One delineator of heterogeneity within children with early-onset antisocial behaviour is a callous and unemotional disposition (Reference Frick and HareFrick & Morris, 2004; Reference Larsson, Andershed and LichtensteinLynam & Gudonis, 2005). This designates a subgroup of children/youths with a more-severe, aggressive and stable pattern of antisocial behaviour and a specific neurocognitive profile indicative of defects in affect processing (Reference Larsson, Andershed and LichtensteinLynam & Gudonis, 2005;Blair, 2006). These are all markers that could be considered precursors of adult psychopathy and as such warrant careful study. We recently conducted the first twin study of callous–unemotional traits and conduct problems in childhood. High levels of callous traits were found to be under strong genetic influence (Reference Trouton, Spinath and PlominViding et al, 2005). This finding was consistent with behavioural genetic studies of psychopathic personality in youth and adults (Bloningen et al, 2003; Reference Price, Freeman and CraigTaylor et al, 2003; Reference Knopik, Alarcon and DeFriesLarsson et al, 2006). Furthermore, when twins with conduct problems were divided according to the presence of callous traits, a strong genetic influence on conduct problems was found.
These results provide strong support for the use of callous–unemotional traits to designate children with early-onset conduct problems who may have distinct causal processes leading to their antisocial behaviour. The present study expanded on these findings by examining the extent of genetic and environmental influences on the relationship between these two important dimensions in 7-year-old twins. Extremes in combination could be highly heritable simply because individual differences across the continuum are highly heritable, even if they are genetically uncorrelated. If common genes are important mediators of the relationship, molecular genetic analyses should focus on finding the common genes that mediate the risk.
Two twin studies to date have addressed the extent of overlap in the genetic influences on callous–unemotional traits and antisocial behaviour/lifestyle (Reference Price, Freeman and CraigTaylor et al, 2003; Reference Knopik, Alarcon and DeFriesLarsson et al, 2006). In both studies the genetic influences on the two domains showed substantial overlap, although independent genetic influences were also observed. Both studies were conducted on youths and young adults only, some of whom may have had a childhood onset to their antisocial behaviour. In addition, neither study focused on extreme of the distributions. Given the risk associated with early-onset antisocial behaviour, we focused on the relationship with callous–unemotional traits in childhood and analysed data from extreme groups in addition to the entire continuum of scores.
METHOD
Participants
Participants were drawn from the Twins Early Development Study (TEDS), a longitudinal study of twin pairs ascertained from population records of twin births in England and Wales between 1994 and 1996 (Reference Taylor, Loney and BobadillaTrouton et al, 2002). The sample consisted of 3434 twin pairs, born between January 1994 and August 1996, who had teacher ratings for callous–unemotional traits and conduct problems. Any twin pairs where either twin had parental reports of medical or neurological conditions were not included (Reference Caspi, Moffitt and MorganDale et al, 1998), leaving a sample of 3232 twin pairs for analysis.
For the bivariate DeFries–Fulker extremes analysis (Defries &Fulker, Reference Dale, Simonoff and Bishop1985, Reference DeFries and Fulker1988), same-gender twin pairs with at least one proband with callous–unemotional traits were included in the trait→conduct problems analysis (selecting on trait and measuring co-twins' conduct problems); pairs with at least one proband with conduct problems were included in the conduct problems→trait analysis (selecting on conduct problems and measuring co-twins' callous–unemotional traits). Probands were selected above the 90th percentile, a cutoff designated as ‘abnormal’ according to the Strengths and Difficulties Questionnaire (SDQ; Reference Frick and MorrisGoodman, 1997). The trait probands scored 1.31 or more standard deviations above the mean on the trait scale (612 probands, 459 twin pairs). The conduct problem probands scored 1.28 or more standard deviations above the mean on the conduct problems scale (444 probands, 364 twin pairs). This selection procedure guaranteed that the probands would score beyond the ‘average range’ (i.e. not within 1 s.d.), yet yielded enough probands to perform the twin analyses.
Zygosity was ascertained by parental ratings with an error rate of 5%, as validated by DNA typing of 8–10 microsatellite polymorphisms (Reference Neale, Boker and XiePrice et al, 2000). Unclear cases were resolved through genotyping a multiplex of 12 highly polymorphic markers (Reference Enders and BandalosFreeman et al, 2003). Despite attrition, the TEDS sample that provided data at 7 years of age is closely matched to UK population in terms of ethnicity and maternal education (Reference GoodmanHarlaar et al, 2005).
Testing procedures
Informed, written consent was obtained from all families who agreed to take part in the study. The families were informed that the TEDS encompasses assessment of cognitive ability, behavioural problems and pro-social behaviours and that all of the data would be anonymised and published in a way that did not identify an individual child. Teachers were approached only if there was family consent for teacher involvement. The consent procedure was approved by the Institute of Psychiatry and Maudsley Ethics Committee.
Measures
Teachers provided ratings of callous–unemotional traits and conduct problems. The response rate of teachers was high: 88% of those approached responded by completing the TEDS assessment. There are several reasons for relying on teacher report. First, teachers are familiar with a broad range of children and have expertise regarding normative child development. Second, twin analyses indicate that teacher ratings show less rater bias than typically found in parent ratings (Reference McGue and BouchardNadder et al, 2001). Third, and most importantly for the purposes of this study, there is evidence that teacher ratings of callous–unemotional traits lead to a more valid differentiation of subgroups of children with conduct problems in pre-adolescent samples (Barry et al, 2000). Consistent with these theoretical reasons for relying on teacher report, parent ratings of callous–unemotional traits and conduct problems showed much poorer levels of internal consistency (α=0.45 and α=0.58 respectively) than teacher ratings (α=0.74 and α=0.71 respectively).
The TEDS 7-year assessment of callous–unemotional traits included three items (‘Does not show feelings or emotions’, ‘Feels bad or guilty if he/she does something wrong’ (reverse scored), ‘Is concerned about how well he/she does at school’ (reverse scored)) from the callous–unemotional traits scales of the Antisocial Process Screening Device (APSD; Reference FrickFrick & Hare, 2001) and four selected items from the Strengths and Difficulties Questionnaire (SDQ; Reference Frick and MorrisGoodman, 1997) (e.g. ‘Considerate of other people's feelings’ (reverse scored)). None of the items overlapped with any of the conduct problem items (see Viding et al (Reference Trouton, Spinath and Plomin2005) for the complete list of items on both scales).
We used the SDQ 5-item scale to assess conduct problems (e.g. ‘Often fights with other children or bullies them’, ‘Often has temper tantrums or hot tempers’). The SDQ is a widely used screening instrument in the UK and its reliability and validity have been demonstrated on a large, national sample (Reference GoodmanGoodman, 2001). Three of the conduct problem items reflected tendency for aggression or bad temper, whereas the remaining two assessed lying and stealing. The callous–unemotional traits and conduct problem scales correlated 0.50 in this sample.
Genetic analyses
ACE model fitting
We fitted a correlated factors model directly to the individual observations by full-information maximum-likelihood function estimation (Reference DeFries, Olson, Pennington, Duane and GrayEnders & Bandalos, 2001) in the program Mx (Reference Nadder, Silberg and RutterNeale et al, 2003).
In addition to yielding maximum-likelihood parameter estimates for the effects of latent additive genetic (A), shared environmental (C), and non-shared environmental (E) influences on callous–unemotional traits and conduct problems, the correlated factors model also provides estimates of the genetic correlation (r g), shared environmental correlation (r c), and non-shared environmental correlation (re) between a pair of measures (see data supplement 1 to the online version of this paper). The genetic correlation indicates the extent to which genetic effects on one measure overlap with genetic effects on another measure.
It is also possible to estimate the extent to which genetic factors contribute to the observed phenotypic correlation between the measures (bivariate heritability). Shared and non-shared environmental mediation of the phenotypic correlation can also be estimated (Reference Nadder, Silberg and RutterNeale et al, 2003).
Because mean effects of age and gender can spuriously inflate twin resemblance, all analyses used age- and gender-adjusted residual scores from multivariate linear regression modelling (Reference Lynam and GudonisMcGue & Bouchard, 1984). Gender-related influences on individual differences can none the less be investigated (see data supplement 2 to the online version of this paper). Footnote *
The relationship of extremes of callous–unemotional traits and conduct problems can be assessed with an extension of the DeFries–Fulker extremes analysis (DeFries & Fulker, Reference Dale, Simonoff and Bishop1985, Reference DeFries and Fulker1988). This addresses the genetic and environmental causes of the mean difference on a quantitative trait score between probands and the rest of the population. Univariate analysis yields a statistic called group differences heritability (h2g), which is the proportion of the phenotypic difference between the probands as a group and the population that can be attributed to genetic factors. The bivariate extension of the group analysis addresses the etiology of co-occurrence of two traits for the extremes of dimensions (Reference DeFries and FulkerDeFries et al, 1991). Rather than selecting probands as extreme on X and comparing the quantitative scores of their monozygotic and dizygotic co-twins on X as in univariate group analysis, bivariate analysis selects probands on X and compares the quantitative scores of their co-twins on Y. The extent to which the cross-twin regression to the population mean is greater for dizygotic co-twins than monozygotic co-twins indicates the extent to which proband deficits in X are a result of genetic factors that also influence the co-twins' quantitative scores on Y (group cross-familiality). An important point to note is that bivariate extremes analysis is not bi-directional. The group genetic correlation can be derived from group heritability estimates (Reference Harlaar, Spinath and DaleKnopik et al, 1997). The DeFries–Fulker regression analysis is performed on same-gender twin pairs and thus a test of gender differences is not incorporated (see data supplement 3 to online version of this paper).
RESULTS
Descriptive statistics
Descriptive statistics for the standardised conduct problems and callous–unemotional traits scores are summarised in Table 1. On both measures, all zygosity and gender groups showed similar mean scores (dizygotic opposite-gender twins showed slightly lower mean scores), but mono- and dizygotic female pairs and dizygotic opposite-gender pairs showed less variance than male mono- and dizygotic pairs, particularly on conduct problems. Although we observed some significant mean differences between our zygosity groups, these are not of a sizeable magnitude and the statistical significance probably reflects our sample size
Table 1 Age and gender-regressed z-scores for callous–unemotional traits and conduct problems according to gender and zygosity 1

| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Standardised score, mean (s.d.) | Monozygotic (n=534) | Dizygotic (n=508) | Monozygotic (n=612) | Dizygotic (n=562) | Dizygotic opposite gender (n=982) | ||
| Callous–unemotional traits2 | 0.05 (1.07) | –0.06 (1.06) | 0.06 (0.96) | –0.02 (0.92) | –0.12 (0.97) | ||
| Conduct problems | 0.00 (1.14) | 0.04 (1.25) | –0.00 (0.81) | –0.01 (0.81) | –0.10 (0.87) | ||
1. One twin from each pair was randomly selected for the analysis. Main effect for zygosity group was found for callous–unemotional traits (F (4, 3157)=4.32, P<0.01 (two-tailed)), reflecting the mean difference between monozygotic males v. dizygotic opposite gender and monozygotic females v. dizygotic opposite gender groups (both comparisons significant after correcting for multiple comparisons at P<0.025 and P<0.01 respectively). Marginal main effect for zygosity was found for conduct problems (F (4, 3157)=2.25, P=0.06 (two-tailed)), reflecting the difference between dizygotic males v. dizygotic opposite gender groups. However, this did not survive correction for multiple comparisons
The phenotypic correlation between callous–unemotional traits and conduct problems scales was moderate (r=0.50 (0.53 for boys, 0.46 for girls)) in this sample. One twin from each pair was randomly selected for the analyses. When we replicated this correlation with the previously unselected twin, the results were very similar (r=0.47 (0.48 for boys, 0.46 for girls)).
Genetic analyses
Although variances and covariances are used in model-fitting analyses of twin data, correlations are useful for comparing resemblances between twins as a function of genetic relatedness. Twin correlations for callous–unemotional traits and conduct problems ratings are shown by gender and zygosity in Table 2. Monozygotic within-trait correlations were consistently greater than the corresponding dizygotic correlations for callous–unemotional traits and for conduct problems, suggesting substantial genetic influence on both. For both, dizygotic opposite-gender correlations were only slightly lower than correlations for dizygotic males and females, suggesting no important qualitative genetic differences between genders. However, quantitative gender differences are suggested by the pattern of correlations for dizygotic males and females, pointing to higher heritability and lower shared environment for males.
Table 2 Within trait (intraclass) and cross-trait twin correlations between callous–unemotional traits and conduct problems according to gender and zygosity 1

| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Monozygotic | Dizygotic | Monozygotic | Dizygotic | Dizygotic opposite gender | |||
| Callous–unemotional traits | 0.72 | 0.32 | 0.67 | 0.44 | 0.32 | ||
| Conduct problems | 0.69 | 0.32 | 0.64 | 0.39 | 0.25 | ||
| Cross-trait | 0.41 | 0.22 | 0.38 | 0.23 | 0.17 | ||
1. n=756–1539 twin pairs/cell, based on pairwise deletion. For each pair of traits, the average of two reciprocal cross-correlations is presented. All correlations significant at P<0.01
Cross-twin, cross-trait correlations for callous–unemotional traits and conduct problems were 0.41 and 0.38, for monozygotic males and females respectively, which were only slightly less than the within-individual correlation of 0.50 (Table 2). The dizygotic cross-trait correlations were only 0.22, 0.23, and 0.17 for males, females and opposite-gender twins respectively. This suggests substantial genetic mediation of the phenotypic correlation. The similar cross-trait correlations for dizygotic twins indicate neither qualitative nor quantitative gender differences.
ACE model-fitting analyses
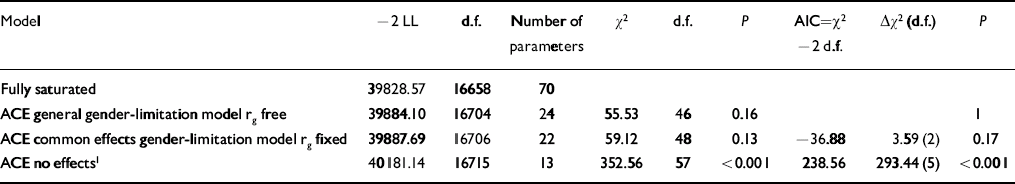
Model fitting statistics comparing the gender-limited bivariate correlated factors model with a fully saturated model, as well as comparing nested submodels are presented in Table 3, with parameter estimates of the best-fitting model in Table 4. (Additional results are available from E.V. upon request). The best-fitting model (with the least number of parameters but no decrease in the model fit as compared with a model with more parameters) indicated that, for both callous–unemotional traits and conduct problems, there were quantitative but not qualitative gender differences. That is, the same genetic influences were important for males and females but in different degrees. The bivariate statistics, however, appeared remarkably similar for both genders.
Table 3 Model fit indices
| Model | –2 LL | d.f. | Number of parameters | χ2 | d.f. | P | AIC=χ2 –2 d.f. | Δχ2 (d.f.) | P |
|---|---|---|---|---|---|---|---|---|---|
| Fully saturated | 39828.57 | 16658 | 70 | ||||||
| ACE general gender-limitation model rg free | 39884.10 | 16704 | 24 | 55.53 | 46 | 0.16 | 1 | ||
| ACE common effects gender-limitation model rg fixed | 39887.69 | 16706 | 22 | 59.12 | 48 | 0.13 | –36.88 | 3.59 (2) | 0.17 |
| ACE no effects 1 | 40181.14 | 16715 | 13 | 352.56 | 57 | <0.001 | 238.56 | 293.44 (5) | <0.001 |
rg free, genetic correlation between dizygotic males and females is allowed to depart from 0.50 (this model allows qualitative and quantitative gender differences); rg fixed, genetic correlation between dizygotic males and females is fixed to 0.50 (this model allows quantitative, but not qualitative gender differences)
1. This model does not allow gender differences
Table 4 Standardised parameter estimates from the full ACE correlated factor model for boys1

| Parameter estimates | Callous–unemotional traits | Conduct problems |
|---|---|---|
| Total variance resulting from | ||
| Additive genetic factors (A) | 0.67 (0.58–0.72) | 0.61 (0.50–0.69) |
| Shared environmental factors (C) | 0.04 (0.00–0.11) | 0.06 (0.00–0.17) |
| Non-shared environmental factors (E) | 0.29 (0.26–0.33) | 0.34 (0.31–0.38) |
| Correlations | ||
| Genetic (rg) | 0.57 (0.48–0.63) | |
| Shared environmental (rc) | 0.56 (0.00–1.0) | |
| Non-shared environmental (re) | 0.40 (0.34–0.46) | |
| Phenotypic relationship mediated by | ||
| Bivariate heritability (biv h2) | 0.71 (0.54–0.80) | |
| Bivariate shared environmental factors (biv c2) | 0.04 (0.00–0.20) | |
| Bivariate non-shared environmental factors (biv e2) | 0.25 (0.20–0.30) |
Tables 4 and 5 show the total variance accounted for by genetic and environmental influences, in boys and girls. As expected from the pattern of cross-twin, within-trait correlations, both callous–unemotional traits and conduct problems were significantly heritable but somewhat more heritable in boys than girls (h2=0.67 and h2=0.61 for boys, and 0.48 and 0.57 for girls, for callous–unemotional traits and conduct problems respectively). Shared environmental influences were not statistically significantly different from zero for boys (c2=0.04 for callous–unemotional traits and c2=0.06 for conduct problems). For girls, there was modest, significant shared environmental influence for callous–unemotional traits (c2=0.20). The shared environmental influence was not significantly different for conduct problems in girls, and the estimated magnitude was similar for that in boys (c2=0.08). Non-shared environmental influences accounted for most of the environmental variance (e2=0.29 and e2=0.34 for boys, and 0.32 and 0.35 for girls, for callous–unemotional traits and conduct problems respectively).
Table 5 Standardised parameter estimates from the full ACE correlated factor model for girls 1

| Parameter estimates | Callous–unemotional traits | Conduct problems |
|---|---|---|
| Total variance resulting from | ||
| Additive genetic factors (A) | 0.48 (0.37–0.60) | 0.57 (0.45–0.68) |
| Shared environmental factors (C) | 0.20 (0.08–0.29) | 0.08 (0.00–0.19) |
| Non-shared environmental factors (E) | 0.32 (0.29–0.35) | 0.35 (0.32–0.38) |
| Correlations | ||
| Genetic (rg) | 0.65 (0.52–0.78) | |
| Shared environmental (rc) | 0.33 (0.00–0.95) | |
| Non-shared environmental (re) | 0.19 (0.12–0.25) | |
| Phenotypic relationship mediated by | ||
| Bivariate heritability (biv h2) | 0.77 (0.58–0.97) | |
| Bivariate shared environmental factors (biv c2) | 0.09 (0.00–0.26) | |
| Bivariate non-shared environmental factors (biv e2) | 0.14 (0.09-0.190) |
1. As the shared environmental estimates for callous–unemotional traits and conduct problems did not significantly differ from 0.00 for boys, it was possible to drop the C path for boys without significant decrease in model fit. The same held for conduct problems for girls, as well as for the rc and biv c2 estimates. In this reduced model, most of the C variance ends up in the A term (results available from E.V.). Despite the acceptability of this nested model in model-fitting terms, we chose to report parameter estimates for the full model, as twin studies are generally underpowered to detect estimates of shared environment
Table 4 also summarises the extent of overlap between genetic and environmental influences. The genetic correlation (r g) is significant as indicated by the confidence intervals and the estimates of 0.57 (boys) and 0.65 (girls) suggesting substantial overlap between genetic influences contributing to individual differences in both boys and girls. The shared environmental correlation (r c) is not significant for either gender. Finally, non-shared environmental influences show significant overlap across callous–unemotional and conduct problems, in slightly greater magnitude for boys (r e= 0.40), than for girls (r e=0.19). The r e estimate could also reflect measurement error common to both domains.
Finally, Table 4 summarises the extent to which genetic and environmental influences mediate the phenotypic relationship. The bivariate heritability estimates (biv h2) of 0.71 (boys) and 0.77 (girls) indicate that the phenotypic relationship between the two traits is primarily mediated genetically for both genders. In other words, co-occurrence of callous–unemotional traits and conduct problems is mainly mediated by genetic influences. Non-shared environmental influences (and common error) make a modest contribution to the phenotypic relationship (biv e2=0.25 (boys) and 0.14 (girls), although the contribution of shared environmental influences is negligible (biv c2=0.04 (boys) and 0.09 (girls)).
DeFries-Fulker extremes analyses
Application of bivariate DeFries–Fulker group analysis selecting on callous–unemotional traits and measuring co-twin conduct problems yielded a bivariate group differences heritability estimate of 76% (95% CI 0.39–1.13). In other words, 76% of the mean difference between the extreme group with regard to callous–unemotional traits and the population on the conduct problems scale can be attributed to genetic factors. The bivariate group shared environment estimate was 4% (95% CI -0.37 to -0.45). The remainder of the mean difference was a result of non-shared environmental factors. The converse analyses – selecting on conduct problems and measuring co-twin callous–unemotional traits –yielded a similar bivariate group differences heritability estimate of 82% (95% CI 0.49–1.14), and bivariate group shared environment estimate of 2% (95% CI -0.31 to 0.35). The extremes genetic correlation estimate is 1, indicating complete commonality of genetic influences at the extremes. The confidence interval for this bivariate DeFries–Fulker extremes estimate of a group genetic correlation has not yet been worked out (Reference Harlaar, Spinath and DaleKnopik et al, 1997) but is likely to be large, and this finding should thus be treated as instructive rather than definitive.
DISCUSSION
As noted previously, children with callous–unemotional traits seem to constitute an important subgroup of children with early-onset conduct problems (Reference Frick and HareFrick & Morris, 2004). Previously, we demonstrated that antisocial behaviour is highly heritable in the group with such traits but not in children with conduct problems only (Reference Trouton, Spinath and PlominViding et al, 2005). The present study attempted to expand on these findings by examining the extent of genetic and environmental influences on the relationship between these two important dimensions in 7-year-old twins.
Our present findings demonstrated, most importantly, that there is substantial genetic overlap between callous–unemotional and conduct problems in both boys and girls. Common genetic influences operate to bring about both of these problems, assessed as a dimension in the entire sample and even more so at the high extremes. These common genetic influences also appear to be largely responsible for the phenotypic relationship. Our study was unique in that its large sample size enabled us to study genetic and environmental influences at the extremes of the distribution, as well as across the entire continuum. We replicated findings from studies of adults and youths which show substantial heritability of individual differences in callous–unemotional traits (Bloningen et al, 2003; Reference Price, Freeman and CraigTaylor et al, 2003; Reference Knopik, Alarcon and DeFriesLarsson et al, 2006) and of genetic mediation of the phenotypic relationship with antisocial behaviour (Reference Price, Freeman and CraigTaylor et al, 2003; Reference Knopik, Alarcon and DeFriesLarsson et al, 2006).
Unlike in an earlier study (Reference Knopik, Alarcon and DeFriesLarsson et al, 2006), there was a gender difference in the magnitude of genetic and shared environmental effects on individual differences in callous–unemotional traits in childhood and this warrants further investigation. One target for future research is to identify specific shared environmental influences that may affect the level of such traits in girls and whether these influences relate to low or high levels (e.g. these could be influences encouraging prosocial behaviour in girls). However, and most importantly, callous–unemotional traits and conduct problems were associated at the phenotypic level in both boys and girls and the mediation of the relationship was strongly driven by common genes for both.
The shared genetic influences suggest that molecular genetic studies should concentrate on polymorphisms associated with callous–unemotional traits and conduct problems.
Shared environmental influences could not be reliably detected as an aetiological factor mediating the relationship between callous–unemotional traits and conduct problems either across the continuum or at the extremes. This does not mean that environmental influences present in the family are not important. However, these influences appear to operate in a child- and trait-specific manner. As an example, parental treatment may differ for twins and this differential treatment may cause differences in levels of callous–unemotional traits and conduct problems considered separately. A recent study demonstrated that elevated maternal negative emotionality was an environmental variable that influenced the extent of differences in conduct problems in genetically identical monozygotic twins (Reference Blonigen, Carlson and KruegerCaspi et al, 2004). Finally, it is likely that the latent addictive genetic influence (‘A’ parameter) also includes effects of gene–environment correlation. For example, children with a particular genotype may evoke a certain reaction from their environment or may actively seek out certain kinds of activities, all of which would reinforce the measured trait.
In line with earlier findings (Reference Price, Freeman and CraigTaylor et al, 2003; Reference Knopik, Alarcon and DeFriesLarsson et al, 2006), not all genetic influences on the individual differences in callous–unemotional traits and conduct problems were overlapping in our study. The non-overlapping genetic variance has been proposed to imply some independence in the underlying biological substrates (Reference Price, Freeman and CraigTaylor et al, 2003). However, both previous studies and our own individual differences analysis addressed the entire continuum of scores. Our analysis of extreme groups suggests that genetic overlap may be complete at the extremes, although we acknowledge that such estimates entail substantial confidence intervals. None the less, we would not rule out the possibility that unique genetic influences may be important.
Some general limitations of the study should be mentioned. Our scale for assessing callous–unemotional traits was not a standard instrument. However, teacher ratings on this scale showed good internal consistency and distinguished an aetiologically distinct group of children with early-onset antisocial behaviour in our earlier study (Reference Trouton, Spinath and PlominViding et al, 2005). Relying on a single source of measurement could be considered a limitation. As the parent ratings of such traits did not show good internal consistency, it seemed dubious to base conclusions on these (Reference Trouton, Spinath and PlominViding et al, 2005). Collection of data at a single age is a limitation, which precludes commenting on the aetiology of the stability of the association or whether the genetic links are of different magnitude in childhood than later in development. We are currently following up the twins at 9 years of age and will thus be able to add a longitudinal aspect in the future.
Within the context of these limitations, the present findings have several important implications. The finding of genetic overlap for callous–unemotional traits and conduct problems suggests that although distinct brain anatomical substrates or cognitive operations may be associated with these dimensions, genetic influences for the two are largely overlapping. Developing a better understanding of genes–brain–cognition–behaviour pathways will enable us to tailor individualised prevention and treatment strategies for children who show the combination of callous–unemotional traits and conduct problems. This genetically vulnerable subgroup with persistent antisocial behaviour requires early intervention. Given the negligible influence of shared environment for the antisocial behaviour in such children (Reference Trouton, Spinath and PlominViding et al, 2005), prevention and treatment programmes may benefit from identifying and targeting child-specific environmental risk factors, such as differential parental treatment or developing programmes that capitalise on the specific cognitive and affective style of the child. For example, programmes that intervene early to promote the development of empathy and the internalisation of values or that use motivational strategies that capitalise on reward-oriented response style and appeal to self-interest may be particularly important for this group of children (Reference Freeman, Smith and CurtisFrick, 2001).
Acknowledgements
We thank the twins, their parents and teachers, Dr Fruhling Rijsdijk for invaluable support with the statistical analyses, and the TEDS team, particularly Patricia Busfield, Jane McKay and Andrew McMillan. The TEDS receives support from the British Medical Research Council (G9424799). Research into development of antisocial behaviour also receives support from the UK Department of Health and Home Office (MRD 12-37). E.V. was supported by an ESRC postdoctoral fellowship during the analysis phase.








eLetters
No eLetters have been published for this article.