INTRODUCTION
Freshwater reservoir effects (FREs) are increasingly acknowledged sources of offsets in radiocarbon (14C) dates on human and some faunal remains. The nature of the effect is well described in the literature (e.g., Keaveney and Reimer Reference Keaveney and Reimer2012; Wood et al. Reference Wood, Higham, Buzilhova, Suvorov, Heinemeier and Olsen2013; Fernandes et al. Reference Fernandes, Rinne, Nadeau and Grootes2016). Briefly, the FRE refers to the apparent, “older” age of samples when part of the carbon in the diet of an individual comes from freshwater resources (such as fish, waterfowl, etc.) with a reservoir offset compared to the 14C age of a contemporaneous purely terrestrial sample obtaining its carbon from the atmosphere. FREs vary geographically and over time and can vary considerable within what are ostensibly single reservoirs, between and within species, and even within single organisms (e.g., Kulkova et al. Reference Kulkova, Mazurkevich, Dolbunova, Regert, Mazuy, Nesterov and Sinai2015; Schulting et al. Reference Schulting, Bronk Ramsey, Scharlotta, Richards, Bazaliiskii and Weber2022), and herein lies the major challenge for chronological determinations and hence for the archaeological interpretations that rely on temporal sequence.
Over the past several decades, a number of studies have highlighted the presence of FREs in various regions of Siberia and the Eurasian Steppe, featuring both modern fish and archaeological samples (shell, human, fish, dog and others; summarized in Svyatko et al. Reference Svyatko, Reimer and Schulting2017a; also Figure 3). Attempts have also been made to analyze the relationship between diets and radiocarbon ages, and to develop regression and Bayesian models to calculate 14C offsets using δ13C and δ15N values (Bronk Ramsey et al. Reference Bronk Ramsey, Schulting, Goriunova, Bazaliiskii and Weber2014; Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii, Goriunova and Weber2014, Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015, Reference Schulting, Bronk Ramsey, Scharlotta, Richards, Bazaliiskii and Weber2022). The analysis of modern aquatic fauna (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a, Reference Svyatko, Schulting, Poliakov and Reimer2017b), as well as alkalinity of water in local reservoirs (Keaveney and Reimer Reference Keaveney and Reimer2012), are informative proxies to assess the extent of modern FREs; however, the values cannot necessarily be extrapolated to prehistoric contexts as the extent of the offsets can change over time (e.g., Ascough et al. Reference Ascough, Cook, Church, Dunbar, Einarsson, McGovern, Dugmore, Perdikaris, Hastie, Friðriksson and Gestsdóttir2010), and these proxies do not account for particular aquatic resources in past human or faunal diets.
Here, we evaluate the extent of the FRE associated with materials from various archaeological sites from Siberia and the Eurasian Steppe, introduce new data, and present an up-to-date summary of the existing FRE data in this region and beyond.
MATERIALS AND METHODS
In total, this study includes 12 locations across the Eurasian Steppe (Figure 3). The analyzed materials represent groups of synchronous samples of purely terrestrial versus aquatic/mixed origin. In cases where the contexts were disturbed (e.g., plundered), only specimens with reliable associations were sampled. The samples (37 in total) include 14 human bone, 1 wood, 15 terrestrial faunal bone, 2 plant macrofossil and 5 fish bone/scale samples.
The sampled archaeological sites date to various periods of the Bronze and Early Iron Ages. The sites are attributed to the Sintashta (21st–18th c. BC), Begazy-Dandybai (2nd mil. BC–8th c. BC), Andronovo (20th–9th c. BC), Samus (15th–13th c. BC), Bulan-Koba (2nd c. BC–5th c. AD), and Tasmola (7th–3rd c. BC) Cultures. The description of the sites and their cultural affiliations are presented in detail in SI 1.
All samples were analyzed in the 14CHRONO Centre for Climate, the Environment and Chronology (Queen’s University Belfast).
Sample Pretreatment
For bone samples, collagen extraction was based on the ultrafiltration method (Brown et al. Reference Brown, Nelson, Vogel and Southon1988; Bronk Ramsey et al. Reference Bronk Ramsey, Higham, Bowles and Hedges2004), which included the following steps: a) bone demineralization in 2% HCl, followed by MilliQ® ultrapure water wash; b) gelatinization in pH=2 HCl at 58°C for 16 hours; c) filtration, using ceramic filter holders, glass filter flasks and 1.2 µm glass microfiber filters; d) ultrafiltration using Vivaspin® 15S ultrafilters with MWCO 30 kDa; 3000–3500 rpm for 30 min; and e) freeze-drying. The dried collagen was stored in a desiccator.
Acid-only pretreatment was used for fish scale samples. The samples were placed in clean 100 mL beakers and immersed in hydrochloric acid (4%, 30–50 mL), followed by deionised water wash until neutral.
For the wood sample, a standard ABA procedure (Mook and Waterbolk Reference Mook and Waterbolk1985) was used. This involves a 4% HCl wash at 80°C, a 2% NaOH wash and another 4% HCl wash at 80°C (1 h for each step), followed by a final rinse in deionized water. For plant macrofossil samples, acid-only pretreatment was used, which included 4% HCl wash at 80°C, and a rinse in deionized water.
Stable Isotope Analysis
Bone collagen stable carbon and nitrogen isotopes were measured in duplicate on a Thermo Delta V Isotope Ratio Mass Spectrometer coupled to a Thermo Flash 1112 Elemental Analyzer peripheral. The measurement uncertainty (± 1SD) of δ13C and δ15N based on 6–10 replicates of seven archaeological bone collagen samples was 0.22‰ and 0.15‰ respectively. The reference standards used were IA-R041 L-Alanine, IAEA-N-2 Ammonium Sulphate, IA-R001 Wheat flour, IAEA-CH-6 Sucrose, and Nicotinamide. Results are reported using the delta convention relative to international standards: VPDB for δ13C and AIR for δ15N (Hoefs Reference Hoefs2009). The results were calibrated using a regression based on the measured and known values of the standards (cf. Coplen et al. Reference Coplen, Brand, Gehre, Gröning, Meijer, Toman and Verkouteren2006).
AMS 14C Dating
Prepared samples were sealed under vacuum in quartz tubes with an excess of CuO and combusted at 850°C. The CO2 was converted to graphite on an iron catalyst using zinc or by the hydrogen reduction method (Slota et al. Reference Slota, Jull, Linick and Toolin1987; Vogel et al Reference Vogel, Southon, Nelson and Brown1984). The pressed graphite “target” was then measured on a 0.5 MV National Electrostatics Compact AMS. The sample 14C/12C ratio was background corrected and normalised to the HOXII standard (SRM 4990C; National Institute of Standards and Technology). The 14C/12C ratio corrected for isotopic fractionation using the AMS-measured δ13C, is equivalent to fraction modern (F14C; Reimer et al. Reference Reimer, Brown and Reimer2004). The 14C age and one-sigma error term were calculated from F14C using the Libby half-life (5568 years) following the conventions of Stuiver and Polach (Reference Stuiver and Polach1977). The statistical proximity of the paired dates was assessed using the Ward and Wilson (Reference Ward and Wilson1978) chi-squared test in CALIB 7.0.
Calculating the Freshwater Reservoir Offset (FRO)
Freshwater reservoir offsets were calculated as the difference in the 14C ages between the terrestrial (faunal/wood) samples and aquatic/mixed (human/fish). FRO uncertainty was calculated using σFRO =
![]() $\sqrt {\sigma _a^2 + \sigma _b^2}$
, where σa and σb are 14C age uncertainties for aquatic/mixed and terrestrial samples. Dates that passed the chi-squared test were interpreted as showing no FRO.
$\sqrt {\sigma _a^2 + \sigma _b^2}$
, where σa and σb are 14C age uncertainties for aquatic/mixed and terrestrial samples. Dates that passed the chi-squared test were interpreted as showing no FRO.
RESULTS AND DISCUSSION
Results
The collagen content of the bone samples varied between 1.3–19.7% (Table 1), meeting the recommended minimum of 1% (van Klinken Reference van Klinken1999). Atomic C:N ratios were all within the accepted range of 2.9–3.6 (mean C:Natomic = 3.2 ± 0.1), indicating well-preserved collagen (DeNiro Reference DeNiro1985). The isotopic results and observed freshwater reservoir offsets are presented in Table 1.
Table 1 AMS 14C dates, stable C and N isotope values, atomic C:N ratios and calculated FRO of the samples.
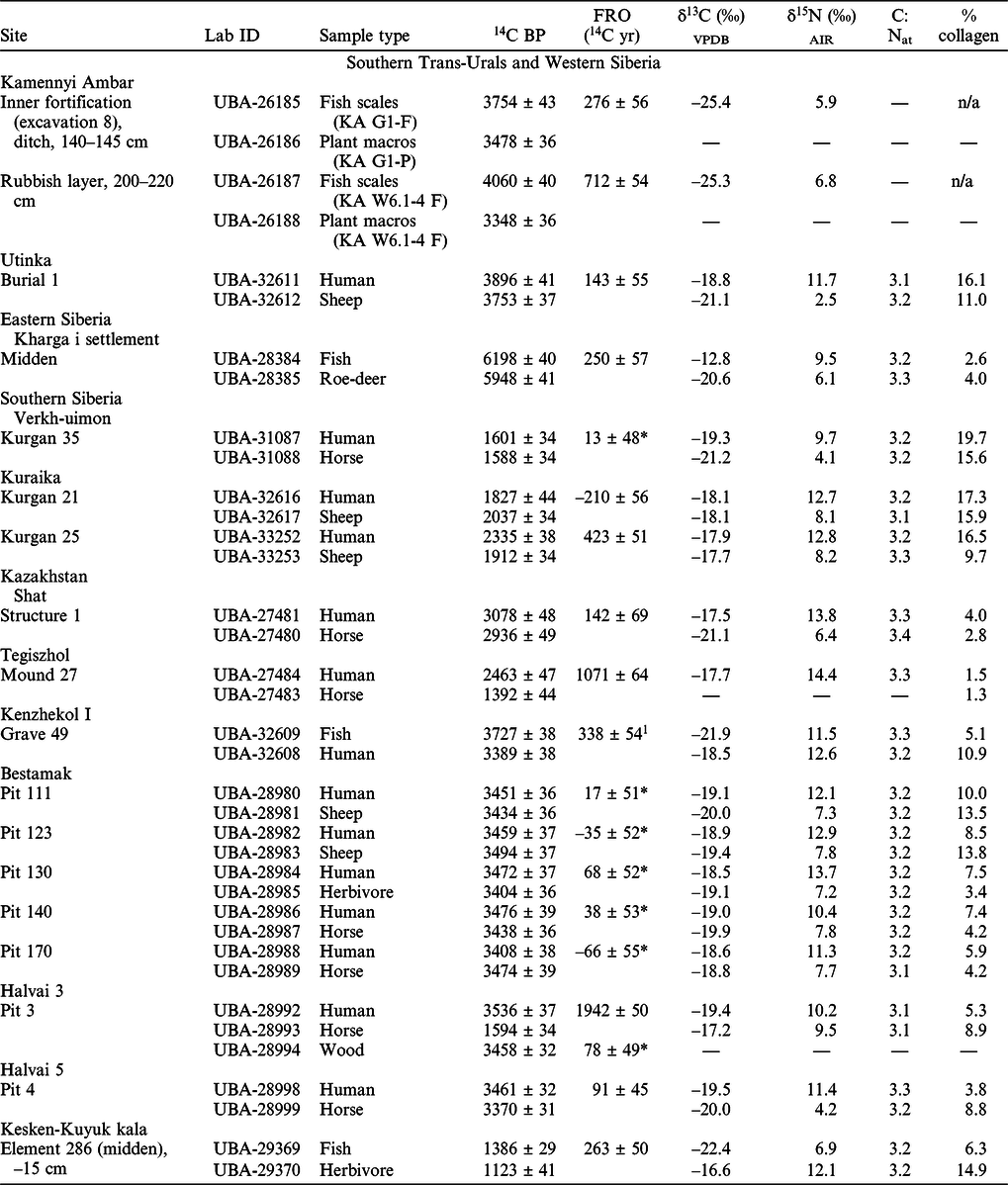
Notes: The cultural affiliation of the sites and specific layers or burials the samples come from is presented in SI 1. The location map of the site is presented in Figure 3.
1 This FRO was calculated between fish and human specimens, the latter possibly being not purely terrestrial sample.
*The dates are statistically indistinguishable at 95% level indicating no demonstrable FRO.
Stable Isotope Values
Stable isotope results (Figure 1) indicate predominately C3-based ecosystems for the sampled sites of the Eurasian Steppe, as expected. Enrichment in both C and N isotopes can only be observed in herbivores from the “marsh town” of Kesken-Kuyuk kala located in the delta (the ancient channel) of the Syr-Darya River. A wide range of nitrogen isotopic values (especially in herbivores) is likely to be the result of climatic variation, specifically aridity (e.g., Hollund et al. Reference Hollund, Higham, Belinskij and Korenevskij2010), as a number of sites are located in arid areas of Kazakhstan. The positive linear correlation between δ13C and δ15N for both herbivores (R2 = 0.730) and humans (R2 = 0.633) is likely related to a comparable gradient (Hollund et al. Reference Hollund, Higham, Belinskij and Korenevskij2010; Schulting and Richards Reference Schulting, Richards, Anthony, Brown, Khoklov, Kuznetsov and Mochalov2016).
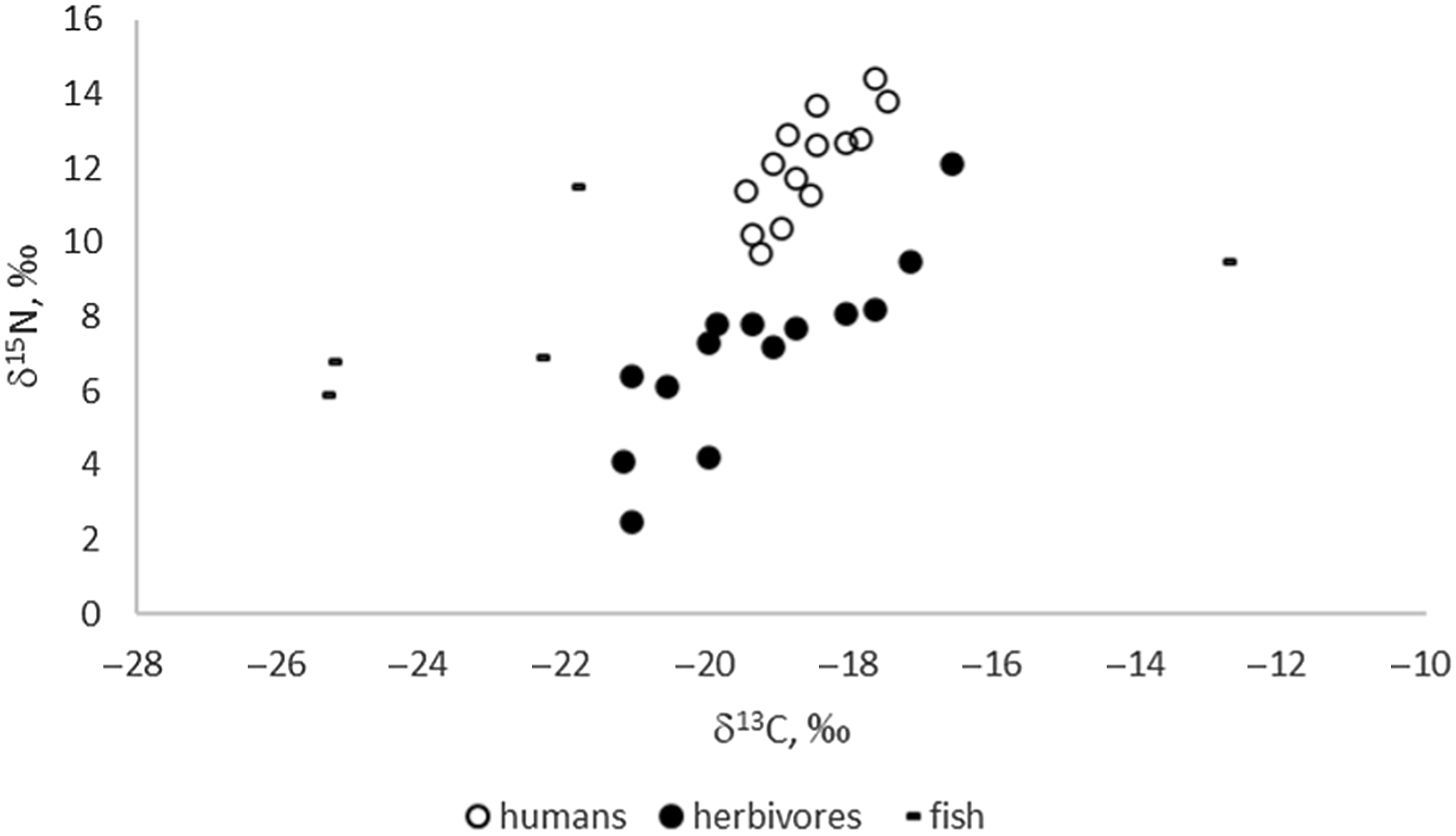
Figure 1 δ13C and δ15N values of the analyzed human and faunal samples (n=33).
Archaeological fish values show great variability in δ15N and δ13C values, as is also the case for modern freshwater fish (Dufour et al. Reference Dufour, Bocherens and Mariotti1999; Svyatko et al. Reference Svyatko, Reimer and Schulting2017a). While the variability in δ15N values is likely related primarily to the trophic level of the fish, δ13C values rather reflect the isotopic ecology of the particular reservoirs (e.g., Dufour et al. Reference Dufour, Bocherens and Mariotti1999; France Reference France1995; Hecky and Hesslein Reference Hecky and Hesslein1995; Spies et al. Reference Spies, Krueger, Ireland and Rice1989; Gu et al. Reference Gu, Schelske and Hoyer1996). It has been shown previously that freshwater reservoirs in the Eurasian Steppe may produce a wide range of δ13C signatures (e.g., Katzenberg and Weber Reference Katzenberg and Weber1999; Svyatko et al. Reference Svyatko, Reimer and Schulting2017a) depending on specific physical and biological factors, yet most reflect C3 ecologies. Our results show elevated δ13C for fish from the settlements of Kharga I (δ13C= –12.8‰) in Eastern Siberia, which corresponds with elevated δ13C data for modern fish from those areas (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a). The isotopic values and FROs of archaeological and modern fish in Siberia and the Eurasian Steppe are further discussed in detail elsewhere (Marchenko et al. Reference Marchenko, Svyatko and Grishin2021).
The mean δ13C and δ15N values for humans are –18.6 ± 1.3‰ and 12.1 ± 2.8‰, respectively; and for herbivores they are –19.3 ± 3.0‰ and 7.1 ± 4.8‰, respectively. We have not undertaken dietary modelling here because there is insufficient isotopic food source data for any of the sites to make this a realistic exercise. In the absence of representative sample sets of triple fish/human/terrestrial specimens, the human-herbivore pairs are not sufficient to provide a regional FRO value but only a minimum. It is impossible to be definite about the amount of fish consumption by humans based on stable isotope values alone, and, therefore, the FRO resulting from terrestrial/human pairs must be considered as a minimum value.
Freshwater Reservoir Effects
The results overall indicate a frequent occurrence of FROs from archaeological sites across the Eurasian Steppe, both for faunal and human samples. However, they are extremely variable, with the largest offset values reaching 1071 ± 64 14C years (human sample, site of Tegiszhol, Kazakhstan). An even larger FRO of 1942 ± 50 14C years, detected in pit 3 at Halvai 3, is likely the result of disturbance of the burial at a later period and intrusion of the animal bone. The 14C date for the wood sample from the same burial is similar to the date from the human sample which indicates an absence of an FRO within the 2σFRO range (78 ± 49), however the dates of paired samples from Halvai 5 are statistically different indicating a potential, albeit small, FRO (FRO=91 ± 45).
Negative FRO values that are within the 2σFRO range, such as those from Bestamak, also indicate the absence of a FRO. The negative FRO values that are larger than 2σFRO indicate that terrestrial samples are older than those containing an aquatic component, which is not theoretically possible if the pairs are contemporaneous. These sample pairs need detailed consideration. This concerns a pair from the site of Kuraika (kurgan 21) in the Altai Mountains, where sheep bone appears to be 210 ± 56 14C years older than associated human sample. The graves had apparently not been disturbed (see SI 1). At the moment it is not clear why the 14C date for the terrestrial sample is older than that of human here, but unrecognized disturbance, or the inclusion of residual material from an earlier grave or settlement, would seem the most likely explanations.
The FROs for archaeological fish vary between 250 ± 57 and 712 ± 54 14C years, with the highest value detected for the site of Kamennyi Ambar, where a measurement on another sample only showed a FRO of 276 ± 56, and underlines the differences that can occur even within the same site. There is often inconsistency in FROs between modern and archaeological fish samples within single areas. For example, within the Kharga I area, the FRO in archaeological fish is 250 14C years while the offset is only 15 14C years in modern fish from the associated lake (Figure 3), although we cannot rule out the possibility that the archaeological fish originated in a different reservoir. It is also possible that the Kharga basin itself exhibits variable reservoir effects (cf. Fernandes et al. Reference Fernandes, Grootes, Nadeau and Nehlich2015, tab. 4), or that the FRO has changed over time (cf. Ascough et al. Reference Ascough, Cook, Church, Dunbar, Einarsson, McGovern, Dugmore, Perdikaris, Hastie, Friðriksson and Gestsdóttir2010).
Logically, FRO values must be lower in humans than in the fish being consumed, as the extent of the human FROs depends on the proportion of fish in the diet. The maximal offset for a human sample determined within this study is 1071 ± 64 14C years (Tegiszhol, Kazakhstan), excluding the pair from Halvai 3 pit 3 discussed above. The results also indicate a moderate positive linear correlation between the size of FROs and both δ13C (R2 = 0.381) and δ15N (R2 = 0.324; Figure 2) for the human samples from this study, although the regressions are heavily weighted by the results from the undisturbed burial from Tegiszhol, Kazakhstan.
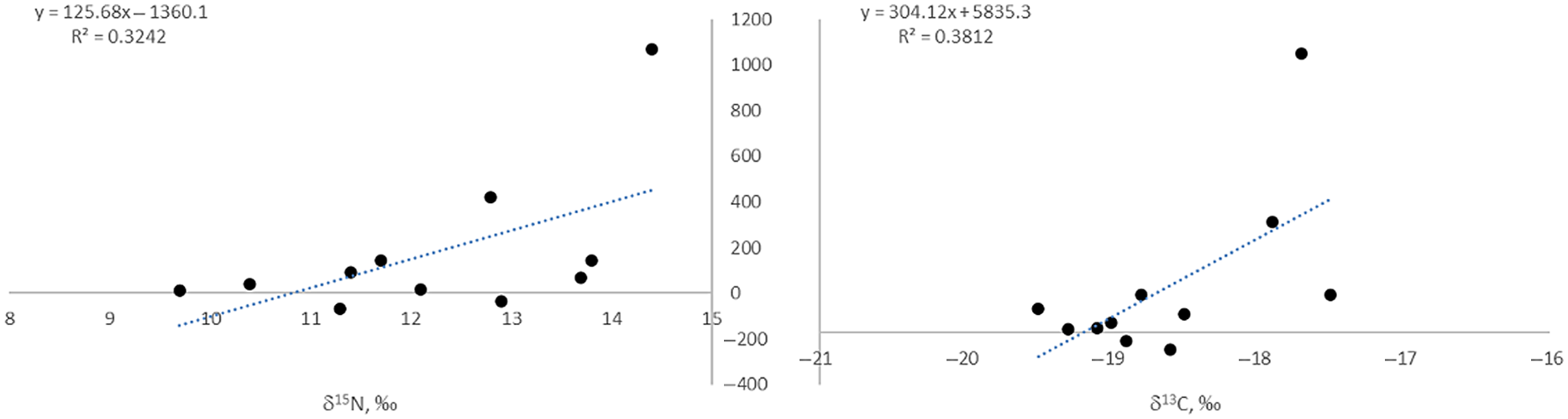
Figure 2 Human δ13C and δ15N plotted against their FRO values. Note that the pair from Halvai 3, and the pair from Kuraika (kurgan 21) with significant negative FRO values were removed, as this would be theoretically impossible.
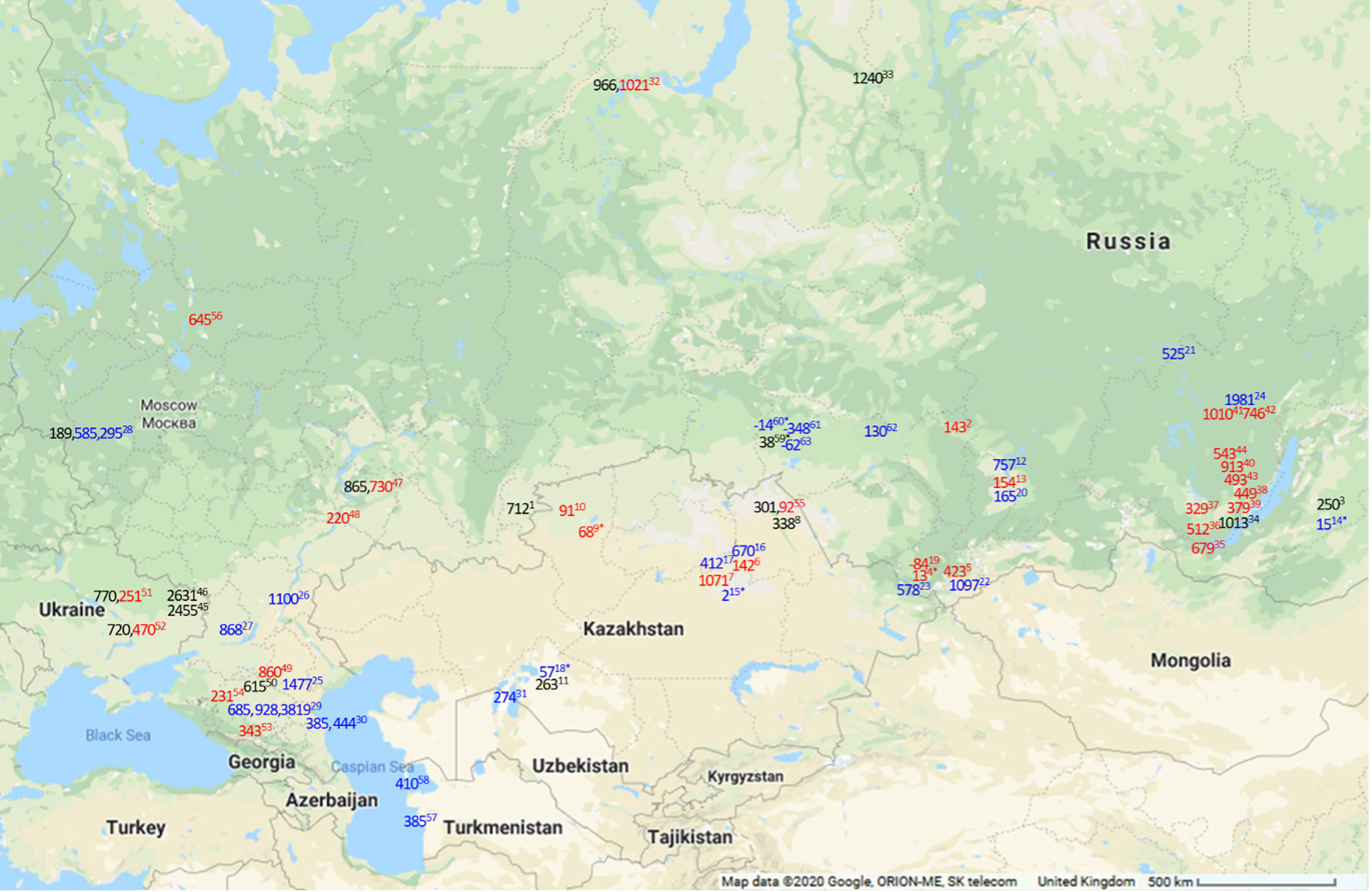
Figure 3 Map of maximal FROs in 14C years for modern (in blue) and maximal observed FROs in archaeological (in red for humans and black for other) samples in Siberia and the Eurasian Steppe. Numbers 1–11 are the sites sampled for current study. Locations presented are approximate. For exact locations see the source studies. 1. Kamennyi Ambar, fish (present study); 2. Utinka, human (present study); 3. Kharga I settlement, fish (present study); 4. Verkh-Uimon, human (present study); 5. Kuraika, human (present study); 6. Shat, human (present study); 7. Tegiszhol, human (present study); 8. Kenzhekol 1, fish (present study); 9. Bestamak, human (present study); 10. Halvai, human (present study); 11. Kesken-Kuyuk kala, fish (present study); 12. Karasuk Bay, modern fish (Svyatko et al. Reference Svyatko, Schulting, Poliakov and Reimer2017b); 13. Abakan 8, human (Svyatko et al. Reference Svyatko, Schulting, Poliakov and Reimer2017b); 14. Kharga Lake, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 15. Kyzylkoi River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 16. Shat River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 17. Nura River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 18. Syr-Darya River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 19. Perviy Mezhelik 1, human (Svyatko et al. Reference Svyatko, Polyakov, Soenov, Stepanova, Reimer, Ogle, Tyurina, Grushin and Rykun2017c); 20. Yenisei River, modern fish (Svyatko et al. Reference Svyatko, Schulting, Poliakov and Reimer2017b); 21. Edarma River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 22. Chuya River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 23. Katun River, modern fish (Svyatko et al. Reference Svyatko, Reimer and Schulting2017a); 24. Lena River, modern fish (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 25. Deed-Khulsun Lake, modern fish (van der Plicht et al. Reference van der Plicht, Shishlina and Zazovskaya2016); 26. Volga River, modern fish (van der Plicht et al. Reference van der Plicht, Shishlina and Zazovskaya2016); 27. Tsimlyansk city, modern algae (van der Plicht et al. Reference van der Plicht, Shishlina and Zazovskaya2016); 28. Serteya II and Serteyka River, food crusts, modern fish, aquatic plant (Kulkova et al. Reference Kulkova, Mazurkevich, Dolbunova, Regert, Mazuy, Nesterov and Sinai2015); 29. Podkumok River, modern fish, aquatic plant matter and water HCO3 (Higham et al. Reference Higham, Warren, Belinskij, Härke and Wood2010); 30. Tyuleniy Island, Sulak River mouth, seal, shell (Olsson Reference Olsson1980; Kuzmin et al. Reference Kuzmin, Nevesskaya, Krivonogov and Burr2007); 31. Kuzhetpes Island, shell (Kuzmin et al. Reference Kuzmin, Nevesskaya, Krivonogov and Burr2007); 32. Ust’-Polui, fish, human (Losey et al. Reference Losey, Fleming, Nomokonova, Gusev, Fedorova, Garvie-Lok, Bachura, Kosintsev and Sablin2018); 33. Mangazeya, fish (Kuzmin et al. Reference Kuzmin, Kosintsev, Boudin and Zazovskaya2020); 34. Sagan-Zaba II, seal (Nomokonova et al. Reference Nomokonova, Losey, Goriunova and Weber2013); 35. Shamanka II, human (Bronk Ramsey et al. Reference Bronk Ramsey, Schulting, Goriunova, Bazaliiskii and Weber2014); 36. Lokomotiv, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii, Goriunova and Weber2014); 37. Ust’-Ida, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii, Goriunova and Weber2014); 38. Kurma XI, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii, Goriunova and Weber2014); 39. Khuzhir-Nuge XIV, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii, Goriunova and Weber2014); 40. Popovskii Lug 2, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 41. Turuka, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 42. Zakuta, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 43. Makrushino, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 44. Ust’ Iamnaia, human (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015); 45. Starobelsk-II, shell (Motuzaite-Matuzeviciute et al. Reference Motuzaite-Matuzeviciute, Lillie and Telizhenko2015); 46. Novoselovka-III, shell (Motuzaite-Matuzeviciute et al. Reference Motuzaite-Matuzeviciute, Lillie and Telizhenko2015); 47. Lebyazhinka V, human, fish (Shishlina et al. Reference Shishlina, van der Plicht and Turetsky2018); 48. Khvalynsk II, human (Shishlina et al. Reference Shishlina, Sevastyanov, Zazovskaya and van der Plicht2014); 49. Peschany V, human (Shishlina et al. Reference Shishlina, Sevastyanov, Zazovskaya and van der Plicht2014); 50. Shakhaevskaya, fish (Shishlina et al. Reference Shishlina, Zazovskaya, van der Plicht and Sevastyanov2012); 51. Dereivka 1, fish, human (Lillie et al. Reference Lillie, Budd, Potekhina and Hedges2009); 52. Yasinovatka, fish, human (Lillie et al. Reference Lillie, Budd, Potekhina and Hedges2009); 53. Klin-Yar, human (Higham et al. Reference Higham, Warren, Belinskij, Härke and Wood2010); 54. Aygurskiy, human (Hollund et al. Reference Hollund, Higham, Belinskij and Korenevskij2010); 55. Shauke, human, fish (Svyatko et al. Reference Svyatko, Mertz and Reimer2015); 56. Minino, human (Wood et al. Reference Wood, Higham, Buzilhova, Suvorov, Heinemeier and Olsen2013); 57. Cheleken Peninsula, shell (Kuzmin et al. Reference Kuzmin, Nevesskaya, Krivonogov and Burr2007); 58. Garabogaz Spit, shell (Kuzmin et al. Reference Kuzmin, Nevesskaya, Krivonogov and Burr2007); 59. Preobrazhenka 6, fish (Marchenko et al. Reference Marchenko, Orlova, Panov, Zubova, Molodin, Pozdnyakova, Grishin and Uslamin2015); 60. Tartas R., fish (Marchenko et al. Reference Marchenko, Svyatko and Grishin2021); 61. Lozhka L., fish (Marchenko et al. Reference Marchenko, Svyatko and Grishin2021); 62. Ob R., fish (Marchenko et al. Reference Marchenko, Svyatko and Grishin2021); 63. Kama R., fish (Marchenko et al. Reference Marchenko, Svyatko and Grishin2021). *The values are statistically non-significant at 95% confidence, indicating the lack of any detectable FRO.
Yet, the major implication here is that the human isotopic values cannot reliably indicate the presence or absence of FROs across such a broad region. Neither does the presence of a FRO in associated archaeological or modern fish necessarily indicate the presence of the offsets in humans, since fish may not have been consumed to any great extent (e.g., 13 14C years in human from Verkh-Uimon versus 578 14C years in local modern fish from the Katun River).
SUMMARY
In recent decades, a number of freshwater radiocarbon offsets have been reported for various modern samples and archaeological sites of Siberia and the Eurasian Steppe region. Plotting our results together with existing FRO data for Siberia and the Eurasian Steppe (Figure 3), several observations can be made:
-
FROs are common but highly variable across the Eurasian Steppe in both modern and archaeological samples including humans. Radiocarbon dates from individuals consuming aquatic sources, such as humans, dogs, bears, beavers, certain birds and terrestrial herbivores (such as elk Alces alces feeding on water plants; e.g., Philippsen Reference Philippsen2019), fish and aquatic mammals, as well as food crusts (e.g., Hart et al. Reference Hart, Taché and Lovis2018), could be misleading;
-
FROs between modern and archaeological samples are often inconsistent within single areas and even within sites, especially in fish;
-
the presence of FROs in local archaeological or modern fish does not necessarily imply the presence of an offset in associated humans, i.e., fish or other aquatic resources do not always feature significantly in the diet;
-
a weak positive relationship has been found between FROs and δ13C or δ15N values of human samples across the region.
From the outlined scenario, it is clear that, when using freshwater/mixed resources for chronological reconstructions, the presence and the variability of FREs need to be explored in depth in each individual area and for each period as the hydrology or carbon sources could change (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015). The latter could be related to a number of factors, such as melting of permafrosts releasing old 14C-depleted carbon into the reservoir (Schulting et al. Reference Schulting, Bronk Ramsey, Bazaliiskii and Weber2015), geothermal activity (e.g., Ascough et al. Reference Ascough, Cook, Church, Dunbar, Einarsson, McGovern, Dugmore, Perdikaris, Hastie, Friðriksson and Gestsdóttir2010), or even changes in the hydrological system of an area (e.g., Marchenko et al. Reference Marchenko, Svyatko and Grishin2021). Bearing this in mind is particularly important for archaeologists because, as mentioned earlier, human remains are very often sampled for 14C dating, and without clear understanding of local FREs chronological reconstructions based on such dates may be unreliable. Without systematic research into the local food chain and isotopic baseline, it is very difficult to predict the extent (or even the presence) of a potential offset in human samples solely from δ13C and δ15N values. This would be especially the case when associated fish isotopic values are close to those for terrestrial fauna, in which case the consumption of fish would be isotopically invisible in humans. The application of other isotopes (δ34S, δ2H), as well as analysis of individual amino acids might help assessing the role of fish in the diet (e.g., Webb et al. Reference Webb, Honch, Dunn, Evershed, Eriksson and Lidén2015; Drucker et al. Reference Drucker, Valentin, Thevenet, Mordant, Cottiaux, Delsate and van Neer2018; Schulting et al. Reference Schulting, Snoeck, Begley, Brookes, Bazaliiskii, Bronk Ramsey and Weber2018). Yet, even when the isotopic values suggest the consumption of freshwater resources, this would not necessarily imply the existence of FROs in human samples.
ACKNOWLEDGMENTS
The study was supported by the Leverhulme Trust grant RPG-2014-08, grant of Ministry of Education and Science of the Russian Federation “Economic and social adaptation of human to climate conditions of the Altai Mountains in the second half of Holocene, №33.1971.2017/4.6”, and by the Russian Science Foundation project №20-18-00179 (Altai State University). We would like to thank Dr Igor Kovtun, Dr Leonid L. Gaiduchenko† and many other colleagues and friends of ours for their advice and help with acquiring samples. We are also very grateful to the anonymous reviewers of the manuscript and to Dr Yaroslav Kuzmin for their effort.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2022.21







