INTRODUCTION
The geology of Yucatan, Mexico, is composed of carbonate and evaporite rocks, forming a karst platform. In this region, there are no rivers; rainwater quickly seeps down to the aquifer. Thus, the primary geochemical process in karst aquifers is the dissolution of carbonate rocks, leaving little residue and a thin or almost non-existent ground cover. As a result, a geological feature of the Yucatan Peninsula is a great density of sinkholes, locally known as cenotes, which give way to large dissolution caves and underground cavities, some of which are linked together in a complex geomorphological structure.
In the Yucatan Peninsula, water of meteoric origin infiltrates and accumulates in the subsoil, forming a freshwater lens that floats on a denser mass of saline water, the origin of which is a marine intrusion through the permeable karstic rock. This vertical stratification is found in coastal systems also known as anchialine systems or subterranean estuaries and has major ecological implications for the cave biodiversity of Yucatan. Although marine intrusion may be found deeper in cenotes that are further from the coast, most cenotes in Yucatan State are exclusively freshwater.
Typhlatya species are found throughout marine and fresh groundwater habitats and are some of the most abundant and widespread stygofauna in the anchialine ecosystems of the Yucatan Peninsula (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020). These crustaceans, of the Atyidae family, are a fundamental part of the food web of anchialine systems, as they feed on bacteria and organic material deposited within the aquifer and transfer energy to higher trophic levels. Figure 1 shows a conceptual framework of radiocarbon (14C) uptake in karstic groundwater fauna.

Figure 1 Conceptual framework of radiocarbon uptake by crustaceans in karstic groundwater: (1) Allochthonous photosynthetic matter; (2) atmospheric CO2 that is permeated into the water table; (3) in situ photosynthesis in the cenote; (4) methane produced from modern sediments and organic matter; (5) radiocarbon-dead carbon which is biosynthesized.
The isotopic signature has been widely used to identify chemical compounds, origin, flux, and transformation in biological and environmental systems. The stable and radioactive isotopes have the same chemical properties of their respective elements but can be distinguished with proper analytical techniques. Carbon isotopes (12C, 13C, and 14C; expressed as δ13C and Δ14C) are commonly used for studying food webs. To identify the carbon sources among ecologically similar species within anchialine systems, the use of δ13C is complemented, with the amount of radiocarbon, as such abundance reflects the modern or old character of the carbon sources. Organic tissues, dissolved organic carbon (DOC), and dissolved inorganic carbon (DIC) contained in the water show values of relative abundancies that can vary from Δ14C= −1000‰ for old carbon, to over +400‰ for modern carbon, offering a greater resolution, compared with δ13C sources (McCallister et al. Reference McCallister, Bauer, Cherrier and Ducklow2004). These parameters provide an opportunity to better understand the role of potential food sources for the Typhlatya genus and the food web dynamics in cenotes.
In this work, we analyzed the 13C and 14C composition of 4 new, and 13 previously-published samples of the Typhlatya genus (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020). The 14C composition of DIC in water from 4 new cenotes located along the Yucatan Peninsula was also included (Table 1). Radiocarbon analysis was carried out at the AMS laboratory (LEMA) of the Institute of Physics of the National Autonomous University of Mexico. The three studied species of the genus Typhlatya: T. mitchelli, T. dzilamensis and T. pearsei (Figure 2b–d), are endemic to the Yucatan Peninsula. Furthermore, T. mitchelli and T. pearsei are under the “special protection” of endangered categories in the local regulation NOM-059-SEMARNAT.
Table 1 δ13C and Δ14C results of Typhlatya biomass and groundwater samples. Local laboratory code, sample identification, site of collection, δ13C obtained by AMS or IRMS in parentheses, the uncertainty of the δ13C measurement, Δ14C and its uncertainty, and the reference if previously published.


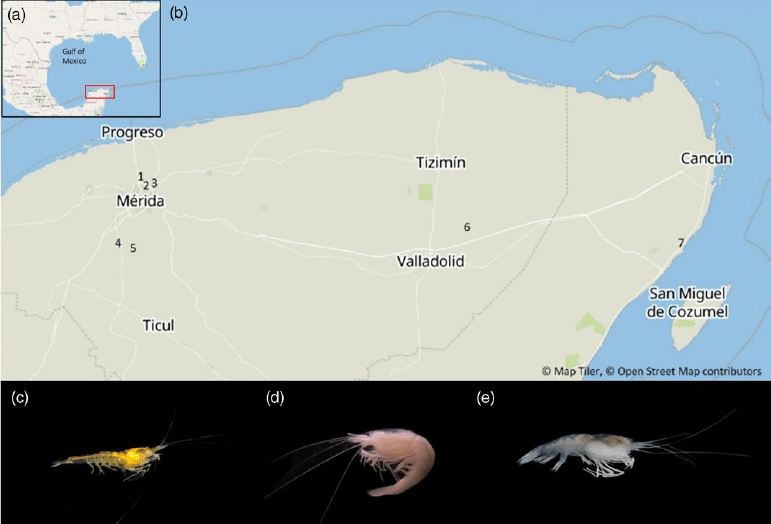
Figure 2 (a) Location of the Yucatan Peninsula within the Gulf of Mexico. (b) Location of sampling sites of Typhlatya specimens and groundwater in the Yucatan Peninsula; cenote Xlakah in dzibilchaltun (1); cenote Xoc (2) and a well from Cholul (3), in Mérida; Tza Itza (4) and Nohmozon (5) in Tecoh; Palomitas (6) in Yalcobá; and Xtabay (7) in Puerto Aventuras. (c) T. mitchelli. (d) T. pearsei. (e) T. dzilamensis. Photo credits: (c): Benjamin Magaña, (d) and (e): EC.
Although these species have been observed to share parts of the subterranean habitat, Benítez et al. (Reference Benítez, Iliffe, Quiroz-Martínez and Alvarez2019) and Chávez-Solís et al. (Reference Chávez-Solís, Solís, Simões and Mascaró2020) showed that the distribution of these species in these particular habitats is not random; T. pearsei is found in the freshwater pools of the cenote, where sunlight allows for in situ photosynthesis and allochthonous input is greatest. T. mitchelli is found in freshwater, mainly in the caverns close to the cenote pools, where allochthonous and photosynthesis derived matter is transported from the cenote towards the cavern. Finally, T. dzilamensis inhabits saline groundwater inside the caves, where allochthonous matter is scarce.
The isotopic values of Typhlatya biomass published in Chávez-Solís et al. (Reference Chávez-Solís, Solís, Simões and Mascaró2020) were incorporated to new biomass samples along with potential carbon sources and were analyzed with Bayesian mixture modeling software SIAR (version 4.2) (Parnell and Jackson Reference Parnell and Jackson2013).
We sought to identify different feeding sources among three species of the Typhlatya genus using isotopic values of potential sources and as biomass adding a new site with different environmental characteristics for T. mitchelli using values of 14C and 13C abundances obtained by AMS, into the Bayesian Mixing Modeling Software SIAR.
MATERIALS AND METHODS
Sample Collection and Habitat
We examined 13C and 14C composition of 4 new and 13 previously published biomass samples of Typhlatya individuals. The new set consisted of T. mitchelli samples collected under the sulfur cloud in cenote Palomitas (Figure 2). This cenote pool is characterized by the presence of a sulfur cloud at 45 m deep.
Water samples from cenotes Palomitas (20.819N, −88.055W), Xlakah (21.090N, −89.598W), Xoc (21.032N, −89.577W), and a well from Cholul (21.047N, −89.553W), were sampled to explore the variation range of 14C within DIC in groundwater of the Yucatan Peninsula (Figure 2). Two replicates were collected in 50 mL falcon tubes and stored at cold temperature in the dark until analysis.
As previously reported in Chavez-Solís et al. (Reference Chávez-Solís, Solís, Simões and Mascaró2020), the Typhlatya species were found in different zones within the anchialine ecosystem: T. pearsei was predominantly found at night in the cenote pool, which is directly under the surface and has the greatest interaction with external factors, may have sunlight incidence, and is the entrance of external input; T. mitchelli in the cavern area, a transitional zone between the cenote pool and the cave, with a moderate amount of light (twilight zone), and T. dzilamensis in the cave where sunlight never reaches. Five T. mitchelli from the cavern of cenote Tza Itza were collected at 13 m of depth (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020), while four T. mitchelli from the cavern of cenote Palomitas were collected at 45 m of depth (new data), just below a sulfur cloud; three T. pearsei were collected from the cenote pool of Nohmozon at 16 m of depth (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020), only 20 km southeast from Tza Itza, and Five T. dzilamensis (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020) were collected from ponderosa system in cave passages adjacent to Xtabay cenote below the halocline at 14 m depth (Figure 2).
Sample Preparation and AMS Analysis
After collection, the Typhlatya samples were rinsed with distilled water and dried at 60°C. For AMS analyses, samples underwent a cleansing with ultrapure water and a chemical treatment using the acid-base-acid (ABA) protocol, to remove salts and other adhered contaminants. Cleaned samples were processed in automated graphitization equipment (AGE III from Ion Plus). The sample (∼1 mg C) was combusted at 950°C in an Elemental Analyzer and the CO2 produced was transferred to a reactor where it reacted with hydrogen in the presence of iron powder to produce pure graphite. Oxalic acid II (NIST SRM 4990C) was used as a primary standard, and phthalic anhydride (without 14C) was used as a blank. The graphite analysis of 14C, 13C, and 12C abundance was performed in a 1 MV accelerator mass spectrometry (AMS) system High Voltage Europe Engineering (HVEE) at the Laboratorio Nacional de Espectrometría de Masas (LEMA) of the Institute of Physics, Universidad Nacional Autónoma de México (UNAM) in Mexico City.
Relative abundance of 13C and 12C isotopes in a sample can be reported in delta notation (d), calculated using the following equation:
Relative abundance of 14C and 12C can be expressed by Δ14C, the 14C isotopic ratio of a material relative to the modern standard after correction for fractionation to δ13C = −25‰:
where fm is the fraction of modern 14C corrected for isotopic fractionation by use of δ13C, and x is the year of deposition (determined from the 210Pb chronology) (Stuiver and Polach Reference Stuiver and Polach1977).
The measured 14C/12C isotopic ratios were corrected for isotopic fractionation using the 13C/12C isotopic ratios measured in the accelerator. Corresponding radiocarbon ages were calculated using computer codes developed at LEMA.
Due to the protected category and the small biomass (∼0.03 gr) of Typhlatya individuals, this study could only sample a small number of individuals, hence the δ13C of only a few samples was analyzed by both AMS and IRMS (Table 1). The AMS δ13C obtained values were in an excellent approximation to the values obtained by isotope ratio mass spectrometry (IRMS) performed at the Laboratory of Stable Isotope Analyses at the Scientific and Technological Park of Yucatan (PCYT, UNAM) (Table 1). Therefore, we used the δ13C values obtained by AMS for the rest of the samples and assumed they were reliable indicators in the analyses of food sources in Typhlatya biomass contribution (Prasad et al. Reference Prasad, Culp and Cherkinsky2019).
We obtained CO2 from DIC in water samples by hydrolysis with 85% orthophosphoric acid in a precleaned flask flushed with helium. The obtained CO2 was then transferred to AGEIII to obtain graphite by bubbling helium gas through the sample. We also processed IAEA-C1 (Marble) and C2 (Travertine) standards in water by acid hydrolysis. The results of the isotopic concentrations are shown in Figure 3, where the values of δ13C for each specimen are plotted against the respective values of Δ14C, the values for the fresh groundwater (FGW) and saline groundwater (SGW) for the different sites are also indicated.

Figure 3 AMS carbon isotopic analysis showing the Δ14C and δ13C composition of Typhlatya biomass and dissolved inorganic carbon contained in groundwater. Full symbols represent Typhlatya biomass data while void symbols represent groundwater data. Modified from Chávez-Solís et al. (Reference Chávez-Solís, Solís, Simões and Mascaró2020).
Mixing Model Analysis
To explain if the lower Δ14C values of T. mitchelli from Palomitas can be linked to a different carbon source than those from Tza Itza, we combined this new group with the previously obtained results into a Bayesian mixing model (Parnell and Jackson Reference Parnell and Jackson2013). This approach allows flexible model specification in a rigorous Bayesian statistical framework to incorporate some features, such as uncertainties, concentration dependence, larger numbers of sources, and more than one group of the same species from different sites. The SIAR software package was used to estimate the source proportions that satisfy mass balance, considering three potential carbon sources that could contribute to the food of Typhlatya species. SIAR package creates an estimated distribution based on the isotopic data from each source and from each biomass and uses that distribution in the model.
To assess the contribution of different carbon sources to Typhlatya species we considered three carbon sources: modern organic matter (OM), modern methanogenic carbon (MM) and ancient methanogenic carbon (AM). The two mixing-model scenarios considered the same OM and MM sources but two different end values for the AM. Biomass values of δ13C and Δ14C, as well as values published for the three potential sources were incorporated into the SIAR software. OM represents a modern carbon pool fixed through photosynthesis by both aquatic algae and phytoplankton located in the cenote pool and by terrestrial plants in the immediate surroundings. MM represents terrestrial methane produced by fermentation of methylated substrates with a modern signature. AM denotes 14C depleted sources due to either the methanogenic decomposition of old organic matter by methane oxidizing bacteria (MOB) (scenario 1), or the assimilation of ancient DIC made available through the dissolution of limestone (scenario 2).
RESULTS AND DISCUSSION
Samples consisted of 4 new and 13 previously published (Chavez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020) Typhlatya biomass samples (Table 1). Additionally, three new and four previously published (Chavez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020) DIC from cenote water samples were analyzed. Carbon composition in Typhlatya biomass can be separated into four groups (Figure 3): FGW T. mitchelli from Tza Itza, had δ13C values ranging from −26.3 to −25.0‰, while T. mitchelli from cenote Palomitas (the new sampling site), had a wider and lower range, from −24.0 to −19.0‰. The FGW T. pearsei from Nohmozon and SGW T. dzilamensis from Ponderosa had markedly lower δ13C values ranging from −35.0 to −41.0‰, and from −31.0 to −44.0‰ respectively. The Δ14C values for T. mitchelli from Tza Itza varied from −3.5 to +19.2‰ while the new collected T. mitchelli from Palomitas had markedly lower values varying from −56.1 to −60.2‰. T. pearsei collected in FGW had Δ14C values from −202 to −166‰. T. dzilamensis collected in SGW showed Δ14C values from −135.5 to −91.5‰. Values of Δ14C from groundwater DIC in all sampled cenotes ranged from −185 to −260‰.
The wide range of δ13C and Δ14C values observed in Typhlatya collected from FGW and SGW environments, reflect a mixture of modern and ancient carbon contributing to their biomass, as previously suggested for other organisms from anchialine environments (Pohlman Reference Pohlman2011).
Mixing Model Analysis
For OM, δ13COM = −25.4‰, and Δ14COM = −4.3‰ values were used in both scenarios. They correspond to the δ13C and Δ14C average values we measured for T. mitchelli from Tza Itza (which were the closest to modern carbon). For MM, we used δ13CMM = −66.3‰ taken from Brankovits et al. (Reference Brankovits, Pohlman, Niemann, Leigh, Leewis, Becker, Iliffe, Alvarez, Lehmann and Phillips2017), and Δ14CMM = −4.3‰ for both scenarios. For scenario 1 we used δ13CAM = −56.3‰ taken from literature Brankovits et al. (Reference Brankovits, Pohlman, Niemann, Leigh, Leewis, Becker, Iliffe, Alvarez, Lehmann and Phillips2017) and Δ14CAM= −1000‰ (older than 50,000 BP), and for scenario 2, we considered Δ14CAM = −260‰, the most negative value that we measured, that corresponds to the DIC from SGW in Xtabay (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020).
Figure 4 shows the Diagnostic Matrix plot that resulted from the Bayesian model. Analyzed Typhlatya are labelled as follows: T. mitchelli (labelled group 1 from Tza Itza and group 4 from Palomitas), T. pearsei (Group 2) and T. dzilamensis (Group 3). The histograms on the diagonal show the relative contribution of each source. The upper triangle shows the contour plots, which indicate how each pair of sources are correlated and the lower triangle indicates the correlation coefficient obtained from the contributions of each source to the diet of each species.

Figure 4 Matrix plots calculated with SIAR for each scenario and source, along with the corresponding correlation factors, and the densities of proportion of each carbon source for each group of Typhlatya for scenarios 1 (top) and 2 (bottom). Typhlatya mitchelli was collected in site 4 (Group 1) and in site 6 (Group 4); T. pearsei was collected in site 5 (Group 2); T. dzilamensis was collected in site 7 (Group 3).
In scenario 1, isotopic signatures of T. mitchelli correspond to modern carbon fixed through photosynthesis by both aquatic algae and phytoplankton. The slight differences allow to elucidate that T. mitchelli from the two cenotes (groups 1 and 4 of Figure 4) depend on the same carbon sources despite the distance between cenotes and the sampling depth, and both uptake OM in a proportion higher than 90%, while the correlation between OM and MM is narrower for group 1 (Tza Itza) than group 4 (Palomitas). For T. mitchelli from Palomitas, it should be noted that its habitat was under a sulfur cloud, which suggests an environment with little organic matter which is reflected in the shape of the surface plots for group 4. These results suggest this population may have modified their diet, feeding almost exclusively on MO, and less MM than what was observed in Tza Itza. The low dispersion observed in the AM histogram suggests that T. mitchelli from Palomitas is consuming approximately 20% of AM. These results show that T. mitchelli incorporates recently fixed organic carbon independently if it is in surface waters of the cenote pool or greater depths in the cavern.
The results obtained for T. pearsei and T. dzilamensis characterized by lower δ13C and Δ14C values, indicate that, while T. mitchelli carbon originates almost exclusively from a modern source, the later species are partially incorporating carbon from different sources. Both species, T. pearsei and T. dzilamensis apparently depend to a certain degree on methane chemosynthetically produced from ancient carbon in the interior of the cavern and cave respectively.
The results obtained from scenario 1 indicate that the OM and MM sources are highly correlated (Figure 4). The isotopic composition of these two sources is very similar in their posterior distributions, indicating that probable solutions could imply the uptake of one or the other sources, but not simultaneously, as they are inversely correlated and spread out in the surface plots (Figure 4). This implies that when T. mitchelli is not consuming MM, it is consuming OM.
For the conditions established in scenario 2, results obtained in this work, corroborate that T. mitchelli from Tza Itza (group 1) feed almost exclusively on OM (Chávez-Solís et al. Reference Chávez-Solís, Solís, Simões and Mascaró2020). However, T. mitchelli from Palomitas (group 4), collected at 45 m depth, and below the sulfur cloud, acquires AM in a greater proportion than those in Tza Itza (approximately 20%). The presence of a sulfur cloud seems to play an important role in this population, probably related to a greater availability of AM in the Palomitas cenote, in contrast to Tza Itza. Nevertheless, the effects observed in the T. mitchelli of Palomitas should not interpreted as depth dependent, and rather on the availability of OM, MM and AM sources. Regarding T. pearsei and T. dzilamensis, the results for scenario 2 indicate similar carbon source incorporation, even though they are living in different salinity layers and several hundred kilometers apart.
In summary, in scenario 1, where the AM source is moderately negative and close to 14CMM values, the model fails to distinguish between the contributions of OM and MM. On the other hand, when AM is fixed with a lower value, as in scenario 2, the model shows that T. mitchelli from Palomitas (group 4) consumes a greater amount of AM. The presence of a sulfur cloud in cenote Palomitas could be related to an anoxic and oligotrophic environment, which could explain why ancient or modern methane incorporation increases its importance for T. mitchelli in this particular habitat.
The incorporation of this new data suggests that T. mitchelli may have the ability to resort to a greater array of carbon sources than previously suggested (Chávez Solís et al Reference Chávez-Solís, Solís, Simões and Mascaró2020). Ecologically, this could imply that Typhlatya species may have a greater feeding plasticity when resources are scarce, and resort to other available feeding sources. Nevertheless, a greater number of individuals from each species and from a greater number of sites would improve our resolution and understanding of the niche breadth of these species, along with the contribution of each source to this abundant and widespread groundwater genus.
CONCLUSIONS
The local environment exerts a great influence on the isotopic content of the biomass of Typhlatya. This suggests that T. mitchelli may resort to methanogenic carbon sources in an oligotrophic environment.
Results obtained in this work with Bayesian inference using SIAR (Parnell and Jackson Reference Parnell and Jackson2013) show similar results as those obtained by Chávez-Solís et al. (Reference Chávez-Solís, Solís, Simões and Mascaró2020) using IsoSource mixing model Software (Phillips and Gregg Reference Phillips and Gregg2003), corroborating the use for this approach in unraveling trophic relations in anchialine systems. The wide range of δ13C and Δ14C values observed in Typhlatya species collected from fresh and saline groundwater environments reflect a mixed contribution of photosynthetic and chemosynthetic derived matter, as well as modern and ancient carbon that contributes particularly to the biomass of each species.
ACKNOWLEDGMENTS
We thank Arcadio Huerta for maintenance and operation of the AMS system and Sergio Martínez for technical assistance. This Project was partially funded by grants from DGAPA PAPIIT IG100619 and CONACyT 315839. We also thank the editor and both anonymous reviewers whose comments and suggestions improved this contribution.
COMPETING INTERESTS
The authors declare no competing interests.