is planned to address these issues.
Our study illustrates the use of voxel-based methods to explore the brain morphology underlying complex behavioural indices affected by ADHD and should encourage its wider application. The results link grey matter volume of a discrete prefrontal-pallidal-temporal circuit to executive performance in ADHD. In older children with ADHD the volume of this circuitry is greater and their reaction times are faster. This has the welcome implication that some features of ADHD may improve with age.
Acknowledgements
This study was supported by a University of Hong Kong Grant to S.E.C. The authors would like to thank Ms Michelle Deng for her help with figure preparation.









eLetters
No eLetters have been published for this article.