Fibromyalgia (FM) is a complex and disturbing chronic pain syndrome. Lacking objective biomarkers, the diagnosis of this disease is based on clinical evaluation and patient report of widespread musculoskeletal pain and concomitant symptoms such as fatigue, insomnia, morning stiffness, emotional distress, and cognitive problems (Galvez-Sánchez & Reyes del Paso, Reference Galvez-Sánchez and Reyes del Paso2020). In Spain the prevalence of FM is 2.45%, being more common in women than in men and concentrating almost half of the cases in the age group of 40–59 years (Font et al., Reference Font, Bordoy, Mas, Seoane-Mato, Álvarez, Delgado, Martínez, Sánchez-Fernández, Rojas, García, Olivé, Rubio, Larrosa, Navarro, Sánchez-Piedra, Díaz-González and Bustabad-Reyes2020). This disease has an important impact on the person’s life. FM patients often have difficulties with their partner, depend on a family member for household chores, and lose their capacity to work (Collado et al., Reference Collado, Gómez, Coscolla, Sunyol, Solé, Rivera, Altarriba, Carbonell and Castells2014).
From a biopsychosocial perspective, FM is considered as result of a multicausal process in which genetic factors, dysfunctions of the central and autonomic nervous systems, neuroendocrine and inmunological aspects, external stressors, and psychological characteristics, among others, may be involved (Bellato et al., Reference Bellato, Marini, Castoldi, Barbasetti, Mattei, Bonasia and Blonna2012). Also, FM is a heterogeneous disorder as evidenced by the wide variety of physical symptoms, functioning limitations, cognitive-affective responses, and comorbidity manifested by patients (Auvinet & Chaleil, Reference Auvinet and Chaleil2012; Belenguer et al., Reference Belenguer, Ramos-Casals, Siso and Rivera2009). Despite research in pharmacological and non-pharmacological treatments, no single option has been identified as universally effective for all FM patients (Okifuji & Hare, Reference Okifuji and Hare2013). For this reason, in recent years the need to establish subgroups of FM patients to guide a more individualized management has been emphasized (Häuser et al., Reference Häuser, Perrot, Clauw and Fitzcharles2018). So, knowing the underlying variability of FM can help to better understand the etiopathogenic mechanisms involved in this syndrome and to design customized treatments that respond optimally to the specific needs of each patient.
Over the past 2 decades, a number of attempts have been made to identify subsets of FM patients who share common characteristics. While some classifications have been performed considering psychophysiological responses (Thieme & Turk, Reference Thieme and Turk2006; Thieme et al., Reference Thieme, Turk, Gracely, Maixner and Flor2015) and history of childhood maltreatment and biomarkers (Loevinger et al., Reference Loevinger, Shirtcliff, Muller, Alonso and Coe2012), most of them have been established according to psychosocial features derived of self-reports measures. In this last set of studies it may be differentiated among approaches focused on symptomatology, focused on personality variables/processes, and mixed.
From an approach focused on symptomatology, and via the Multidimensional Pain Inventory (MPI) that allows to identify 3 subgroups (dysfunctional, interpersonally distressed, and adaptive coppers), in FM patients Turk et al. (Reference Turk, Okifuji, Sinclair and Starz1996) observed that dysfunctional and interpersonally distressed subgroups reported higher pain, disability, and depression, and interpersonally distressed subgroup also reported significantly lower marital satisfaction, and Thieme et al. (Reference Thieme, Turk and Flor2004) found that dysfunctional subgroup mainly reported anxiety, interpersonally distressed subgroup mood disorders, and adaptive coppers subgroup reduced comorbidity with mental disorders. Considering the Fibromyalgia Impact Questionnaire (FIQ), De Souza et al. (Reference de Souza, Goffaux, Julien, Potvin, Charest and Marchand2009) identified in FM patients two subgroups: Type I characterized by low levels of anxiety, depression and morning tiredness, and Type II characterized by high levels of pain, fatigue, morning tiredness, stiffness, anxiety and depression. Later studies have broadened the characteristics of this typology (Calandre et al., Reference Calandre, García-Carrillo, García-Leiva, Rico-Villademoros, Molina-Barea and Rodríguez-López2011; Salgueiro et al., Reference Salgueiro, Aira, Buesa, Bilbao and Azkue2012; Triñanes et al., Reference Triñanes, González-Villar, Gómez-Perretta and Carrillo de la Peña2014). For example, Calandre et al. (Reference Calandre, García-Carrillo, García-Leiva, Rico-Villademoros, Molina-Barea and Rodríguez-López2011) found that Type II showed greater medical comorbidities and prescribed drugs, worse sleep quality, more physical ill and psychological distress. Applying the FIQ-revised it was identified three subgroups (Salaffi et al., Reference Salaffi, Mozzani, Draghessi, Atzeni, Catellani, Ciapetti, Di Carlo and Sarzi-Puttini2016) and a four-cluster solution (reflecting different levels of FM severity) validated taking into account clinical measures, economic costs, inflammatory markers levels, and gray matter volumes (Pérez-Aranda et al., Reference Pérez-Aranda, Andrés-Rodríguez, Feliu-Soler, Núñez, Stephan-Otto, Pastor-Mira, López-Roig, Peñacoba, Calandre, Slim, Salgueiro, Feixas and Luciano2019).
From an approach focused on personality variables/processes several categorizations of FM patients have been established. For example, Torres et al. (Reference Torres, Bailles, Valdés, Gutiérrez, Peri, Arias, Gómez and Collado2013) via the NEO Five-Factor Inventory identified two clusters, one of them showed higher neuroticism and lower extraversion and worse pretreatment clinical state including more psychosocial problems, and the other presented better adaptation. Similarly, Gonzalez et al. (Reference Gonzalez, Novo and Peres2020) using the Minnesota Multiphasic Personality Inventory–2 found two groups, one of them characterized by higher negative emotionality and introversion, presented a more disturbed profile in terms of general maladjustment, symptomatic behavior and clinical problem areas.
From a mixed approach, some studies have performed classifications of FM patients based on psychological features and other clinical aspects. Solutions between three and five clusters have been established considering musculoskeletal, non-musculoskeletal and cognitive/psychological domains (Wilson et al., Reference Wilson, Robinson and Turk2009), descriptors of sensory abnormalities and comorbities (Rehm et al., Reference Rivera and González2010), personal/family history of medical/psychopathological comorbidities, disease evolution, clinical scales and somatic symptoms (Docampo et al., Reference Docampo, Collado, Escaramís, Carbonell, Rivera, Vidal, Alegre, Rabionet and Estivill2013), pain, fatigue, function, sleep disturbance, depression, anxiety, dyscognition and stiffness (Vincent et al., Reference Vincent, Hoskin, Whipple, Clauw, Barton, Benzo and Williams2014), cognitive performance, physical performance, anxiety and depression (Follick et al., Reference Follick, Cherry, Rutledge, Zettel-Watson and Jones2016), pain, functioning, anxiety, depression and social support (Yim et al., Reference Yim, Lee, Park, Kim, Nah, Lee, Kim, Lee, Hong, Kim, Lee, Kim, Joung, Kim and Lee2017), and pain intensity, pain unpleasantness, fatigue, anxiety and depression (Bartley et al., Reference Bartley, Robinson and Staud2018). Possibly this mixed approach, compared to the previous ones, has the advantage of capturing the patterns of manifestations of FM in a more complete and varied way, showing greater ecological validity.
From this approach are of special interest the classifications of FM patients that have included cognitive-affective variables related to pain. Note that according to the influential Fear-Avoidance Model of Musculoskeletal Pain (Leeuw et al., Reference Leeuw, Goossens, Linton, Crombez, Boersma and Vlaeyen2007) these variables (e.g., pain catastrophizing, fear of pain and pain vigilance) are involved in the exacerbation of pain experience and disability. Giesecke et al. (Reference Giesecke, Williams, Harris, Cupps, Tian, Tian, Gracely and Clauw2003) considering mood, cognitive and pain sensitivity variables identified via a hierarchical cluster analysis (HCA) in 97 FM patients three subgroups: Subgroup 1 exhibited moderate anxiety, depression, pain catastrophizing and perceived control over pain, and low tenderness; Subgroup 2 exhibited high anxiety, depression and tenderness, the highest pain catastrophizing, and the lowest perceived control over pain; and Subgroup 3 exhibited normal anxiety and depression, very low pain catastrophizing, and the highest perceived control over pain and tenderness. However, the Giesecke et al.’s three-cluster model was not replicated by Luciano et al. (Reference Luciano, García-Forero, Cerdà-Lafont, Peñarrubia-María, Fernández-Vergel, Cuesta-Vargas, Ruíz, Rozadilla-Sacanell, Sirvent-Alierta, Santo-Panero, García-Campayo, Serrano-Blanco, Pérez-Aranda and Rubio-Valera2016) in 160 FM patients. Applying a HCA a two-cluster solution (functional and hypersensitive groups) emerged, whereas using a latent profile analysis (LPA) a three-class model was identified: Functional profile defined by moderate tenderness, distress and pain catastrophizing, dysfunctional profile defined by high tenderness, distress and pain catastrophizing, and highly dysfunctional and distressed profile defined by high tenderness and extremely elevated distress and pain catastrophizing (Luciano et al., Reference Luciano, García-Forero, Cerdà-Lafont, Peñarrubia-María, Fernández-Vergel, Cuesta-Vargas, Ruíz, Rozadilla-Sacanell, Sirvent-Alierta, Santo-Panero, García-Campayo, Serrano-Blanco, Pérez-Aranda and Rubio-Valera2016). Plazier et al. (Reference Plazier, Ost, Stassijns, de Ridder and Vanneste2015) based on physical symptoms, disability, health status, pain catastrophizing, pain vigilance, and emotional distress applying a HCA in 65 FM patients identified three subgroups: Subgroup 1 reflected high mood and catastrophizing-related symptoms and decreased mental health, Subgroup 2 reflected high fatigue and decreased physical health, and Subgroup 3 reflected a mixture of these two subgroups. Estévez-López et al. (Reference Estévez-López, Segura-Jiménez, Álvarez-Gallardo, Borges-Cosic, Pulido-Martos, Carbonell-Baeza, Aparicio, Geenen and Delgado-Fernández2017) based on declarative memory, active lifestyle, physical fitness, fatigue, psychological distress, pain catastrophizing, and resilience applied a HCA in 486 FM patients and identified five subgroups: Adapted, fit, poor performed, positive and maladapted. Finally, Muller et al. (Reference Muller, Chan, Iwanaga, Wu, Chen, Lee, Tao, Rumrill and Bezyak2020) considering self-efficacy, resilience, coping, social support, social stigma, perceived stress, anxiety, cognitive impairment, pain intensity, fatigue, and sleep problems, and via a HCA in 302 FM patients identified three subgroups reflecting moderate, least and many biopsychosocial problems, but in this study pain appraisal variables were not included.
Although previous research considered important psychological characteristics associated with FM to establish subgroups, only a few studies contemplated cognitive-affective variables related to pain and none of them included pain acceptance, an important protective factor from FM impact. In the last few years pain acceptance has been proposed as a crucial concept that extends the Fear-Avoidance Model of Musculoskeletal Pain (Crombez et al., Reference Crombez, Eccleston, Van Damme, Vlaeyen and Karoly2012), and as one of the interrelated processes (along with cognitive defusion, flexible present-focused attention, self-as-observer, values, and committed action) included in the Psychological Flexibility Model (McCracken & Morley, Reference McCracken and Morley2014) for the management of chronic pain. Pain acceptance is defined as the willingness to live with pain with no need to try to change it (Thompson & McCracken, Reference Thompson and McCracken2011) and evidence suggests that contributes in a powerful way to developing a more constructive pain experience. In FM patients several studies found that pain acceptance was associated with lower emotional distress and disability (Lami et al., Reference Lami, Martínez, Miró, Sánchez and Guzmán2018), and better functional level and less symptoms (Tangen et al., Reference Tangen, Helvik, Eide and Fors2020), and that pain-related psychological flexibility and general psychological acceptance were related to functional status (Trainor et al., Reference Trainor, Baranoff, Henke and Winefield2019). Surprisingly, despite this evidence, no study has considered pain acceptance as a variable of interest in the configuration of FM subgroups.
To understand better the heterogeneity of FM the present cross-sectional study analyze the role of pain acceptance along with other cognitive-affective and physical variables in the configuration of differentiated subsets of patients. The selection of grouping variables was in line with previous research (mixed approach) and additionally incorporated pain acceptance as a key feature that had not been considered so far in this type of study. Knowing how these variables and their combinations reflect specific clinical clusters that entail certain needs and require personalized therapeutics can be of great practical interest. Further, it is well known that FM is frequently linked to high functional impairment and psychological distress, so in order to evaluate how well the clustering findings match to this prior knowledge disability and psychopathology were taken as criteria for external validation. Thus, the objectives of this study were: i) to identify subgroups of FM patients characterized by a specific constellation of physical manifestations (pain, fatigue and poor sleep quality) and cognitive-affective responses to pain (pain catastrophizing, pain vigilance, self-efficacy in pain management, and pain acceptance). ii) to analyze whether the FM subgroups identified differ in disability and psychopathology. We hypothesized that FM patients with more favorable cognitive-affective responses will show better adaptation to the disease (expressed as less disability and psychopathology).
Method
Patients
Considering that female sex is the variable most associated with FM in prevalence reports in Spain (e.g., Font et al., Reference Font, Bordoy, Mas, Seoane-Mato, Álvarez, Delgado, Martínez, Sánchez-Fernández, Rojas, García, Olivé, Rubio, Larrosa, Navarro, Sánchez-Piedra, Díaz-González and Bustabad-Reyes2020) in the present study only women were included. Participants were recruited from the Virgen de las Nieves University Hospital (Rheumatology Service) and several FM associations. The inclusion criteria to participate in this study were the following: (a) Be a woman between 18 and 67 years old; (b) have adequate reading comprehension; and (c) have a diagnosis of FM according to the criteria of the American College of Rheumatology (Wolfe et al., Reference Wolfe, Smythe, Yunus, Bennett, Bombardier, Goldenberg, Tugwell, Campbell, Abeles, Clark, Fam, Farber, Fiechtner, Franklin, Gatter, Hamaty, Lessard, Lichtbroun, Masi and Sheon1990, Reference Wolfe, Clauw, Fitzcharles, Goldenberg, Katz, Mease, Russell, Russell, Winfield and Yunus2010). The presence of other rheumatic/pain problems, relevant medical diseases or pregnancy, psychological disorder with severe symptoms, and history of alcoholism/substance addiction were exclusion criteria. Applying a consecutive sampling and the eligibility criteria, the final sample included 163 women with FM (see Appendix).
Measures
Measures for Cluster Derivation: Physical Symptoms and Cognitive-Affective Variables related to Pain
McGill Pain Questionnaire-Short Form (MPQ-SF; Melzack, Reference Melzack1987). The MPQ-SF evaluates pain experience with 15 sensory/affective descriptors, pain intensity during the previous week with a visual analogue scale (VAS) from no pain (1) to extreme pain (10), and pain intensity at the time of the test. In this study the VAS was selected considering that offers a more stable measure of pain and that it is in line with the time range of evaluation of the remaining measures used. The VAS has good sensitivity and specificity, and these values are considerably similar to those of dolorimetry (Marques et al., Reference Marques, Assumpção, Matsutani, Pereira and Lage2008).
Multidimensional Fatigue Inventory (MFI; Smets et al., Reference Smets, Garssen, Bonke and de Haes1995; adaptation by Fillion et al., Reference Fillion, Gélinas, Simard, Savard and Gagnon2003). The MFI comprises 20 items that evaluate general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. Items are assessed on a scale ranging from not agree (1) to completely agree (5). The Spanish version of the MFI showed good reliability, and construct validity in FM patients (Munguía-Izquierdo et al., Reference Munguía-Izquierdo, Segura-Jiménez, Camiletti-Moirón, Pulido-Martos, Álvarez-Gallardo, Romero, Aparicio, Carbonell-Baeza and Delgado-Fernández2012). In this study the general fatigue subscale was used (scores can vary from 1 to 5, high scores indicating greater fatigue). This domain was selected because includes general statements concerning to person functioning and it is the scale that shows better test-retest reliability and the highest associations to the VAS for global fatigue (Munguía-Izquierdo et al., Reference Munguía-Izquierdo, Segura-Jiménez, Camiletti-Moirón, Pulido-Martos, Álvarez-Gallardo, Romero, Aparicio, Carbonell-Baeza and Delgado-Fernández2012).
Pittsburgh Sleep Quality Index (PSQI; Buysse et al., Reference Buysse, Reynolds, Monk, Berman and Kupfer1989). The PSQI includes 19 items that explore subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The total-scale score ranges from 0 to 21 (high scores indicating greater disturbance). The Spanish version of the PSQI showed adequate internal consistency, test-retest reliability, and convergent validity in FM patients (Hita-Contreras et al., Reference Hita-Contreras, Martínez-López, Latorre-Román, Garrido, Santos and Martínez-Amat2014).
Pain Catastrophizing Scale (PCS; Sullivan et al., Reference Sullivan, Bishop and Pivik1995). The PCS consists of 13 items that evaluate magnification, rumination, and helplessness. Items are assessed on a scale from not at all (0) to all the time (4), and the total-scale score ranges from 0 to 52 (high scores indicating greater pain catastrophizing). The Spanish version of the PCS showed good internal consistency, test-retest reliability and sensitivity to change in FM patients (García-Campayo et al., Reference García-Campayo, Rodero, Alda, Sobradiel, Montero and Moreno2008).
Pain Vigilance and Awareness Questionnaire (PVAQ; McCracken, Reference McCracken1997). The PVAQ includes 16 items that assess awareness, consciousness, vigilance and observation of pain. Items are measured on a scale from never (0) to always (5), and the total-scale score ranges from 0 to 80 (high scores indicating greater pain vigilance). The Spanish version of the PVAQ showed good internal consistency, convergent validity, and divergent validity in FM patients (Martínez, Miró, Sánchez, Lami, et al., Reference Martínez, Miró, Sánchez, Lami, Prados and Ávila2014).
Chronic Pain Self-efficacy Scale (CPSS; Anderson et al., Reference Anderson, Dowds, Pelletz, Edwards and Peeters-Asdourian1995). The CPSS comprises 19 items that explore self-efficacy expectations regarding pain management, coping with symptoms, and physical function. Items are measured on a scale from totally incapable (0) to totally capable (10), and the total-scale score ranges from 0 to 190 (high scores indicating greater self-efficacy). The Spanish version of the CPSS showed good construct validity and internal consistency (Martín-Aragón et al., Reference Martín-Aragón, Pastor, Rodríguez-Marín, March, Lledó, López-Roig and Terol1999).
Chronic Pain Acceptance Questionnaire (CPAQ; McCracken et al., Reference McCracken, Vowles and Eccleston2004). The CPAQ includes 20 items that assess activity engagement and pain willingness. Items are measured on a scale from never true (0) to always true (6), and the total-scale ranges from 0 to 120 (high scores indicating greater pain acceptance). The Spanish version of the CPAQ showed adequate test-retest reliability, internal consistency, and construct validity in FM patients (Rodero et al., Reference Rodero, García-Campayo, Casanueva, López del Hoyo, Serrano-Blanco and Luciano2010).
Measures for External Validation: Disability and Psychopathology
Fibromyalgia Impact Questionnaire (FIQ; Burckhardt et al., Reference Burckhardt, Clark and Bennett1991). The FIQ consists of 10 items that explore the current health status of FM patients. Item 1 assesses functional capacity for daily living ranged from always (0) to never (3). Items 2 and 3 ask the patient to mark the number of days in last week he/she felt well/unable to work (ranged from 0 to 7 days). Items 4 through 10 are scales marked in 10 levels which evaluate work difficulty, pain, fatigue, morning tiredness, stiffness, anxiety, and depression. The total-scale score ranges from 0 to 100 (high scores indicating greater impact). The Spanish version of the FIQ showed good test-retest reliability, internal consistency, validity, and sensitivity to change (Rivera & González, Reference Rivera and González2004).
Symptom Checklist–90–Revised (SCL–90–R; Derogatis, Reference Derogatis1994). The SCL–90–R evaluates psychopathological symptoms through 90 items estimated on a scale from not at all (0) to very much or extremely (4). It includes 9 primary dimensions (somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation and psychoticism) and 3 global indices (global severity index, positive symptom total, and positive symptom distress index, PSDI). In this study the PSDI was used (the score ranges from 0 to 4, high scores indicating greater distress). The PSDI measures the intensity of the symptoms perceived and reflects the person’s characteristic style of expression psychic distress. The Spanish version of the SCL–90–R showed adequate reliability and validity (González de Rivera et al., Reference González de Rivera, de las Cuevas, Rodríguez-Abuín and Rodríguez-Pulido2002).
Procedure
Participants were administered an interview to gather information on demographic and clinical data. After the interview a booklet of self-report measures about physical symptoms, cognitive-affective variables, disability and psychopathology was delivered that the person had to complete at home, setting a new appointment for delivery within a maximum of one week (or facilitating any other way of delivery). The study was approval by the Ethics Committee of the Universidad de Granada, and all patients signed an informed consent document to collaborate in the study.
Data Analysis
Since it is necessary to have a sample size of at least 2 m participants (where m = number of clustering variables) for a cluster analysis (Formann, Reference Formann1984), in the present study, m = 7 and a minimum of 128 subjects was required. Therefore, the sample recruited (n = 163 women with FM) was adequate.
All analyses were performed using the SPSS 20.0 software. For statistical significance a level of p < .05 was considered. As a preliminary step missing values of data were imputed with fully conditional specification method (MCMC), and outliers were identified using Mahalanobis distance and excluded of the sample. Pearson correlation coefficient between the measures for cluster derivation was computed to confirm that data were suitable to perform the cluster analysis. In order to establish FM groups a HCA (agglomerative method) was performed. Squared Euclidean distance was considered in the proximities matrix. To form clusters at each stage Ward’s method was applied. Standardized data (z-scores) were used to minimize the bias associated to different metric of variables. The optimal number of clusters was determined observing the percentage change in agglomeration coefficient between successive cluster solutions, and considering the clinical interpretability and parsimony of data (minimal number of groups). To explore the differences between FM groups in the variables for cluster derivation and in the variables for external validation, multivariate analyses of variance (MANOVAs) were computed. The differences between FM groups in each variable were examined via one-way ANOVAs (with Scheffé test for post-hoc contrasts). Finally, a discriminant function analysis was conducted to examine the relative weight of each clustering variable in discriminating between the FM groups.
Results
Description of the Sample
The characteristics of the sample (n = 163) are presented in Table 1. The patients were women with an average age of 50.98 years. The majority was married (82.2%), and had primary/secondary education (61.1%), with 34.2% in active work status and 23.6% in sick leave due to disability. The patients had high/moderated scores in pain intensity and general fatigue, and poor sleep quality (cut-off point in PSQI > 5; Buysse et al., Reference Buysse, Reynolds, Monk, Berman and Kupfer1989). The levels of pain catastrophizing and pain vigilance were moderated and similar to those presented by FM patients from the study by Crombez et al. (Reference Crombez, Eccleston, van den Broeck, Goubert and van Houdenhove2004). The levels of pain self-efficacy and pain acceptance were moderated and higher than those reported by FM patients in other studies (Lledó-Boyer et al., Reference Lledó-Boyer, Pastor-Mira, Pons-Calatayud, López-Roig, Rodríguez-Marín and Bruehl2010; Rodero et al., Reference Rodero, García-Campayo, Casanueva, López del Hoyo, Serrano-Blanco and Luciano2010). The FM impact was severe (cut-off point in FIQ ≥ 59; Bennett et al., Reference Bennett, Bushmakin, Cappelleri, Zlateva and Sadosky2009), and the level of psychopathology corresponded to 85th percentile of the group of healthy women (González de Rivera et al., Reference González de Rivera, de las Cuevas, Rodríguez-Abuín and Rodríguez-Pulido2002).
Table 1. Demographic and Clinical Characteristics of FM Patients (n = 163)
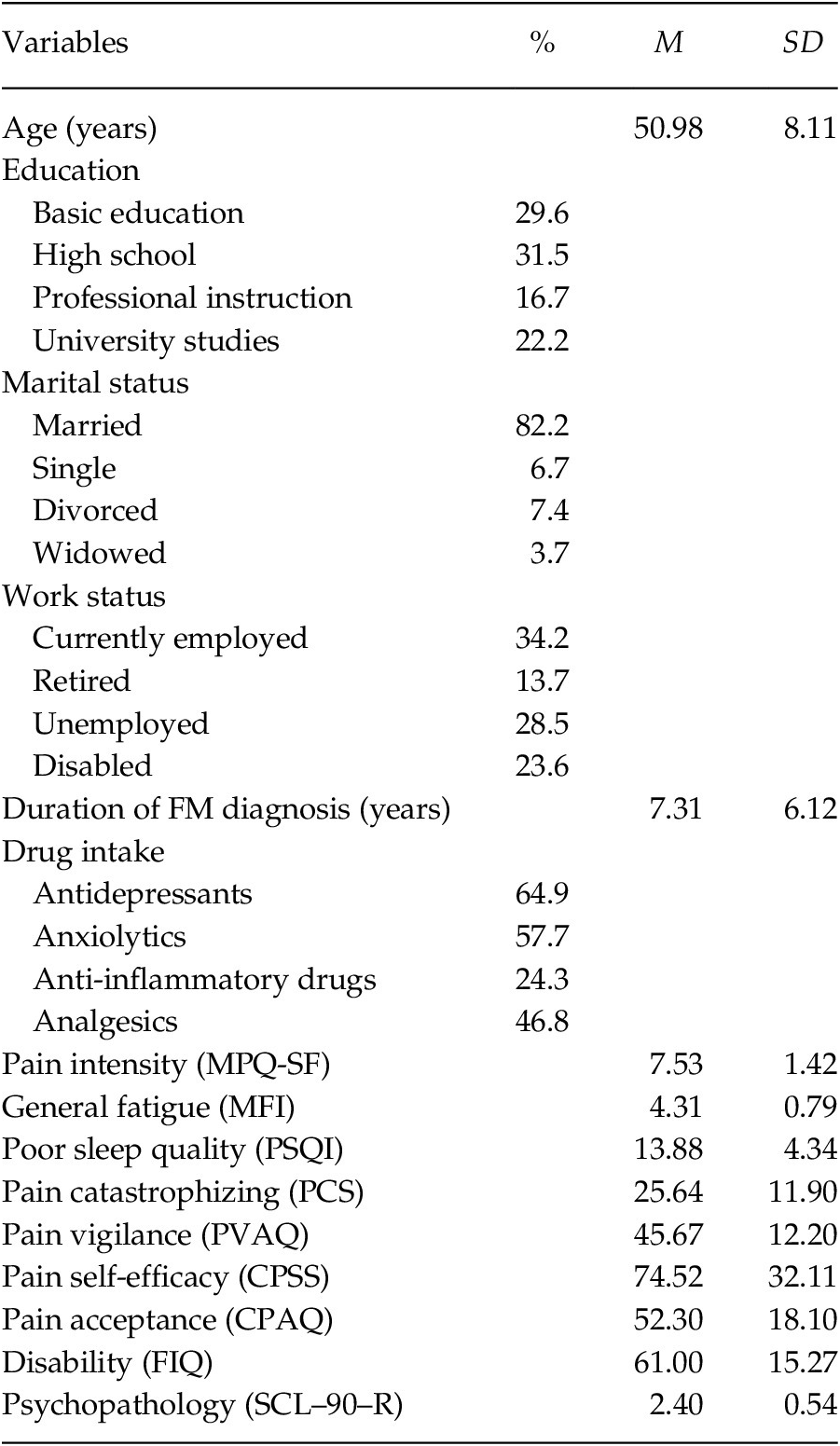
Note. MPQ-SF = McGill Pain Questionnaire-Short Form; MFI = Multidimensional Fatigue Inventory; PSQI = Pittsburgh Sleep Quality Index; PCS = Pain Catastrophizing Scale; PVAQ = Pain Vigilance and Awareness Questionnaire; CPSS = Chronic Pain Self-efficacy Scale; CPAQ = Chronic Pain Acceptance Questionnaire; FIQ = Fibromyalgia Impact Questionnaire; SCL–90–R = Symptom Checklist–90–Revised.
Clustering
In the sample, 2.7% of lost values were identified and imputed. Two participants were excluded because they presented atypical values, leaving the sample consisting of 161 patients, an appropriate size to proceed with the cluster analysis. Correlational analysis revealed significant correlations between all variables, ranging from r = –.56 (p < .01) to r = .57 (p < .01), except between pain intensity and general fatigue (r = .08, p = .309) and between sleep quality and pain vigilance (r = .10, p = .193).
Considering the agglomeration coefficients the first marked percentage change occurred from Stage 2 (coefficient = 0.000) to Stage 3 (coefficient = 0.119), so the solution of 3-cluster solution was identified as the most appropriated. Group 1 was integrated by 72 participants, Group 2 by 19, and Group 3 by 70.
The MANOVA confirmed that the set of variables for the clusters derivation differentiated the groups, Wilks’ λ = .17, F(14, 304) = 31.50, p < .001, ηp2 = .59. Specifically, the one-way ANOVAs showed significant differences between the groups in each variable, between F(2, 158) = 11.19, p < .01 and F(2, 158) = 94.93, p < .01 (see Table 2 and Figure 1). Considering the post-hoc contrasts, Group 1 differed significantly from the other groups in all aspects considered. Group 1 showed greater pain intensity and general fatigue, worse quality of sleep, greater pain catastrophizing and pain vigilance, and lower pain self-efficacy and pain acceptance than Groups 2 and 3. Group 1 can be defined by high physical and psychological affectation. Taking into account the post-hoc contrasts, Group 2 differed significantly from Group 3 in several aspects. Group 2 showed less general fatigue, better quality of sleep, and greater pain self-efficacy than Group 3; while Group 3 presented less pain catastrophizing than Group 2. So, Group 2 can be defined by low physical affectation and high pain self-efficacy, and Group 3 by moderate physical affectation and low pain catastrophizing.
Table 2. Comparison between FM Groups in Measures for Cluster Derivation
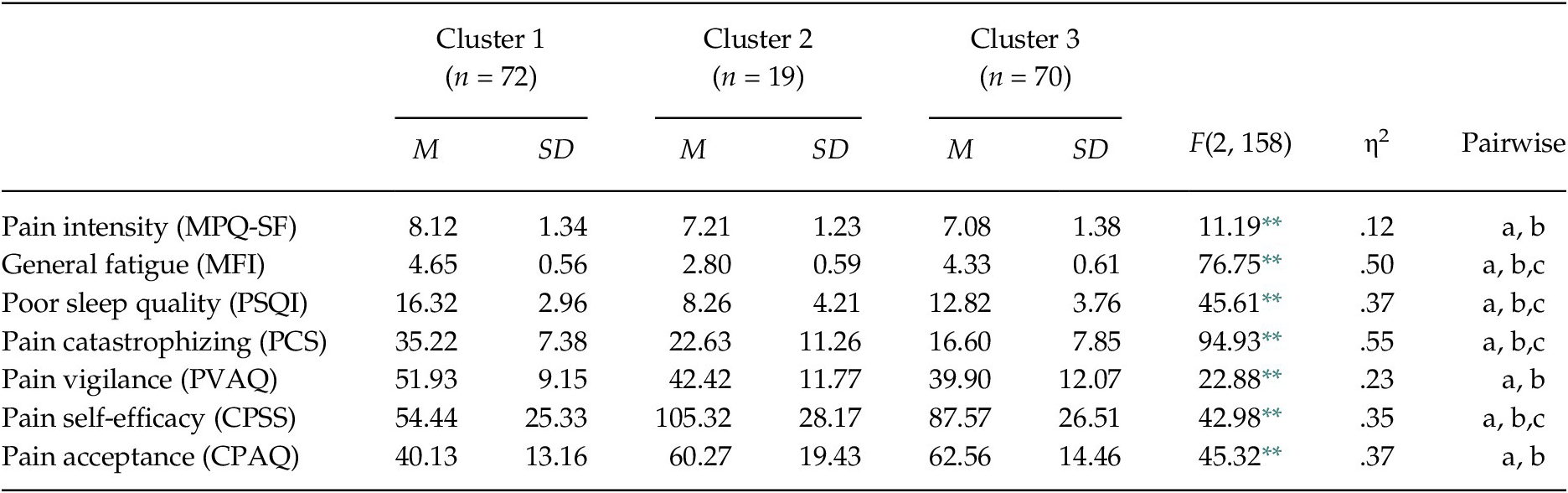
Note. MPQ-SF = McGill Pain Questionnaire-Short Form; MFI = Multidimensional Fatigue Inventory; PSQI = Pittsburgh Sleep Quality Index; PCS = Pain Catastrophizing Scale; PVAQ = Pain Vigilance and Awareness Questionnaire; CPSS = Chronic Pain Self-efficacy Scale; CPAQ = Chronic Pain Acceptance Questionnaire. Significant post-hoc contrasts (Scheffé test, p < .05): (a) Cluster 1 vs. Cluster 2; (b) Cluster 1 vs. Cluster 3; (c) Cluster 2 vs. Cluster 3. The observed power in all analyses was > .95, calculated with p = .05.
** p < .001.
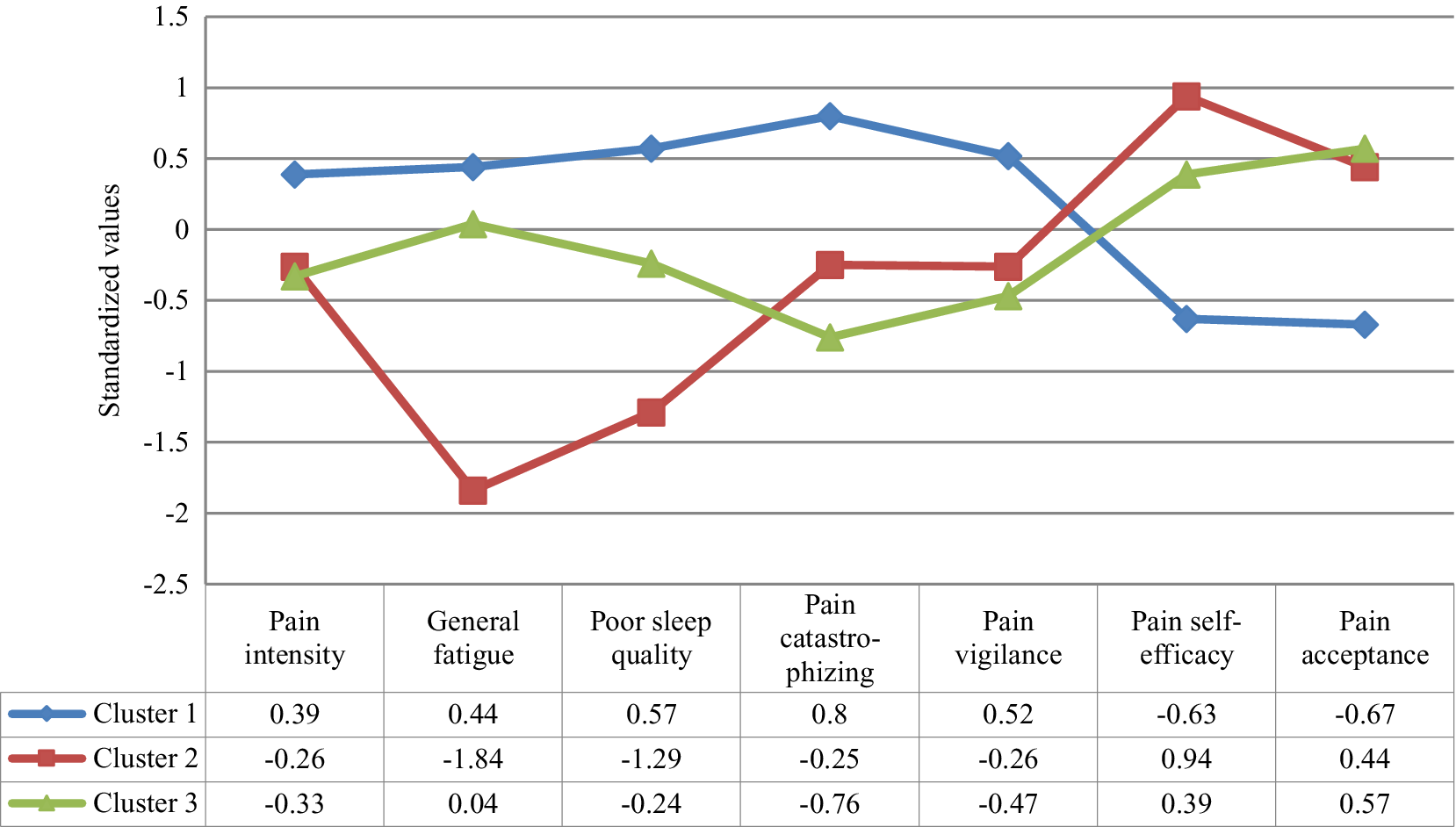
Figure 1. Profile of the Clusters in the Clinical Grouping Variables
To identify the differentiating capacity of the variables, a discriminant analysis was carried out (see Table 3). Considering the greater load of each variable throughout the clusters, the findings revealed that: In Group 1 the most relevant variable was pain acceptance (–0.66); in Group 2 were pain intensity, general fatigue, poor quality of sleep (–0.37, –3.79 and –2.02, respectively) and pain self-efficacy (0.91); and in Group 3 were pain catastrophizing and pain vigilance (–1.38 and –0.11, respectively).
Table 3. Coefficients of the Discriminant Functions of each Cluster
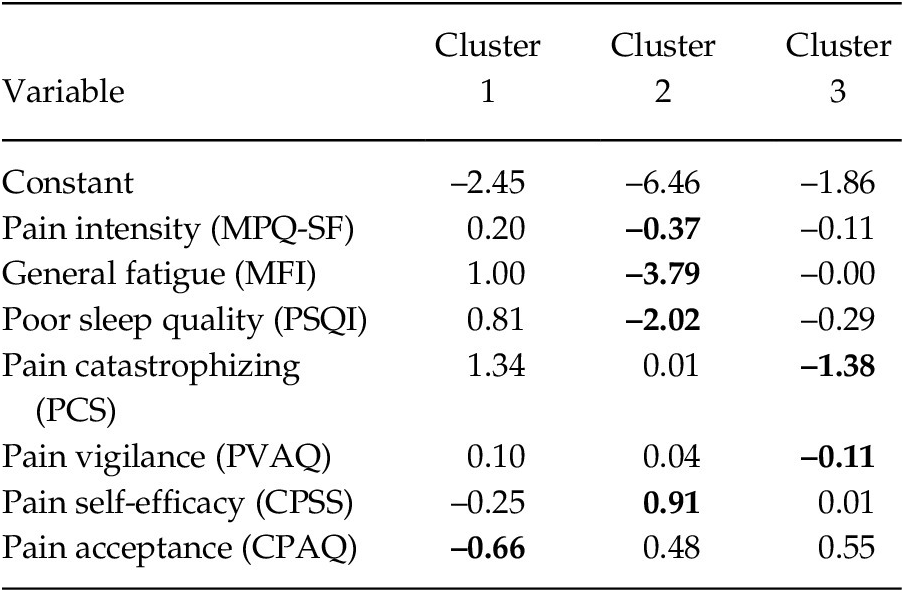
Note. MPQ-SF = McGill Pain Questionnaire-Short Form; MFI = Multidimensional Fatigue Inventory; PSQI = Pittsburgh Sleep Quality Index; PCS = Pain Catastrophizing Scale; PVAQ = Pain Vigilance and Awareness Questionnaire; CPSS = Chronic Pain Self-efficacy Scale; CPAQ = Chronic Pain Acceptance Questionnaire. The values of the variables with the highest coefficients are shown in bold.
External Validation of Clusters
The MANOVA indicated that the external validation variables differentiated between the clusters, Wilks’ λ = .72, F(4, 314) = 14.09, p < .001, ηp2 = .15. The one-way ANOVAs showed significant differences between the groups in disability F(2, 158) = 14.46, p < .01, and psychopathology F(2, 158) = 22.88, p < .01 (see Table 4). The post-hoc contrasts showed that Group 1 had greater disability and psychopathology than Groups 2 and 3.
Table 4. Comparison between FM Groups in Measures for External Validation
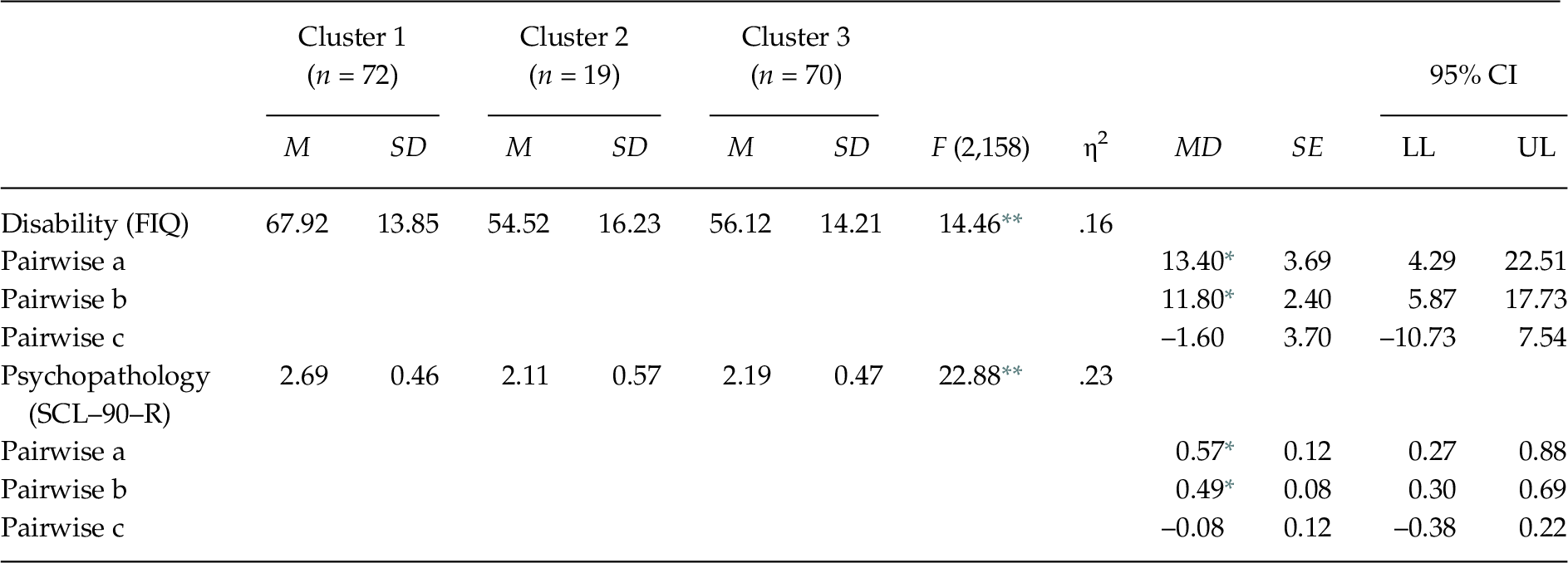
Note. FIQ = Fibromyalgia Impact Questionnaire; MD = Mean Difference; SCL–90–R = Symptom Checklist–90–Revised. Significant post-hoc contrasts (Scheffé test, p < .05): (a) Cluster 1 vs. Cluster 2; (b) Cluster 1 vs. Cluster 3; (c) Cluster 2 vs. Cluster 3. The observed power in all analyses was > .95, calculated with p = .05.
* p < .05;
** p < .001.
Differences in Demographic Variables between Clusters
The groups did not differ significantly in age F(2, 160) = 1.44, p = .241, education χ2(10) = 12.26, p = .268, nor marital status χ2(8) = 8.25, p = .409, however they differed in work status χ2(10) = 18.97, p = .041, with the highest percentage of disabled in Group 1.
Discussion
This is the first study that examines the profiles of FM patients taking into account the role of pain acceptance, together with another key psychological variables (pain catastrophizing and pain vigilance) contemplated in the fear-avoidance model of musculoskeletal pain (Leeuw et al., Reference Leeuw, Goossens, Linton, Crombez, Boersma and Vlaeyen2007).
Cluster analysis in the FM sample revealed three conglomerates based on the severity of physical symptoms and cognitive-affective variables related to pain. Group 1 was characterized by highest levels of pain intensity, general fatigue, poor sleep quality, pain catastrophizing, and pain vigilance, and lowest levels of pain self-efficacy and pain acceptance. Group 2 showed the lowest level of general fatigue, moderated levels of pain intensity, pain catastrophizing, pain vigilance and pain acceptance, and the highest levels of sleep quality and pain self-efficacy. Group 3 displayed by the lowest level of pain intensity, pain catastrophizing and pain vigilance, moderated levels of general fatigue, sleep quality, and pain self-efficacy, and the highest level of pain acceptance. Significant differences were observed in all variables between Group 1 and Groups 2 and 3, with Group 1 showing the most dysfunctional profile. Likewise, significant differences were observed between Group 2 and Group 3, with Group 2 exhibiting less general fatigue, better sleep quality, and greater pain self-efficacy than Group 3, and Group 3 showing less pain catastrophizing than Group 2. The variables with greater discriminative power were general fatigue, sleep quality and pain catastrophizing. Regarding the external validity of the identified conglomerates, only significant differences were found between Group 1 and Groups 2 and 3, with Group 1 showing the highest levels of FM impact and psychopathological distress. Probably the greater self-efficacy and less catastrophizing could account for the better clinical status of patients in Groups 2 and 3, confirming the hypothesis raised.
Taking into account the most dysfunctional profile represented by Group 1, the low pain acceptance seems to be a relevant variable linked to severity of FM, which is in line with previous research (e.g., Lami et al., Reference Lami, Martínez, Miró, Sánchez and Guzmán2018; Tangen et al., Reference Tangen, Helvik, Eide and Fors2020; Trainor et al., Reference Trainor, Baranoff, Henke and Winefield2019). However, beyond the low pain acceptance, other processes of psychological inflexibility may be involved in FM experience. For example, there is evidence that cognitive fusion is associated with poorer functioning indirectly through FM symptoms (Écija et al., Reference Écija, Luque-Reca, Suso-Ribera, Catala and Peñacoba2020). The contribution of other facets of psychological inflexibility is a relevant issue that needs further research.
Greater attention requires the distinction between Groups 2 and 3 that reflect more adaptive profiles. In fact, the differences between both clusters correspond to pain self-efficacy and the most discriminating variables (general fatigue, poor sleep quality and pain catastrophizing), which is in line with the classifying role of these features showed in previous studies (e. g., Estévez-López et al., Reference Estévez-López, Segura-Jiménez, Álvarez-Gallardo, Borges-Cosic, Pulido-Martos, Carbonell-Baeza, Aparicio, Geenen and Delgado-Fernández2017; Giesecke et al., Reference Giesecke, Williams, Harris, Cupps, Tian, Tian, Gracely and Clauw2003; Luciano et al., Reference Luciano, García-Forero, Cerdà-Lafont, Peñarrubia-María, Fernández-Vergel, Cuesta-Vargas, Ruíz, Rozadilla-Sacanell, Sirvent-Alierta, Santo-Panero, García-Campayo, Serrano-Blanco, Pérez-Aranda and Rubio-Valera2016; Muller et al., Reference Muller, Chan, Iwanaga, Wu, Chen, Lee, Tao, Rumrill and Bezyak2020; Plazier et al., Reference Plazier, Ost, Stassijns, de Ridder and Vanneste2015). It is possible that the peculiarities of Groups 2 and 3 are due to the fact that pain self-efficacy and pain catastrophizing and its complex interaction play a different role in fatigue, sleep quality and pain, determining differentiated profiles. In this sense, it was observed in patients with chronic pain that pain self-efficacy attenuated pain intensity’s direct effect on depressive symptoms and its indirect effect on depressive symptoms via pain catastrophizing and both effects were reduced in a dose-dependent manner (Cheng et al., Reference Cheng, Leung, Chan, Chen, Chow, Chung, Law, Lee, Leing and Tam2018). Investigating the pathways of reciprocal influence between physical factors and protective and exacerbating cognitive-affective factors using structural equations models can shed light on this question in FM.
In general, the findings of the present study show that pain acceptance is not an especially critical variable in the establishment of clinical profiles in FM patients. Rather, it seems that its potential contribution is partially overshadowed by the more powerful action of other pain-related cognitive-affective variables such as catastrophizing and self-efficacy. This trend was also observed in previous studies that showed the relevant contribution of self-efficacy on pain intensity and functional/psychological adjustment to FM and the less salient role of pain acceptance (Sahar et al., Reference Sahar, Thomas and Clarke2016), and that pain catastrophizing mediated the relationship between pain and depression/anxiety but pain acceptance no played a mediating role in these relationships (Lami et al., Reference Lami, Martínez, Miró, Sánchez and Guzmán2018). Acceptance may have a greater favourable influence when combined with other psychological features of resilience, although this is something that research needs to examine.
The present study replicated the findings of Giesecke et al. (Reference Giesecke, Williams, Harris, Cupps, Tian, Tian, Gracely and Clauw2003), Plazier et al. (Reference Plazier, Ost, Stassijns, de Ridder and Vanneste2015), and Luciano et al. (Reference Luciano, García-Forero, Cerdà-Lafont, Peñarrubia-María, Fernández-Vergel, Cuesta-Vargas, Ruíz, Rozadilla-Sacanell, Sirvent-Alierta, Santo-Panero, García-Campayo, Serrano-Blanco, Pérez-Aranda and Rubio-Valera2016) regarding the number of groups identified and the relevance of pain catastrophizing in the profiling of the conglomerates. However, it was not possible a detailed comparison between the present study and previous studies given the different variables considered and instruments used to evaluate the same variable, which may mean that the same construct is not being captured in all the studies.
Identification of profiles of FM patients based on clinically important features have practical value in terms of patient-orientated therapeutic approaches. Some studies have observed differential response to interdisciplinary treatment among FM patients classified according to the MPI. Turk et al. (Reference Turk, Okifuji, Sinclair and Starz1998) reported that the dysfunctional subgroup exhibited significant reductions in pain, psychological distress, and disability, the interpersonally distressed subgroup showed no changes in these parameters, and the adaptive copper subgroup only reported significant improvements in pain. Verra et al. (Reference Verra, Angst, Brioschi, Lehmann, Keefe, Staal, de Bie and Aeschlimann2009) reported that the greatest improvements in pain severity, pain interference and physical functioning were showed by the dysfunctional group.
Other studies have recommended specific therapeutic strategies based on the FM profiles. Giesecke et al. (Reference Giesecke, Williams, Harris, Cupps, Tian, Tian, Gracely and Clauw2003) suggested that patients with moderate distress might not need cognitive-behavioral therapy (CBT), patients with high distress might benefit from CBT and antidepressant and other psychotropic drugs, whereas patients with normal distress but highest hyperalgesia could better respond to antidepressant drugs with analgesic properties. Thieme et al. (Reference Thieme, Turk and Flor2004) proposed that dysfunctional patients might benefit from a therapy focused on modification of solicitous responses by significant others and the management of anxiety disorders associated; interpersonally distressed patients might benefit from a therapy focused on interpersonal problem-solving and communication skills and the management of comorbid depression; and adaptive coppers patients might benefit from an approach focused on exercise and support without additional psychological therapy. Luciano et al. (Reference Luciano, García-Forero, Cerdà-Lafont, Peñarrubia-María, Fernández-Vergel, Cuesta-Vargas, Ruíz, Rozadilla-Sacanell, Sirvent-Alierta, Santo-Panero, García-Campayo, Serrano-Blanco, Pérez-Aranda and Rubio-Valera2016) suggested that education, aerobic exercise and some medications would be sufficient for the functional group, and CBT or acceptance and commitment therapy could be additional beneficial components for dysfunctional and highly dysfunctional and distressed groups, in low-intensity and high-intensity, respectively. Yim et al. (Reference Yim, Lee, Park, Kim, Nah, Lee, Kim, Lee, Hong, Kim, Lee, Kim, Joung, Kim and Lee2017) recommended for highly dysfunctional patients intensive pharmacological therapy, for lower dysfunctional patients non-pharmacological therapy (CBT, exercise and self-management strategies) and for the middle dysfunctional patients pharmacological and/or non-pharmacological interventions based on the dominant symptoms.
The FM profiles identified in the present study seem to show a continuum of severity and a remarkable concordance between the levels of physical symptoms and the levels of cognitive-affective variables related to pain. So, matching clinical needs of FM patients with the psychological strategies can lead to better care of the patients. Group 1 that presents the greatest physical and psychological affectation, and is the conglomerate in which the low pain acceptance becomes more relevant, could benefit from CBT for insomnia (Martínez, Miró, Sánchez, Díaz-Piedra, et al., Reference Martínez, Miró, Sánchez, Díaz-Piedra, Cáliz, Vlaeyen and Buela-Casal2014) or CBT for pain and insomnia (Prados et al., Reference Prados, Miró, Martínez, Sánchez, Lami and Cáliz2020) (education, sleep hygiene, bed time restriction, stimulus control, physiological deactivation techniques, activity-rest adjustment, communication and problem solving training, and cognitive restructuring to change dysfunctional thoughts related to pain and sleep) combined with elements of acceptance/mindfulness-based interventions (Veehof et al., Reference Veehof, Trompetter, Bohlmeijer and Schreurs2016) (training in psychological flexibility via acceptance of experience, cognitive defusion, present moment awareness, sense of self as-context, valued life directions and committed actions, and training in intentional and non-judgmental awareness via meditation). Groups 2 and 3 that have a more functional profile than Group 1, would require a less intensive and prolonged therapeutic approach in which the components of CBT would be differentially emphasized depending on the preponderance of the symptoms. For Group 2, characterized by low physical affectation and moderate pain catastrophizing, cognitive restructuring focused on replacing dysfunctional thoughts about pain for more adaptive ones would be the best option. For Group 3, characterized by a moderate physical affectation and a low psychological affectation, strategies aimed at improving sleep (sleep hygiene, bed time restriction, stimulus control, and physiological deactivation techniques), and an adequate regulation of levels of activity and rest to alleviate fatigue would be recommended. Consider that Group 2 is probably an “atypical” group due to its better level of adaptation, since the usual is that FM notably affects the physical and emotional functioning of the person. In fact, most of the FM patients perceive themselves with moderate to severe disability (Horta-Baas & Romero-Figueroa, Reference Horta-Baas and Romero-Figueroa2019), and show more difficulty adjusting to the disease and generally use poorer strategies to cope with pain than patients with other rheumatic diseases (Bucourt et al., Reference Bucourt, Martaillé, Goupille, Joncker-Vannier, Huttenberger, Réveillère, Mulleman and Courtois2019).
In the last years the CBT has acquired an increasingly prominent role in the integral approach to FM and can be an excellent treatment option for many patients with this pain condition. In this sense, several evidence-based clinical guidelines assign the highest level of recommendation to aerobic exercise, CBT, amitriptyline, and multicomponent treatment in the management of FM (Thieme et al., Reference Thieme, Mathys and Turk2017). Also, several studies show the efficacy and tolerability of CBT and acceptance-based therapies in reducing key clinical manifestations of FM compared to control interventions (see review by Bernardy et al., Reference Bernardy, Klose, Welsch and Häuser2018), and there is evidence to reveals that acceptance and commitment therapy (ACT) is beneficial in improving physical and emotional functioning in FM patients (see review by Hegarty et al., Reference Hegarty, Fletcher, Conner, Stebbings and Treharne2020). However, and although the differential efficacy between CBT and mindfulness-based therapies in chronic pain has been examined, no support of clear differences between both approaches has been found (see review by Pardos-Gascón et al., Reference Pardos-Gascón, Narambuena, Leal-Costa and van-der Hofstadt-Román2021). Thus, it is necessary to acquire more knowledge about “what works for whom” and the delimitation of clinical profiles in FM patients can be key to making decisions about the care of these patients.
This study presents several limitations. All measures were based on self-reports, and other objective measures to evaluate physical symptoms (e.g., tenderness via algometer or sleep quality via polysomnography) and the impact of FM (e.g., frequency of medical visits, medications consumption, days off work) would have provided relevant complementary information. Also, biomarkers were not available in this study. The sample consisted of middle-aged adult women with FM, so the findings could not be transferred to patients with other demographic characteristics. Finally, FM patients were not suffered associated diseases, so it is possible that FM patients with important comorbidity (physical and/or mental) could show different profiles to those identified in the present study.
With a view to future research, it would be necessary to examine the consistency of the clusters identified in other samples of FM patients, the confirmation of the optimal number of clusters via complementary statistical methods (e.g., latent profile analysis), the replicability of findings using revised versions of the self-reports (e.g., FIQ-revised), as well as the stability along the time of the clusters. Likewise, and given the key role of fatigue in FM patients (Velasco-Furlong et al., Reference Velasco-Furlong, Gutiérrez-Hermoso, Mateos-Pintado, Garvi-de Castro, Blanco-Rico, Sanromán-Canelada, López-Roig, Pastor-Mira and Peñacoba-Puente2020), it would be of great interest to examine whether the dimensionality of fatigue is also reflected in the establishment of clusters. It would also be of remarkable clinical utility to establish the predictive value of the classification generated by analyzing the differential response of each subgroup to CBT. In addition, it would be convenient to include other variables such as resilience (Alonso-Tapia et al., Reference Alonso-Tapia, Garrido-Hernansaiz, Rodríguez-Rey, Ruiz Díaz and Nieto2018) and stress biomarkers (Arco García et al., Reference Arco García, González Pérez, Henares Extremera, García León and Peralta-Ramírez2020) for the establishment of subgroups.
In conclusion, there were at least three subgroups of FM patients based on the pattern of physical and cognitive-affective characteristics with important implications for individuals and health care system. The assessment of psychological aspects is crucial to delineate the optimal management of patients according to their clinical needs. Future research faces the challenge of identifying and validating which psychological therapies can be most beneficial in each profile.
Appendix
Data Transparence
The present study is part of a larger research on psychological evaluation and intervention in fibromyalgia (FM). A part of the FM patients of this study participated in a psychometric study aimed at establishing the reliability and validity of Spanish adaptation of several cognitive-affective measures about pain. In addition, a part of the FM patients of this study was included in a randomized controlled trial to compare the efficacy of various psychological intervention programs. Since these are studies with very different objectives and methodology, the data collected have been (and/or will foreseeably be) presented in separate papers. The present study constitutes an original use of data, does not overlap with the aforementioned studies, and has not been published previously.







