Long-standing evidence from Papez Reference Papez1 and others supports an essential role for the cingulum bundle in the neural system subserving emotional regulation, implicating it in the emotional dysregulation of bipolar disorder. The cingulum has long fibres providing frontotemporal connections and shorter fibres connecting adjacent portions of the cingulate cortex. The anterior cingulum subregion is especially implicated in the pathophysiology of bipolar disorder, as it provides substantial connections from the anterior cingulate cortex to the orbitofrontal cortex, as well as to mesial temporal and striatal structures. Reference Mufson and Pandya2 These brain regions are central in emotional regulation, and abnormalities within each of these structures have been demonstrated in bipolar disorder. Reference Blumberg, Kaufman, Martin, Whiteman, Zhang, Gore, Charney, Krystal and Peterson3–Reference Yurgelun-Todd, Gruber, Kanayama, Killgore, Baird and Young9 There is less evidence to support the involvement of the posterior cingulum, although neuroimaging findings have demonstrated abnormal posterior cingulate cortex anatomy and function in bipolar disorder. Reference Kaur, Sassi, Axelson, Nicoletti, Brambilla, Monkul, Hatch, Keshavan, Ryan, Birmaher and Soares10,Reference Atmaca, Ozdemir, Cetinkaya, Parmaksiz, Belli, Poyraz, Tezcan and Ogur11
Evidence from post-mortem histological and structural neuro-imaging studies supports the involvement of white matter in the pathophysiology of bipolar disorder. Decreases in glial density in anterior cingulate and orbitofrontal cortical subregions were observed in bipolar disorder. Reference Ongur, Drevets and Price12,Reference Rajkowska13 Oligodendrocyte abnormalities in bipolar disorder are increasingly implicated by reports of decreased oligodendrocyte density, as well as reduced expression of oligodendrocyte- and myelination-related genes, in the frontal cortex. Reference Tkachev, Mimmack, Ryan, Wayland, Freeman, Jones, Starkey, Webster, Yolken and Bahn14,Reference Uranova, Vostrikov, Orlovskaya and Rachmanova15 Magnetic resonance imaging (MRI) studies of bipolar disorder provide further evidence for white matter abnormalities, including abnormalities in the volume and structural integrity of frontal white matter. Reference Adler, Holland, Schmithorst, Wilke, Weiss, Pan and Strakowski16–Reference Haznedar, Roversi, Pallanti, Baldini-Rossi, Schnur, Licalzi, Tang, Hollander and Buchsbaum20
Diffusion tensor imaging presents the opportunity to measure the organisation of fibres within specific white matter tracts. Reference Basser, Mattiello and LeBihan21 Fractional anisotropy is an indirect measure of the coordinated directionality and coherence of fibres within a white matter fibre bundle. Reference Beaulieu22 Decreases in fractional anisotropy have been detected in disorders of central nervous system myelination, Reference Harsan, Poulet, Guignard, Steibel, Parizel, de Sousa, Boehm, Grucker and Ghandour23–Reference Filippi, Cercignani, Inglese, Horsfield and Comi25 suggesting that fractional anisotropy is a measure sensitive to myelination abnormalities. In this study, a diffusion tensor imaging method that provides accurate isolation of the cingulum bundle was used to measure fractional anisotropy in the anterior and posterior cingulum to test the hypothesis that bipolar disorder is associated with abnormalities in the structural integrity of the cingulum bundle. Anterior cingulum deficits in bipolar disorder were anticipated.
Methods
Participants
The bipolar disorder group included 42 participants (mean age 32.6 years (s.d.=10.1), 69% female) recruited from the Yale University School of Medicine Medical Center (New Haven, Connecticut) the Veterans Affairs Connecticut Healthcare System (West Haven, Connecticut) and the Greater New Haven community. The healthy comparison group included 42 participants (mean age 28.7 years (s.d.=9.10), 64% female) who were recruited from the community with neither personal history of a DSM–IV Axis I disorder nor a history of a mood, psychotic, anxiety or substance misuse disorder in their first-degree family members. The Structured Clinical Interview for DSM–IV Axis I disorders version 2.0 (SCID) Reference First, Spitzer, Gibbon and Williams26 confirmed the presence or absence of DSM–IV Axis I disorders. No participants had a history of neurological illness, head trauma with loss of consciousness exceeding 5 min or major medical disorder, with the exception of five female participants with bipolar disorder with treated hypothyroidism. After a complete description of the study, written informed consent was obtained from all participants in accordance with the human investigation committees of the Yale University School of Medicine and the Department of Veterans Affairs.
Twenty-five (60%) participants with bipolar disorder met criteria for rapid cycling. At the time of scanning, 11 (26%) participants with bipolar disorder met DSM–IV criteria for a current manic/mixed or hypomanic episode, 9 (21%) for a depressive episode and 22 (52%) were euthymic. Comorbidity included panic disorder (4 participants with bipolar disorder, 10%) and post-traumatic stress disorder (2 participants, 5%). Seven (17%) participants with bipolar disorder were unmedicated. Psychotropic medications prescribed to the remaining participants with bipolar disorder included lithium carbonate (n=11, 26%), anticonvulsants (n=20, 48%), atypical antipsychotics (n=19, 45%), antidepressants (n=17, 40%), benzodiazepines (n=8, 19%) and levothyroxine sodium (n=5, 12%).
Magnetic resonance imaging acquisition
Diffusion-weighted images were acquired on a 3T Trio MR scanner (Siemens, Erlangen, Germany) with a single-shot echo planar imaging sequence in alignment with the anterior commissure–posterior commissure plane. Diffusion sensitising gradients were applied along 32 non-colinear directions uniformly distributed on a unit sphere, with b-value=1000 s/mm2, together with an acquisition without diffusion weighting (b-value=0) (repetition time (TR)=7400 ms, time to echo (TE)=115 ms, field of view (FOV)=256 × 256 mm2, matrix=128 × 128, slice thickness= 3 mm without gap, 40 slices, 1 average).
Diffusion tensor imaging processing
Diffusion tensor imaging data were processed with BioImage Suite for Windows (www.bioimagesuite.org). Diffusion-weighted data were first interpolated to 2 mm thickness along the coronaloblique direction with a within-plane resolution of 1 mm × 1 mm, and denoised by a three-dimensional isotropic Gaussian kernel with sigma 1 mm full-width-at-half-maximum. After diagonalisation of diffusion tensor imaging, diffusion eigenvectors and corresponding eigenvalues (λ1, λ2, λ3) were acquired. Fractional anisotropy was calculated according to the following formula: Reference Basser, Mattiello and LeBihan21
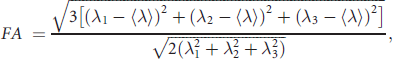
The absolute red-green-blue colour-encoding scheme defined the directionality of the principal eigenvector: Reference Pajevic and Pierpaoli27 left–right fibres in red, anterior–posterior fibres in green and superior–inferior fibres in blue. The colour-coded diffusion tensor images provided excellent distinction between the cingulum and nearby bundles such as the corpus callosum. The cingulum bundle was delineated to include voxels containing fibres travelling in the anterior–posterior direction that also exhibited fractional anisotropy greater than 0.2 in order to exclude gray matter. Reference Bonekamp, Nagae, Degaonkar, Matson, Abdalla, Barker, Mori and Horska28 The cingulum was further subdivided into anterior and posterior sections by the coronal-oblique slice perpendicular to the anterior commissure–posterior commissure line and passing through its midpoint (the mid-anterior commissure–posterior commissure slice) (see online Fig. DS1). Then, mean fractional anisotropy was calculated separately for anterior cingulum and posterior cingulum regions of interest, each in the right and left hemispheres. Specifically, fractional anisotropy for the anterior cingulum regions of interest was calculated as the mean cingulum fractional anisotropy over five coronal slices (sampled every three slices with 6 mm intervals between sampled slices) anterior to the mid-anterior commissure–posterior commissure slice; fractional anisotropy in the posterior cingulum region of interest was calculated as the mean cingulum fractional anisotropy over five coronal slices (sampled every three slices with 6 mm intervals between sampled slices) posterior to and including the midanterior commissure–posterior commissure slice. High interrater reliability for manual delineation on the coronal slices was obtained with intraclass correlation coefficients of 0.92–0.95.
Statistical analysis
All data were analysed using SAS, version 9.1 for Windows. Fractional anisotropy values were tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. The primary statistical mixed model (PROC MIXED) tested whether the bipolar disorder and healthy control groups differed in regional fractional anisotropy values. The model included data from all participants (n=84), a fixed effect of diagnosis (bipolar disorder and healthy controls) and random participant effects. Repeated measures were performed over the spatial domain of region (anterior and posterior cingulum) and hemisphere (right and left) and were included as within-participant factors in the model. Age and gender served as covariates, and all twoand three-way interactions were fitted in the final models. The correlation structure of the data was modelled by random effects for participant and by unstructured variance–covariance matrix for observations on the two cingulums within each hemisphere. The latter variance–covariance structure was the best fitting according to the Akaike Information Criterion. Only significant results (P<0.05) involving diagnosis are reported below. Least squares means and standard errors were calculated from the mixed model for regional fractional anisotropy values and plotted to interpret diagnosis effects.
Post hoc exploratory analyses were performed for potential main effects of clinical variables among bipolar disorder participants. Clinical factors examined included presence or absence of rapid cycling, mood state at the time of scanning and medication status at scanning.
Results
The bipolar disorder and healthy control groups did not differ significantly in age or gender (P>0.05 for both). Data adhered to a normal distribution as assessed.
The main effect of diagnosis was significant (F(1,240)=6.33, P=0.013), as was the diagnosis by region interaction (F(1,240)=5.1, P=0.025). The difference of least squares means between the diagnostic groups (Fig. 1) indicated that the stronger contribution to group differences was derived from smaller anterior cingulum fractional anisotropy values in the bipolar disorder group compared with the healthy control group. Anterior cingulum fractional anisotropy was decreased significantly in the bipolar disorder group compared with the healthy control group (F(1,240)=9.36, P=0.003); posterior cingulum fractional anisotropy was decreased to a lesser extent in the bipolar disorder group compared with the healthy control group, and the difference was not significant (F(1,240)=2.81, P=0.10). Exploratory analyses did not reveal any significant main effects of clinical factors within the bipolar disorder group on anterior cingulum fractional anisotropy values, including presence or absence of rapid cycling (P=0.40), mood state at the time of scanning (P=0.91) and medication status at scanning (P=0.77).
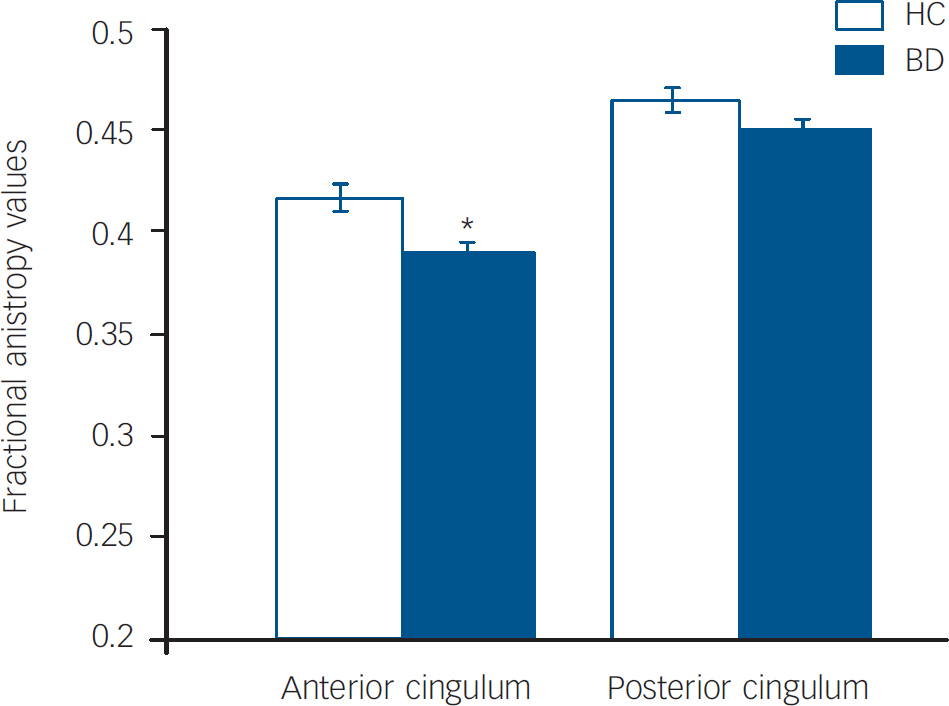
Fig. 1 Least squares mean fractional anisotropy values in the anterior and posterior cingulum and standard errors for the bipolar disorder group (n=42) and the healthy control group (n=42).
Means are adjusted for age, gender and hemisphere.
*The effect of diagnosis was significant in the anterior cingulum (P<0.05).
Discussion
We found decreased fractional anisotropy values in the anterior cingulum in participants with bipolar disorder compared with healthy controls, supporting the presence of abnormalities in the structural integrity of anterior cingulum in bipolar disorder. Our findings extend the body of evidence demonstrating abnormalities in the disorder in the morphology and function within anterior paralimbic and mesial temporal lobe structures Reference Blumberg, Kaufman, Martin, Whiteman, Zhang, Gore, Charney, Krystal and Peterson3–Reference Yurgelun-Todd, Gruber, Kanayama, Killgore, Baird and Young9 to suggest that these are accompanied by substantial abnormalities in the white matter that connects them via the anterior cingulum. This is consistent with the growing evidence in bipolar disorder suggesting that neuronal abnormalities may be accompanied by the presence of frontotemporal glial abnormalities, with increasing implication of oligodendrocyte involvement. Reference Ongur, Drevets and Price12–Reference Uranova, Vostrikov, Orlovskaya and Rachmanova15 Further study of neuronal–glial interactions in bipolar disorder may be important in elucidating its pathophysiology. Moreover, given its role in providing major connections between frontotemporal structures subserving emotional regulation, further study of the anterior cingulum may illuminate mechanisms underlying circuitry dysfunction that contribute to the emotional dysregulation characteristic of bipolar disorder.
The findings are consistent with previous diffusion tensor imaging reports consistent with abnormalities in individuals with bipolar disorder in the structural integrity of frontal white matter including ventral regions, as well as areas that contain frontostriato-thalamic projections. Reference Adler, Holland, Schmithorst, Wilke, Weiss, Pan and Strakowski16–Reference Beyer, Taylor, MacFall, Kuchibhatla, Payne, Provenzale, Cassidy and Krishnan18,Reference Haznedar, Roversi, Pallanti, Baldini-Rossi, Schnur, Licalzi, Tang, Hollander and Buchsbaum20 This, however, is the first report that we are aware of to specifically examine the cingulum bundle with diffusion tensor image methodology in individuals with bipolar disorder and to report anterior cingulum fractional anisotropy abnormalities. The region of interest method employed has the strong advantage of providing excellent, reliable delineation of the cingulum. However, it is possible that regional abnormalities extend to subgenual subregions further ventral than studied herein.
The specific cellular abnormalities that underlie differences in fractional anisotropy cannot be concluded from this study. Although the organisation of myelinated fibres within white matter bundles is thought to be the major contribution to fractional anisotropy values, and the findings are consistent with reports of decreases in frontal oligodendrocytes in the disorder, Reference Uranova, Vostrikov, Orlovskaya and Rachmanova15 other microstructural components of white matter fibres such as axonal membranes, microtubules and neurofilaments could potentially affect fractional anisotropy measures. Reference Beaulieu22 Further, a recent study by Houenou et al Reference Houenou, Wessa, Douaud, Leboyer, Chanraud, Perrin, Poupon, Martinot and Paillere-Martinot29 that employed diffusion tensor image tractography methodology demonstrated an increased number of reconstructed fibres between the left subgenual cingulate and left amygdalo-hippocampal, supporting the presence of macrostructural abnormalities in connectivity in bipolar disorder. Reference Houenou, Wessa, Douaud, Leboyer, Chanraud, Perrin, Poupon, Martinot and Paillere-Martinot29 This suggests the importance of examination of both micro- and macrostructure of white matter connectivity in future studies of bipolar disorder.
We did not detect significant main effects of clinical variables such as presence or absence of rapid cycling, mood state or medication status within the bipolar disorder group on the anterior cingulum fractional anisotropy values. However, our ability to detect effects of these factors might have been limited by inadequate power and heterogeneous bipolar disorder participant samples. A previous diffusion tensor imaging report of frontal white matter abnormalities in medication-naïve adolescents with bipolar disorder suggests that white matter abnormalities may be early manifestations of the disorder that are not related to repeated episodes or medication exposure. Reference Adler, Adams, DelBello, Holland, Schmithorst, Levine, Jarvis and Strakowski17
Conclusions
Our findings indicate the presence of abnormalities in the structural integrity of the anterior cingulum in bipolar disorder. Further understanding of abnormalities in anterior cingulum white matter may prove important in the treatment of mood disorders. For example, a deep brain stimulation study that targeted white matter proximal to the anterior cingulum, albeit in a more ventral region, showed effectiveness in treating depression. Reference Mayberg, Lozano, Voon, McNeely, Seminowicz, Hamani, Schwalb and Kennedy30 This suggests that a focus of future research on white matter in the anterior cingulum may help to elucidate the pathophysiology underlying neural circuitry abnormalities in bipolar disorder and point to new treatment strategies.
Acknowledgements
The authors were supported by grants from the National Institute of Mental Health R01MH69747 (H.P.B.), R01MH070902 (H.P.B.), the Department of Veterans Affairs CareerDevelopment (H.P.B.), Merit Review (H.P.B.) and Research Enhancement Award Programs (REAP) (H.P.B., L.G.C.), the National Alliance for Research in Schizophrenia and Depression (Great Neck, New York) (H.P.B., J.H.K.), The Ethel F. Donaghue Women's Investigator Program at Yale (New Haven, Connecticut) (H.P.B.) and the Klingenstein Foundation (J.H.K.), Howard Hughes Medical Institute Fellowship (M.P.S.). BioImage Suite was supported in part by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering under the grant R01EB006494 (X.P.).
This article is dedicated to Ms Kathleen Colonese who was devoted to helping those suffering from psychiatric illnesses and to advancing the field of bipolar disorder research. The authors thank Cheryl Lacadie, Karen Martin, Terry Hickey and Hedy Sarofin for their technical expertise, Allison McDonough and Lindsay Warren for assistance with the study, and the people who participated in this study.




eLetters
No eLetters have been published for this article.