Introduction
Birds are involved in the short- and long-distance dispersal of plant diaspores (seeds and fruits) (Viana et al., Reference Viana, Gangoso, Bouten and Figuerola2016; Godínez-Alvarez et al., Reference Godínez-Alvarez, Ríos-Casanova and Peco2020). Diaspores can be transported after attaching to the animal's body (epizoochory) or after being consumed (endozoochory) (Viana et al., Reference Viana, Gangoso, Bouten and Figuerola2016). However, fruits and seeds must withstand mechanical digestion in the muscular stomach (gizzard) of birds (Ziswiler and Farner, Reference Ziswiler, Farner, Farner and King1972). Therefore, diaspores require protection, and those that effectively pass through a bird's digestive system exhibit diverse morphological modifications in the pericarp and/or seed coat. They often possess one or more layers of cells with thick lignified cell walls or produce mucilaginous envelope (Costea et al., Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Numerous studies have shown that diaspores that are protected in this manner can pass through the digestive system and germinate after being deposited in a suitable area (Barnea et al., Reference Barnea, Yom-Tov and Friedman1990; Rodríguez-Pérez et al., Reference Rodríguez-Pérez, Riera and Traveset2005; Vazačová and Münzbergová, Reference Vazačová and Münzbergová2013; Godínez-Alvarez et al., Reference Godínez-Alvarez, Ríos-Casanova and Peco2020; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). The passage of seeds through the digestive system can act in two main ways. It can decrease the germination when, for example, the seed spends a longer time in the gut or it can increase the germination due to the chemical or mechanical abrasion. It is also possible that the way through the digestive system has neutral effect on the seeds (Traveset, Reference Traveset1998).
The mucilage envelope performs diverse functions. Its hydration and water storage abilities may create optimal conditions for diaspore germination, while adhesive properties enable diaspore attachment to soil or animal bodies (epizoochoric dispersal) (Grubert, Reference Grubert1974; Kreitschitz, Reference Kreitschitz and Gorb2009; Western, Reference Western2012; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). The chemical composition of mucilage is a mixture of diverse polysaccharides that occur in different proportions in the envelope, determining the mucilage type: cellulosic, pectic or hemicellulosic (Kreitschitz, Reference Kreitschitz and Gorb2009; Western, Reference Western2012). Cellulose mucilage consists mainly of pectins, but also some hemicelluloses and cellulose fibrils are present and are typical of plant groups such as Asteraceae (e.g. Artemisia sp.), Brassicaceae (e.g. Arabidopsis sp., and Capsella sp.) and Lamiaceae (e.g. Ocimum sp. and Salvia sp.). Cellulose forms long fibrils that serve as scaffolds for the other components (Western, Reference Western2012; Kreitschitz and Gorb, Reference Kreitschitz and Gorb2018). The mucilage of Linum sp. is dominated by pectins and is classified as pectic type (Naran et al., Reference Naran, Chen and Carpita2008; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). The mucilage envelope of Plantago sp. diaspores is dominated by hemicelluloses and is classified as hemicellulose type (Saeedi et al., Reference Saeedi, Morteza-Semnani, Ansoroudi, Fallah and Amin2010; Phan et al., Reference Phan, Tucker, Khor, Shirley, Lahnstein, Beahan, Bacic and Burton2016). The different structures of mucilage polysaccharides (mainly pectins and hemicelluloses) and their proportions, as well as the presence of cellulose fibrils, determine the rheological and physical characteristics of mucilage, such as viscosity, friction and adhesion (Naran et al., Reference Naran, Chen and Carpita2008; Saeedi et al., Reference Saeedi, Morteza-Semnani, Ansoroudi, Fallah and Amin2010; Kreitschitz et al., Reference Kreitschitz, Kovalev and Gorb2015, Reference Kreitschitz, Kovalev and Gorb2016; Phan et al., Reference Phan, Tucker, Khor, Shirley, Lahnstein, Beahan, Bacic and Burton2016).
Diaspores passing through an animal's digestive system can suffer from damage such as abrasion, seed/coat fractures or even loss of parts (Beveridge, Reference Beveridge1964; Barnea et al., Reference Barnea, Yom-Tov and Friedman1990; Cochrane et al., Reference Cochrane, Friend and Hill2005; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Notably, mechanical processing of diaspores can be an indispensable element of their dispersal and colonizing success as damage to or loss of the hard, thick coat may enable subsequent germination (Izhaki and Safriel, Reference Izhaki and Safriel1990; Fukui, Reference Fukui1996; Traveset et al., Reference Traveset, Riera and Mas2001; Cochrane et al., Reference Cochrane, Friend and Hill2005; Costa et al., Reference Costa, Ramos, da Silva, Timoteo, Araújo, Felgueiras, Rosa, Matos, Encarnação, Tenreiro and Heleno2014, Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019). Our previous study (conducted exactly in the same experimental conditions like the present experiment, Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) revealed that a mucilaginous envelope, owing to its low friction, provides favourable conditions for diaspores to pass through the pigeon digestive system (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). By testing the endozoochoric potential of mucilaginous seeds, we showed that 13.5% of the recovered diaspores of three Plantago (P. lanceolata, P. psyllium and P. ovata) taxa with hemicellulose mucilage that passed through the pigeon digestive system were able to germinate. In contrast, few non-mucilaginous seeds (Brassica napus, Nigella sativa and Amaranthus albus) passed through the digestive system (0.1% of recovered seeds). The results of our previous study clearly showed that the mucilage envelope plays a protective role for diaspores in endozoochory and that endozoochoric diaspore dispersal depends on the mucilage type (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021).
The fleshy fruits are in the first line of these diaspores which attract diverse consumers (also birds) and thus increasing the probability of seed dispersal (Schaefer and Ruxton, Reference Schaefer, Ruxton, Schaefer and Ruxton2011). Mucilaginous diaspores are sparsely reported among the many seeds found in animal droppings. This may be because the role of the mucilage envelope in diaspore dispersal has not been previously examined in detail. Previous studies have mainly focused on the diaspore condition, survival number and taxonomic status (family, genus and species). However, mucilaginous diaspores have been collected from the droppings of diverse animals, including mammals such as cattle and sheep and birds such as bullfinches (Pyrrhula pyrrhula), green finches (Chloris chloris), grey partridges (Perdix perdix), house sparrows (Passer domesticus), European greenfinches pigeons (Columba ssp.) and mallards (Anas platyrhynchos). Among the diverse seeds collected, those from Plantago were also found in the droppings (Eber, Reference Eber1962; Cavers et al., Reference Cavers, Bassett and Crompton1980; Vazačová and Münzbergová, Reference Vazačová and Münzbergová2013; Orłowski et al., Reference Orłowski, Czarnecka, Goławski, Karg and Paneket2016; Lovas-Kiss et al., Reference Lovas-Kiss, Vizi, Vincze, Molnár and Green2018a,Reference Lovas-Kiss, Sánchez, Wilkinson, Coughlan, Alves and Greenb). The results of our previous study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) showed that the diaspores of Plantago sp. survived being passed through the pigeon digestive system and were represented at the highest rate.
The present work is a continuation of our previous experimental study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021), in which we attempted to determine whether the presence of a mucilage envelope around diaspores improves their endozoochoric dispersal capability. The main objective of the present study was to evaluate the performance of diaspores devoid of their mucilage envelope in passing through the digestive tract of pigeons and maintaining their ability to germinate. Additionally, we investigated whether non-mucilaginous diaspores covered with an artificial mucilage envelope can better withstand passage through the bird's digestive system.
Material and methods
Plant material
We used commercially supplied mucilaginous diaspores of the following taxa: Linum usitatissimum (Market Hall, Wrocław, Poland), Plantago lanceolata (Pflanzen-Vielfalt, Germany), P. ovata, P. psyllium (FLOS, Poland) (like in previous experiment Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Additionally, we used P. ovata husk (Intenson Europe, Poland) as ‘artificial’ mucilage. The following non-mucilaginous seed were also tested: B. napus, N. sativa, A. albus (Market Hall, Wrocław, Poland) (like in the previous experiment, Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) We did not test the intact mucilaginous seeds studied previously (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) that is Ocimum basilicum, Salvia hispanica, Lepidium sativum, because it was difficult to remove the cellulose mucilage from these diaspores without damaging them mechanically. The germination ability of all seeds was tested before they were used in the experiment. Mucilage envelope was visualized using 0.1% aqueous solution ruthenium red (Sigma-Aldrich) (Braune et al., Reference Braune, Leman and Taubert1975). The images were documented with a light microscope connected to the camera (Zeiss Axioplan, AxioCam MRc, Carl Zeiss Microscopy, GmbH, Germany).
Experimental design
In this study, we used a group of domestic pigeons (Columba livia domestica) from a private colony of one of the co-authors (E. Haase), situated close to the Kiel University campus in Germany. Bird feeding followed the protocol described in our previous study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Nine pigeons were used per plant species, and a total of 2700 seeds (prepared as described below) of each species were packed into gelatin capsules. Each pigeon was fed one/two capsule(s) containing 300 seeds, and the number of each type of seed that passed through the digestive system intact was recorded. The droppings were collected during 24 h after feeding (for the details, see Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Additionally, seed germination tests were performed as described below. Summarizing – we used 300 seeds per one pigeon what makes: nine pigeons × 300 seeds = 2700 seed in total (per one plant species).
Preparation of seeds through natural mucilage removal
We removed the natural mucilage from seeds of P. lanceolata, Plantago ovata, Plantago psyllium, and L. usitatissimum. Mucilage was removed from the diaspore surface using a magnetic stirrer. The diaspores were hydrated with distilled water (30 min, at room temperature), mixed with fine sand, and stirred for 2.5–3.0 h at 100 rpm at room temperature. We used fine sand because it binds well to mucilage and helps remove it from the diaspore surface. Finally, the mixture was put on the sieve and using a pestle and sand the mucilage was grinding and finally separated from the seeds. Diaspores were washed with tap water, placed in a petri dish, and air-dried for at least 24 h at room temperature. Subsequently, diaspores were examined using a stereomicroscope (Leica M205A, Leica DFC420 camera, software LAS V3.8, Leica Microscopy GmbH, Wetzlar, Germany), and those with visible mucilage or noticeable cracks or breaks were removed. The mucilage of L. usitatissimum was difficult to remove using water (at room temperature); therefore, the seeds were stirred in water at 55–60°C for approximately 3 h, which easily removed the mucilage. Seeds were examined and sorted as described above. The prepared seeds were packed into gelatin capsules (300 seeds = one portion = one pigeon).
Preparation of seeds through artificial mucilage application
We used commercially supplied P. ovata husk as an artificial mucilage and applied it to the non-mucilaginous seeds of B. napus, N. sativa and A. albus to investigate its impact on the survival rate of diaspores passed through a pigeon's digestive system. First, the weight of 300 P. ovata seeds (with mucilage) was calculated. Then the seeds were weighed after mucilage removal and the difference in weight was calculated (23%). This determined the amount of artificial mucilage that was added to the non-mucilaginous seeds (23% of the dry weight of 300 non-mucilaginous diaspores). We calculated the exact mass of the artificial mucilage for every 300 non-mucilaginous seeds according to the following proportion:
x is the mass of the ‘artificial’ mucilage.
Seeds (300 seeds = one sample = one pigeon) were prepared in two ways: (1) seeds were mixed with dry artificial mucilage and packed into the capsules and (2) seeds were mixed with hydrated artificial mucilage to cover their surface and then air-dried for 24 h before being packed into the capsules. Non-mucilaginous seeds and the same seeds covered with hydrated artificial mucilage and air-dried were visualized using Scanning Electron Microscope. The samples were glued to the SEM stubs with a carbon-containing double-sided adhesive conductive tape. They were coated with gold-palladium (film thickness 10 nm) using a Leica EM SCD 500 High Vacuum Sputter Coater (Leica Microsystems GmbH, Wetzlar, Germany) and visualized in the SEM (Hitachi S-4800, Hitachi High-Tech. Corp., Tokyo, Japan).
Germination test for seeds that passed through the digestive systems of pigeons
Seeds that passed through the pigeon's digestive system intact were collected and subjected to a germination test. The germination test followed the protocol described in our previous study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Germination energy (the number of seeds germinated after a defined short period of time, 5 days) and germination strength (the number of seeds germinated after a defined longer period of time, 21 days, after which all viable seeds should have germinated) were calculated. Germinated seeds (identified by a visible radicle) were counted (International Rules of Seeds Evaluation, 1997; Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021; Winiarczyk et al., Reference Winiarczyk, Skrzypczak, Jaroszuk-Ściseł and Bocianowski2014). Mouldy seeds were removed from the experiment to avoid further microbial or fungal contamination. As controls, we used demucilaginated seeds of the same taxa as well as non-mucilaginous seeds, with and without artificial mucilage that had not been fed to pigeons. We tested the same number of seeds in the control groups as that collected from pigeons. In the case of seeds with artificial mucilage, we did not add the mucilage to the control seeds due to the difficulty of estimating the dry mucilage weight for one to three seeds. However, we do not believe that such a minimal amount of artificial mucilage would greatly influence the germination rate.
Additional germination test of mucilaginous versus demucilaginated, non-mucilaginous seeds and seeds with ‘artificial’ mucilage
We performed this test to determine how the absence/presence of natural and artificial mucilage envelope influences seed germination. In our experiment, only a few seeds from each group (demucilagined seeds, seeds with ‘artificial’ mucilage) passed the digestive system of the pigeons. This was not sufficient to evaluate the germination ability of the samples. We decided to perform additional tests (G1, G2) to check, whether the seeds with mechanically removed mucilaginous envelope and the seeds with artificial mucilage are able to germinate. The results are summarized in Tables 4 and 5 for the original seeds used in the experiment. We tested 30 seeds per all of here used species for (a) mucilaginous seeds, (b) demucilaginated seeds, (c) non-mucilaginous seeds, (d) seeds with dry artificial mucilage and (e) seeds with hydrated and dried out artificial mucilage. The seeds were let for germination at the same conditions (21–22°C and 16 h long day). We did not test L. usitatissimum, because the seeds of this species were demucilaginated using warm water (50–60°C), which likely affected their viability.
Statistical analysis
To determine our main hypothesis how many seeds passed through the digestive system intact, we performed a chi-square test in R (R Core Team, 2017). With the same test, we also analysed the results of the present study in comparison with those of our previous experiment (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). However, we did not conduct statistical analyses of the germination rate of seeds that passed through the digestive systems of the pigeons as only a few intact seeds were collected during the experiment.
Results
Seeds collected from pigeons
In Fig. 1, we demonstrated the mucilage envelope morphology of the studied taxa. The most abundant mucilage was typically of P. ovata and P. psyllium and less abundant was observed in P. lanceolata. Seeds of L. usitatissimum produced a smaller envelope, in compare to Plantago taxa, which is very often not evenly distributed on the seeds surface with thicker and thinner places (Fig. 1).

Figure 1. Mucilage envelope morphology of studied taxa. (A) Linum usitatissimum, (B) Plantago lanceolata, (C) Plantago ovata, and (D) Plantago psyllium. Staining with ruthenium read revealed the mucilage envelope. ss - seed; mu - mucilage.
In the first part of the experiment, where we used mucilaginous seeds with removed mucilage envelope, we obtained results that are shown in Table 1. The results obtained for the seeds with artificial mucilage are presented in Table 2. In Table 3, we summarized the results from both experiments (previous – Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021 and present).
Table 1. Germination test for naturally mucilaginous seeds (control) with their mucilage removed passed through the digestive system of pigeons

Note: The number of germinated seeds is provided instead of percentage values due to the low number of seeds passing through the digestive system intact.
For the germination test of control seeds, we used the same count of seeds as collected from the pigeons (see also Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021).
Table 2. Germination test for non-mucilaginous seeds (control) with an artificial mucilage passed through the digestive system

For the germination test of control seeds, we used the same count of seeds as collected from the pigeons (see also Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021).
Abbreviations: control, untreated seeds; DH, dry husk; HDH, hydrated and dried husk.
Table 3. Comparison of the results from both experiments – seeds passed the digestive system
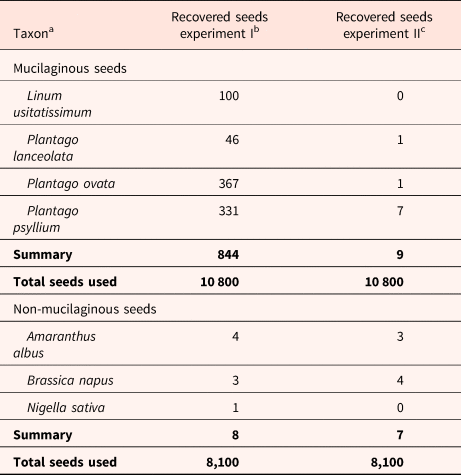
a 2700 seeds was used per each plant species.
b Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021.
c Present data.
The number of seeds for the demucilaginated collected from pigeon droppings and the germination results are shown in Table 1. From all the demucilaginated Plantago sp. seeds fed to pigeons, we obtained nine that passed through the digestive system intact. No L. usitatissimum samples were obtained. The highest count (seven seeds) was recorded for P. psyllium. However, none of the seeds that passed through the pigeon digestive system germinated. Among the control samples (demucilagined seeds), from seven seeds of P. psyllium, only one seed germinated.
The number of seeds with artificial mucilage collected from pigeon droppings and the germination result are presented in Table 2. From all the seeds with artificial mucilage fed to pigeons, we obtained seven seeds that passed through the digestive system intact that is six that were prepared with dry mucilage and one that was prepared with hydrated and dried mucilage. Of the collected seeds, only three germinated (Table 2). The rest did not germinate, and after some time, the seeds were covered with fungi or bacteria.
The seed surface of non-mucilaginous seeds differed from almost smooth in A. albus, through the delicate sculptured surface of B. napus to the distinct sculpture of N. sativa (Fig. 2A–C). After coating the seeds with artificial mucilage (hydrated and air-dried), it was visible that the mucilage did not cover the entire seed surface (Fig. 2A′–C′).

Figure 2. Non-mucilaginous seeds before (A–C) and after coating with artificial mucilage A′–C′. A–A′ Amaranthus albus, B–B′ Brassica napus, C–C′ Nigella sativa. Artificial mucilage formed discontinuous layer on the seed surface. ss - seed; mu - mucilage.
Statistical analysis showed that there was no significant difference between tested seed groups according to their ability to pass through the bird digestive system (demucilaginated seeds versus seeds with artificial mucilage) (χ2(2) = 4.37, P = 0.113). The germination rate was not analysed statistically because of the small number of seeds obtained. For further analysis, we compared the results of this study to those of our previous experiment (Table 3) (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021), in which we collected 844 mucilaginous seeds (2700 seeds per plant species/four plant species/10 800 total used seeds) of the same four plant species (L. usitatissimum, P. ovata, P. lanceolata and P. psyllium) from bird droppings. In the current study, we obtained eight demucilaginated seeds (2700 seeds per plant species/four plant species/10 800 total used seeds) that passed through the digestive system. The statistical analysis showed a statistically significant difference between these results – the count of passed mucilaginous versus demucilaginated seeds (χ2(1) = 849, P < 0.001).
Furthermore, we found that there was no significant difference (χ2(1) = 1.88, P = 0.171) (see also Supplement 1 – Table 1. Analysis of Two-Way Table) between the count of passed non-mucilaginous seeds collected in the previous study (8 seeds, 0.10%; 2700 seeds per plant species/three plant species/8100 total used seeds) (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) and non-mucilaginous seeds coated with artificial mucilage collected in the present study (7 seeds, 0.04%; 2700 seeds per plant species/three plant species/8100 total used seeds). Additionally, in the present study, we tested two methods of applying artificial mucilage. However, there was no apparent difference in the influence of these methods on the ability of the seed to pass through the pigeon digestive system.
In our experiment, only a few seeds from each group (demucilaginated seeds and seeds with artificial mucilage) passed through the pigeon digestive system. However, this was not sufficient to evaluate the germination rate of the samples. We performed additional tests (G1, G2) to determine whether seeds with mechanically removed mucilaginous envelopes and seeds with artificial mucilage were able to germinate. The results are summarized in Tables 4 and 5 for the original seeds used in the experiment. The seeds were germinated under the following conditions (21–22°C and 16 h of light).
Table 4. Germination tests (G1) of mucilaginous (intact) and demucilagined seeds

Table 5. Germination test (G2) of non-mucilaginous control seeds and seeds with artificial mucilage

Abbreviations: control, normal, non-mucilaginous seeds; DH - dry husk (‘artificial’ mucilage) added to the seeds; HDH - seeds covered with hydrated (‘‘artificial’) mucilage and dried.
Additional germination test
The germination of demucilaginated seeds (G1) differed markedly between Plantago species (Table 4). The intact control seeds (with mucilage) of Plantago taxa germinated well (Table 4). Among demucilagined samples stronger germination ability was observed only for P. lanceolata, whereas P. ovata and P. psyllium did not germinate. Almost all demucilaginated seeds of P. ovata and P. psyllium were covered (at the end of the test) with a mouldy/bacterial biofilm. A whitish to whitish-rose biofilm was visible on the seed surface.
In the germination test (G2) of non-mucilaginous control seeds as well as those with artificial mucilage, the germination rate was relatively high in almost all samples (Table 5). However, the germination rate of one sample of B. napus (DH-dry husk) was slightly lower (60%).
Discussion
Our experiment clearly demonstrated that in mucilaginous seeds, the removal of the mucilage envelope drastically increased seed damage caused by the digestive system of the pigeon. Very few seeds passed through the pigeon digestive system intact. In this study, we collected only nine seeds from the 10 800 seeds fed to pigeons (0.8%) compared to the 844 seeds with intact mucilage envelopes (7.8%) collected in our previous study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). In the current study, the nine seeds collected from the pigeon droppings failed to germinate. Although the germination proportion of control seeds was also very low, the extremely low number of demucilaginated seeds that passed through the bird digestive system showed that the original mucilage bound to the seed coat can have a strong influence on the dispersal success of the plant by birds. The results of our previous studies (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) revealed clearly supporting role of mucilage envelope in the seeds' passage through the pigeon's digestive system. It can be also important for the diaspore dispersal. Whereas here, where the seeds lack the mechanism (mucilage envelope) preventing them against digestion in the bird's gut the results demonstrate that granivorous birds play a minor role in the seeds' dispersal.
Mucilage envelopes can be beneficial to young seedlings in several ways. They have a positive effect on the microbial community, supporting seed germination and seedling emergence and providing protection from pathogens (Hu et al., Reference Hu, Zhang, Baskin, Baskin, Wang, Liu, Du, Yang and Huang2019). In Artemisia sphaerocephala, seedling emergence was higher for mucilaginous seeds than for demucilaginated seeds (Hu et al., Reference Hu, Zhang, Baskin, Baskin, Wang, Liu, Du, Yang and Huang2019). Substantial decreases in demucilaginated seed germination have also been observed in Lepidium perfoliatum (Zhou et al., Reference Zhou, Xing, Zhao, Liu, Gu and Lan2022). Our previous and current results support these observations. Seeds that passed through the digestive system and still possessed mucilage germinated well, for example, in the Plantago species (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Those that lost their mucilage envelope (L. usitatissimum) did not germinate (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021).
The results of the additional germination test showed that the demucilaginated seeds of two Plantago taxa did not germinate at all. The seeds of P. lanceolata, which produced the smallest mucilage envelope, were an exception. Although mucilage was removed from the seed surface, many seeds of this species (73%) germinated. We propose that the seed mucilage of P. lanceolata plays a supporting role in germination, rather than an essential role. In contrast, the seeds of P. psyllium and P. ovata produce an abundant mucilage envelope, which appears to be crucial for seedling emergence.
Zhou et al. (Reference Zhou, Xing, Zhao, Liu, Gu and Lan2022) noted many positive effects of mucilage on the germination of L. perfoliatum seeds and determined that seed mucilage is essential for the rapid and efficient germination of intact seeds. Mucilage is beneficial for germination in different ways, such as allowing germination under abundant or excess water or at higher temperatures (Zhou et al., Reference Zhou, Xing, Zhao, Liu, Gu and Lan2022).
Diverse substances with antifungal and antimicrobial effects are produced in the seed coat (pericarp) (Costea et al., Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019). For example, antimicrobial compounds have been found in the mucilage of Dillenia indica seeds. Therefore, mucilage may protect seeds against soil pathogens (Dasanayaka et al., Reference Dasanayaka, Jinadasa, Jayasuriya and Phartyal2022). Based on our observation (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021) that mucilaginous seeds moulded less frequently than seeds without mucilage, we concluded that the complete loss of mucilage leads to the loss of antifungal/antimicrobial protection at least in selected taxa (P. psyllium, P. ovata).
A total of seven non-mucilaginous seeds (A. albus, B. napus, N. sativa) passed through the pigeon digestive system intact, which was very similar to our previous results (eight seeds from the same species) (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). However, in the present experiment, we attempted to support the passage of non-mucilaginous seeds through the pigeon digestive system by applying artificial mucilage. Our experiments demonstrated that artificial mucilage added as a cover for the seeds (not mechanically bound to the seed coat) did not enhance the passage of seeds through the bird's digestive system. In the current study, microscopic examination revealed that the artificial mucilage did not cover the entire seed surface. During drying, the mucilage shrank, likely because many seeds were only partially covered with mucilage. In naturally mucilaginous diaspores, the mucilage envelope is a natural integrative part of the seed/fruit coat and is typically connected to the next layer of the coat (Kreitschitz, Reference Kreitschitz and Gorb2009; Western, Reference Western2012). Very often the outer layers of the diaspores (exotesta and exocarp) are cuticularized. This provides them with hydrophobic or superhydrophobic properties (Barthlott, Reference Barthlott1981) and protects the diaspores against pathogens present in the soil (Serrano et al., Reference Serrano, Coluccia, Torres, L'Haridonand and Métraux2014; Costea et al., Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019). We suppose that such hydrophobic properties can make it difficult to attach (hydrated) artificial mucilage to the seeds' surface.
It is known that, for example, flax mucilage exhibits laxative properties, what is an advantage in treating constipation (Chand et al., Reference Chand, Chopra, Talwar, Homroy, Singh, Dhiman and Payyunni2024). However, it must not suggest that the mucilaginous seeds can move faster through the (bird's) digestive system. We can suppose that the mucilaginous seeds due to the mucilage presence and the very low friction (Kreitschitz et al., Reference Kreitschitz, Kovalev and Gorb2015, Reference Kreitschitz, Kovalev and Gorb2016) can just easily pass the digestive system and not faster. However, at list, a small sped-up cannot be excluded.
Costea et al. (Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019) studied the structure of seeds with no mucilage or fleshy tissues that could support endozoochory and showed that among the tested seeds of diverse taxa, survival was considerably higher owing to the presence of (a) a thick cuticle, (b) one or several lignified cell layers and (c) diverse combinations of other architectural elements. Germination was affected to different degrees after gut passage, from increased to decreased germination (Costea et al., Reference Costea, El Miari, Laczkò, Fekete, Molnár, Lovas-Kiss and Green2019).
The mucilage envelope appears to be an effective adaptive feature for plant dispersal. The epizoochoric distribution of mucilaginous diaspores between the Hawaiian islands due to the presence of a mucilage envelope has been observed in some Euphorbia taxa (Carlquist, Reference Carlquist1966 – Part III). The endozoochoric distribution of Plantago diaspores (P. lanceolata and P. major) is supported by the fact that they are found in the droppings of birds (including pigeons) (Cavers et al., Reference Cavers, Bassett and Crompton1980). The presence of mucilage allows them to pass through the digestive system, as shown in our previous study (Kreitschitz et al., Reference Kreitschitz, Haase and Gorb2021). Phylogenetic studies of Plantago sp. on oceanic islands have provided further evidence for the supporting role of mucilage in diaspore dispersal. A large number of single-island endemic taxa exist among approximately 250 Plantago species. The colonization of islands by Plantago taxa was likely possible because of the transport of diaspores (internally and externally) by migratory birds (Ivanycki Ahlstrand et al., Reference Ivanycki Ahlstrand, Verstraete, Hassemer, Dunbar-Co, Hoggard, Meudt and Rønsted2019). It can be supposed that the diaspores of these endemic taxa are no longer able to produce mucilage. They may have lost this ability after reaching and colonizing new island habitats. This speciation process can explain their endemism and indicates the supporting (for seed dispersal) and protective (against digestion by vertebrates) characteristics of the seed mucilage envelope. The results of our previous and current studies provide significant confirmation supporting existing theories about efficient seed dispersal in Plantago sp. and other plant species with mucilaginous seed envelopes. As it was observed in Nepetoidea, the mucilage production may evolve very quickly as a consequence of adaptation to different biological conditions. The mucilage production can be lost quickly if the costs of its production under certain conditions are too high (Ryding, Reference Ryding2001). There are also known species which produce seeds with mucilaginous cells but without mucilage, for example, Artemisia vulgaris and Artemisia princeps. This can be also an effect of adaptation to other (more humid) habitats which do not require the presence of a mucilage envelope (Kreitschitz, Reference Kreitschitz2012).
Conclusions
The mucilage envelope is a multifunctional material that is important for diaspore dispersal, germination, and protection against pathogens. Its presence can play an important role in plant speciation. The results of this study revealed the essential role of mucilage envelope in diaspore dispersal via endozoochory. Diaspores that were mechanically stripped of mucilage seldom passed through the digestive system intact. However, artificial mucilage applied as a protective layer to non-mucilaginous diaspores did not substantially impact the number that passed through the pigeon digestive system. Therefore, only the original mucilage envelope, strongly bound to the seed coat, supports diaspore passage through the digestive system of birds. This study confirmed the important role of the mucilage envelope in diaspores. The results of our study can be valuable in the analysis of the seeds’ and fruits’ dispersal ways in the world (e.g. bird migrations versus plant dispersal).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0960258524000187.
Financial support
This work was supported by Biuro Projektu Inicjatywa Doskonałości – Uczelnia Badawcza, Uniwersytet Wrocławski (BPIDUB.4610.38.2022.TW, umowa stażowa nr 38/2022) to AK.
Ethics approval
Our experiment was conducted in accordance with German law (Interne Versuchsnummer: 1063, ‘Die Rolle des Schleims in der Endozoochorie/Role of the mucilage in the endozoochory’, MELUR Aktenzeichen: V 241 – 41520/2017).
Conflicts of interest
None declared.










