The loss of wild bee diversity is one of the most pressing contemporary challenges for biodiversity conservation due to the importance of wild pollinators for ecosystems (via wild plant pollination) and society (via crop pollination) (Zattara and Aizen, Reference Zattara and Aizen2021). Agricultural intensification and urbanization push species to fragment vegetation remaining in the landscape, often loosing large habitat patches that can support wild bee populations (Kennedy et al., Reference Kennedy, Lonsdorf, Neel, Williams, Ricketts, Winfree, Bommarco, Brittain, Burley, Cariveau, ısa Carvalheiro, Chacoff, Cunningham, Danforth, Dudenh, Elle, Gaines, Garibaldi, Gratton, Holzschuh, Isaacs, Javorek, Jha, Klein, Krewenka, Mandelik, Mayfield, Morandin, Neame, Otieno, Park, Potts, Rundl, Saez, Steffan-Dewenter, Taki, Felipe Viana, Westphal, Wilson, Greenleaf and Kremen2013; Ferreira et al., Reference Ferreira, Boscolo, Carvalheiro, Biesmeijer, Rocha and Viana2015). Climate change pushes species to northerly latitudes, creating new habitats for species to exploit, whereas the previous habitat may become too warm or dry (Marshall et al., Reference Marshall, Perdijk, Dendoncker, Kunin, Roberts and Biesmeijer2020).
The role of urban habitats such as gardens for supporting species conservation or migration is scarce in evidence though wide in speculation (Hall et al., Reference Hall, Camilo, Tonietto, Ollerton, Ahrné, Arduser, Ascher, Baldock, Fowler, Frankie, Goulson, Gunnarsson, Hanley, Jackson, Langellotto, Lowenstein, Minor, Philpott, Potts, Sirohi, Spevak, Stone and Threlfall2017; Banaszak-Cibicka et al., Reference Banaszak-Cibicka, Twerd, Fliszkiewicz, Giejdasz and Langowska2018). Some work has documented how gardens can be hotspots for bee and plant diversity (Baldock et al., Reference Baldock, Goddard, Hicks, Kunin, Mitschunas, Morse, Osgathorpe, Potts, Robertson, Scott, Staniczenko, Stone, Vaughan and Memmott2019), with gardeners cultivating previously undocumented plant species within their yards (Taylor and Mione, Reference Taylor and Mione2019; Seitz et al., Reference Seitz, Buchholz, Kowarik, Herrmann, Neuerburg, Wendler, Winker and Egerer2022). Yet we have little evidence of how gardens may provide nectar, pollen and nesting resources for wild bees whose populations may be changing in diversity and distribution under land-use change. Furthermore, it is an open question as to whether gardens are rare resource patches in the cityscape that promote and sustain rare species populations, or whether gardens are ecological traps with rare species documented as remains of declining populations. Monitoring bee diversity in urban gardens can provide needed insight into species change (Baldock, Reference Bates, Sadler, Fairbrass, Falk, Hale and Matthews2011).
This article reports on the finding of a bee species previously undocumented in the city of Berlin, Germany to explore the role of urban gardens in species change. We investigated the species richness of wild bees (Clade: Anthophila) in 18 urban community garden sites distributed across Berlin, Germany's largest metropolitan region. Berlin's community gardens are an ever–popular novel urban ecosystem type situated on vacant lots, brownfields, wastelands, rooftops and parking lots (Kowarik, Reference Kowarik2011).
We surveyed wild bees three times between May and August 2020 during good weather for bee activity (minimum 15°C, low wind, no rain and dry vegetation) (Bates et al., Reference Bates, Sadler, Fairbrass, Falk, Hale and Matthews2011). We used standard passive trapping methods (15-cm-diameter plastic bowls, spray-painted in UV-bright yellow, white and blue placed 72 h) and netting methods (two observers walked through a 20 × 20 m observation plot for 60 min and identified species observed on flowers, netting individuals unidentified to species). We documented 102 wild bee species, and the comprehensive results of this research are presented in detail elsewhere (the authors, in review).
Discovery of Lasioglossum limbellum
This report provides initial evidence of a previously unrecorded wild bee species, Lasioglossum limbellum (Morawitz, 1876) in Berlin, documented within a very urban area in the city (84% impervious surface within 500 m of the garden). A female individual was found in a community garden in the Neukölln district in June 2020 using pan traps (Fig. 1). To our knowledge, this is the first documentation of this bee species in the Berlin region, and in northern Germany. The recorded distribution of the species spans warm localities in the Western Palearctic, from eastern Austria to China (Kansu), south to Israel, and north to Ukraine (Kiev); a subspecies is distributed from Morocco to Malta, from northwestern Spain to Austria, north to Guernsey and around Cologne, Germany (Ebmer, Reference Ebmer1997; Pauly, Reference Pauly2016). In Germany, the species was previously restricted to Southern Germany (Pauly, Reference Pauly2016), is rare with a moderate decline, and was listed as endangered (level 3) on the 2007 Red List of bees in Germany (Westrich et al., Reference Westrich, Frommer, Mandery, Riemann, Ruhnke, Saure and Voith2008). It is a small (8-10 mm), short-tongued solitary species belonging to the Halictidae, and can be identified by minute tranverse ridges usually at the sides of tergite 1, with translucent orange hind margins of tergites 1-4 that create a distinct banded appearance (Westrich, Reference Westrich2018). The top of the propodeum is smooth, especially along the hind margin. Females have a rounded face, while males have more oval faces and have short dark antennae. Females fly beginning in April, while males at the beginning of August (Westrich, Reference Westrich2018).
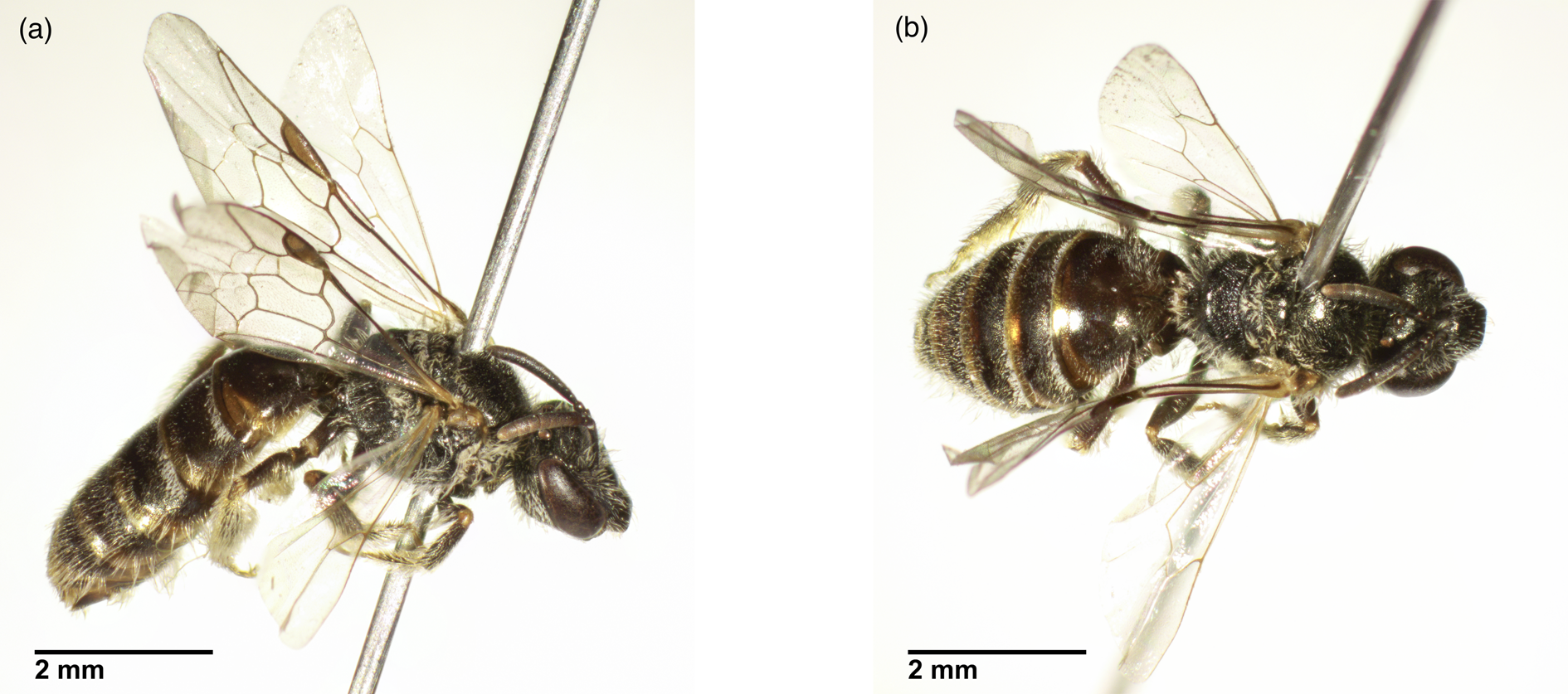
Fig. 1. Images of the bee specimen from the lateral (a) and dorsal (b) perspective. Images taken using a Motic SMZ-171 microscope (Motic Europe, Barcelona, Spain) with the Toupcam U3CMOS 16000KPA (ToupTek Photonics, Zhejiang, P. R. China), and processed using ToupView (Touptek) for photo acquisition with manual Focus stacking (16 Photos). The images where further processed using Lightroom (Adobe Inc., San José, California, USA) for images colour, brightness, sharpness, and control of chromatic aberations. The images were stacked in Photoshop (Adobe Inc.) and the scalebar (images shown to scale) was added from a measure slide. Imaging and processing by: Kenneth Kuba.
In the European habitats in which this species is found, it is strongly associated with soft rock cliffs and sandy and coastal grassland (Petanidou and Ellis, Reference Petanidou and Ellis1993; Sárospataki and Fazekas, Reference Sárospataki and Fazekas1995)—rare or functionally nonexistent in most urban areas. The bee colonizes sandy quarries, and constructs its nests in self-dug cavities in the steep walls from loam, sandy loam and sand material (Scheuchl and Willner, Reference Scheuchl and Willner2015; Westrich, Reference Westrich2018). As a polylectic species, it forages primarily on composites (e.g., Taraxacum officinale, Picris Hieracioides, Chichorium intybus), and pollen is collected on the hind legs and abdomen (Westrich, Reference Westrich2018).
Garden features suggest role in species conservation
The context of this garden raises interesting questions about how novel urban ecosystems that are created on concrete surfaces from natural elements could support biodiversity given their local vegetation and structural features. The garden (Vollguter Gemeinschaftsgarten) is a community project located in Berlin's Kindl Kiez, collectively managed by a group of citizens using ecological and permaculture practices. The garden community describes itself as a platform for experimentation, to explore the worlds of plants and herbs and where to build installations from recycled material (http://www.vgg.green). This civic engagement has produced an ecosystem of annual and perennial vegetation within raised beds in an area of otherwise concrete with little nearby vegetation (Fig. 2).

Fig. 2. Community garden in Berlin, Germany where bee was discovered; the garden exists on concrete slabs (a) and is created out of annual and perennial flowering vegetation in makeshift beds and pots (b). Photos: the author.
Despite being small, the garden is cultivated with many perennial and wild plant species that are high in pollinator attractiveness. We simultaneously documented 70 plant species within the garden using random sampling, including composite flowering species Chichorium intybus and the common Taraxacum officinale (Appendix 2), evidence that pollen and nectar resources associated with this species were available.
Of note, at the time of this research, an entire underground parking structure was being excavated right next to the garden. Previous studies have shown that demolition areas can provide nesting resources for ground-nesting bees (Seitz et al., Reference Seitz, van Engelsdorp and Leonhardt2019). Perhaps the construction activity was providing materials to nest.
Urban landscape features suggest gardens are stepping stones
This finding opens up interesting questions in Island Biogeography and within the broader context of the ‘SLOSS’-debate. In urban planning and ecology, an open question is what is the role of ‘single large or several small’ habitats in urban landscapes (Fattorini, Reference Fattorini2016; Wintle et al., Reference Wintle, Kujala, Whitehead, Cameron, Veloz, Kukkala, Moilanen, Gordon, Lentini, Cadenhead and Bekessy2019). Can small patches or ‘urban islands’ like gardens support populations as resource-providing ‘stepping stones’ in the landscape, or do we just need one large habitat as a ‘source’? Are urban gardens ecological sinks or ecological traps, where species are attracted but the habitat cannot sustain the population? In cities, high amounts of imperviousness create a matrix that may be very difficult for species with low dispersal distances to traverse and maintain their populations. In this case, the garden is surrounded by high amounts of impervious surface. Yet it neighbors one of the largest habitats in central Berlin, Tempelhofer Feld (1000 m away), along with several other small green spaces including St.-Jakobi Kirchhof, St. Thomas-Kirchhof and Volkspark Hasenheide nearby (within 500 m). Tempelhofer Feld may be an important source habitat for many green space islands within this urban landscape. We cannot say whether the garden functions as purely a stepping stone across the urban landscape, or whether the garden can essentially function as the entire habitat for a bee species. The local context of the garden—small, raised beds on concrete, approximately 30 m2 in size—may suggest that the garden is only as a stepping stone or even an ecological trap. Though for small species such as those of Lasioglossum with limited dispersal ability (couple hundred meters), this patch could serve as an entire habitat.
Open questions on the role of urban gardens in species conservation
Environmental change from land-use change is impacting species distribution and diversity worldwide. For wild bees, it is still largely unclear how urbanization may hinder (via densification) or support (via ‘stepping stones’) species conservation. This documentation provides preliminary evidence of how urban gardens may play a role in changes in bee species diversity. We cannot confirm any information on population numbers with this discovery, and the lack of historical collection records and wild bee monitoring across the regions and in diverse habitats means that we cannot confirm prior distributions or occurrences in the region. Nevertheless, it is critical to report such novel work to highlight the observations that we see occurring in urban ecosystems on changes in species diversity and distribution. This work opens the door to new investigations in urban environments to investigate, for example, whether species moving to new areas may be likely to use transformed novel ecosystems in cities. This discovery opens the question about the role of urban gardens in our city landscapes—even if only stepping stones—as important ecosystems for species ecology and conservation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1742170522000199.
Acknowledgements
Thank you to Vollguter Gemeinschaftsgarten and the community gardeners for supporting this work in their garden. Thank you to Ulrike Sturm and the Museum für Naturkunde in Berlin for project support. Thank you to Frank Koch and Christian Schmid-Egger for bee species identification. Thank you to Carolina Achilles, Sascha Buchholz, Anika Gathof, Anita Grossman, Julia Felderhoff, Ingo Kowarik, Johann Herrmann, Moritz von der Lippe, Martin Penzel, Birgit Seitz, Leonie Neuerburg, Julian Wendler and Leonie Winker for supporting field and lab work. Thank you to Kenneth Kuba for specimen imaging and processing. Thank you to the editor and two reviewers for their constructive comments.
Financial support
This work was funded by an International Postdoctoral Fellowship from the Technical University of Berlin.
Conflict of interest
No competing interests to declare.





